Non-Classical HLA Class 1b and Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Classical HLA Class I Molecules (HLA-Ia)
3. Non-Classical HLA Class I Molecules (HLA-Ib)
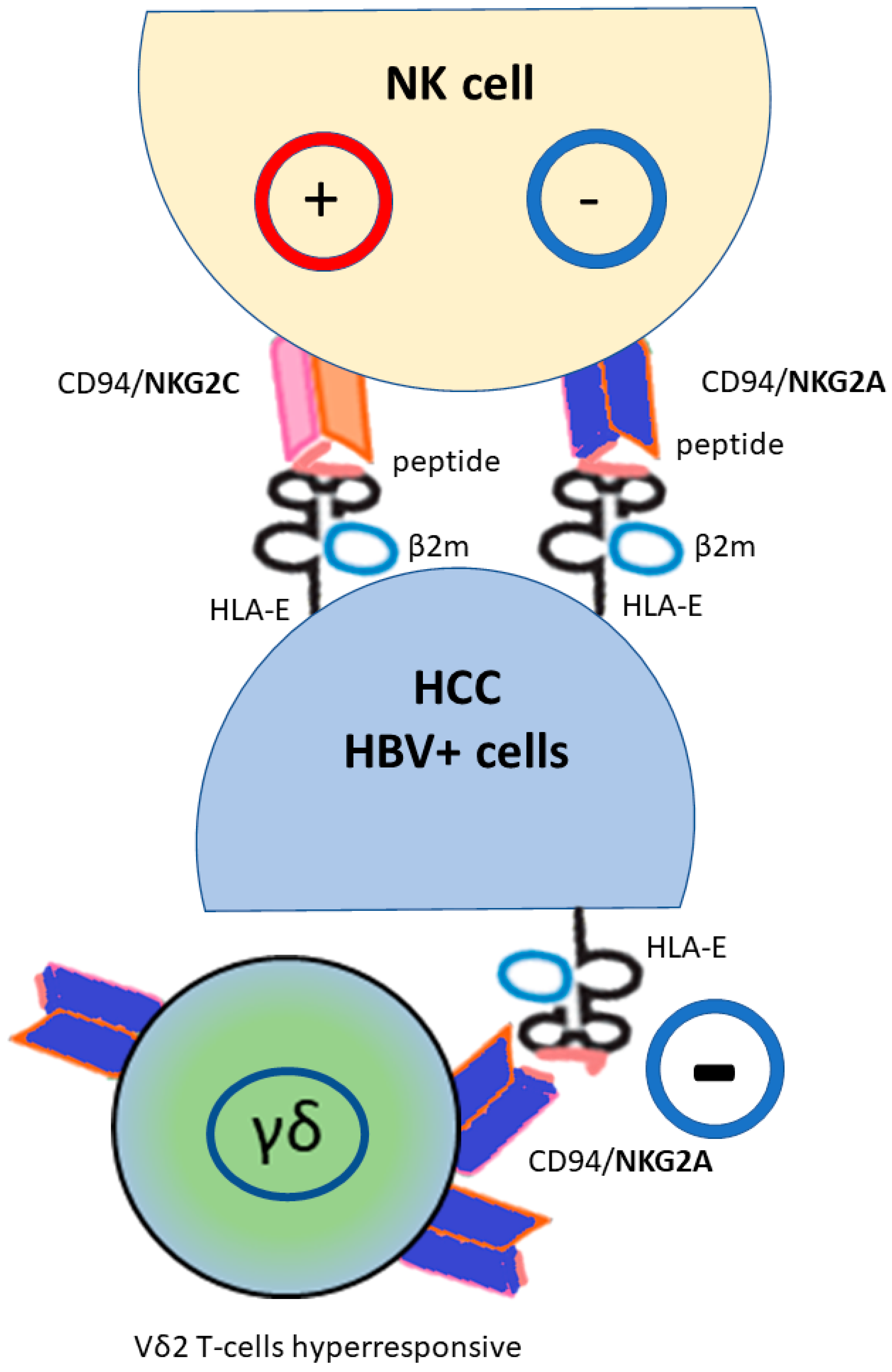
4. NK and NK-like T-Cells
5. Non-Classical NKG2A HLA-E as a Novel Potential Checkpoint Targeted Therapy for HCC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samant, H.; Amiri, H.S.; Zibari, G.B. Addressing the worldwide hepatocellular carcinoma: Epidemiology, prevention and management. J. Gastrointest. Oncol. 2021, 12, S361–S373. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, A.; De Re, V.; Steffan, A.; Ravaioli, M.; Miolo, G.; Leone, P.; Racanelli, V.; Uzzau, A.; Baccarani, U.; Cescon, M. Carcinogenesis and Metastasis in Liver: Cell Physiological Basis. Cancers 2019, 11, 1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.S.; Villeret, F.; Decaens, T.; Merle, P.; Nahon, P. Immunotherapy in hepatocellular carcinoma: How does underlying liver disease influence therapeutic strategy and outcomes? Liver Int. 2023, 43, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef]
- Tian, Z.; Hou, X.; Liu, W.; Han, Z.; Wei, L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Xu, J.; Wang, F.; Shi, M.; Zhang, Y.; Li, S.-P.; Zheng, L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum. Pathol. 2009, 40, 381–389. [Google Scholar] [CrossRef]
- Moeini, A.; Torrecilla, S.; Tovar, V.; Montironi, C.; Andreu-Oller, C.; Peix, J.; Higuera, M.; Pfister, D.; Ramadori, P.; Pinyol, R.; et al. An Immune Gene Expression Signature Associated With Development of Human Hepatocellular Carcinoma Identifies Mice That Respond to Chemopreventive Agents. Gastroenterology 2019, 157, 1383–1397.e11. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y.; et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.J.; Nicoletti, P.; Chalasani, N.; Serrano, J.; Stolz, A.; Daly, A.K.; Aithal, G.P.; Dillon, J.; Navarro, V.; Odin, J.; et al. Minocycline hepatotoxicity: Clinical characterization and identification of HLA-B∗35:02 as a risk factor. J. Hepatol. 2017, 67, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirasawa, M.; Hagihara, K.; Abe, K.; Ando, O.; Hirayama, N. Interaction of Nevirapine with the Peptide Binding Groove of HLA-DRB1*01:01 and Its Effect on the Conformation of HLA-Peptide Complex. Int. J. Mol. Sci. 2018, 19, 1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monshi, M.M.; Faulkner, L.; Gibson, A.; Jenkins, R.E.; Farrell, J.; Earnshaw, C.J.; Alfirevic, A.; Cederbrant, K.; Daly, A.K.; French, N.; et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology 2013, 57, 727–739. [Google Scholar] [CrossRef]
- Jee, A.; Sernoskie, S.C.; Uetrecht, J. Idiosyncratic Drug-Induced Liver Injury: Mechanistic and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2954. [Google Scholar] [CrossRef]
- Daly, A.K.; Day, C.P. Genetic association studies in drug-induced liver injury. Drug Metab. Rev. 2012, 44, 116–126. [Google Scholar] [CrossRef]
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef]
- Umemura, T.; Joshita, S.; Saito, H.; Wakabayashi, S.-I.; Kobayashi, H.; Yamashita, Y.; Sugiura, A.; Yamazaki, T.; Ota, M. Investigation of the Effect of KIR–HLA Pairs on Hepatocellular Carcinoma in Hepatitis C Virus Cirrhotic Patients. Cancers 2021, 13, 3267. [Google Scholar] [CrossRef]
- Joshita, S.; Ota, M.; Kobayashi, H.; Wakabayashi, S.-I.; Yamashita, Y.; Sugiura, A.; Yamazaki, T.; Tanaka, E.; Umemura, T. Association analysis of KIR/HLA genotype with liver cirrhosis, hepatocellular carcinoma, and NUC freedom in chronic hepatitis B patients. Sci. Rep. 2021, 11, 21424. [Google Scholar] [CrossRef]
- Khakoo, S.I.; Thio, C.L.; Martin, M.P.; Brooks, C.R.; Gao, X.; Astemborski, J.; Cheng, J.; Goedert, J.J.; Vlahov, D.; Hilgartner, M.; et al. HLA and NK Cell Inhibitory Receptor Genes in Resolving Hepatitis C Virus Infection. Science 2004, 305, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Hydes, T.J.; Moesker, B.; Traherne, J.A.; Ashraf, S.; Alexander, G.J.; Dimitrov, B.D.; Woelk, C.H.; Trowsdale, J.; Khakoo, S.I. The interaction of genetic determinants in the outcome of HCV infection: Evidence for discrete immunological pathways. Tissue Antigens 2015, 86, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Vázquez, A.; Rodrigo, L.; Martínez-Borra, J.; Pérez, R.; Rodríguez, M.; Fdez-Morera, J.L.; Fuentes, D.; Rodríguez-Rodero, S.; González, S.; López-Larrea, C. Protective Effect of the HLA-Bw4I80 Epitope and the Killer Cell Immunoglobulin-Like Receptor 3DS1 Gene against the Development of Hepatocellular Carcinoma in Patients with Hepatitis C Virus Infection. J. Infect. Dis. 2005, 192, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Zignego, A.L.; Izzo, F.; Tornesello, M.L.; Buonaguro, F.M.; Mangia, A.; Sansonno, D.; et al. Genetic Diversity of the KIR/HLA System and Susceptibility to Hepatitis C Virus-Related Diseases. PLoS ONE 2015, 10, e0117420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bona, D.; Aiello, A.; Colomba, C.; Bilancia, M.; Accardi, G.; Rubino, R.; Giannitrapani, L.; Tuttolomondo, A.; Cascio, A.; Caiaffa, M.F.; et al. KIR2DL3 and the KIR ligand groups HLA-A-Bw4 and HLA-C2 predict the outcome of hepatitis B virus infection. J. Viral Hepat. 2017, 24, 768–775. [Google Scholar] [CrossRef]
- Fan, Q.R.; Long, E.O.; Wiley, D.C. Crystal structure of the human natural killer cell inhibitory receptor KIR2DL1–HLA-Cw4 complex. Nat. Immunol. 2001, 2, 452–460. [Google Scholar] [CrossRef]
- Husain, Z.; Levitan, E.; Larsen, C.; Mirza, N.M.; Younes, S.; Yunis, E.J.; Alper, C.A.; Dubey, D.P. HLA-Cw7 zygosity affects the size of a subset of CD158b+ natural killer cells. J. Clin. Immunol. 2002, 22, 28–36. [Google Scholar] [CrossRef]
- Boudreau, J.E.; Hsu, K.C. Natural killer cell education in human health and disease. Curr. Opin. Immunol. 2018, 50, 102–111. [Google Scholar] [CrossRef]
- Hansasuta, P.; Dong, T.; Thananchai, H.; Weekes, M.; Willberg, C.; Aldemir, H.; Rowland-Jones, S.; Braud, V.M. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur. J. Immunol. 2004, 34, 1673–1679. [Google Scholar] [CrossRef]
- Bowness, P.; Ridley, A.; Shaw, J.; Chan, A.T.; Wong-Baeza, I.; Fleming, M.; Cummings, F.; McMichael, A.; Kollnberger, S. Th17 Cells Expressing KIR3DL2+ and Responsive to HLA-B27 Homodimers Are Increased in Ankylosing Spondylitis. J. Immunol. 2011, 186, 2672–2680. [Google Scholar] [CrossRef] [Green Version]
- Mouchet, N.; Vu, N.; Turlin, B.; Rioux-Leclercq, N.; Jouneau, S.; Samson, M.; Amiot, L. HLA-G Is Widely Expressed by Mast Cells in Regions of Organ Fibrosis in the Liver, Lung and Kidney. Int. J. Mol. Sci. 2021, 22, 12490. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, N.; Zhu, Q.; Li, Z.; Zhang, G.; Chen, J.; Lv, Y.; Wang, Y.; Liu, Z.; Hao, C. Association of serum soluble human leukocyte antigen-G levels with chronic hepatitis B virus infection. Clin. Exp. Med. 2014, 14, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, S.; Jia, S.; Zhu, Z.; Gao, X.; Dong, D.; Gao, Y.; Rizzo, R.; Bergamini, G.; Bortolotti, D.; et al. Association of HLA-G3′ UTR 14-bp Insertion/Deletion Polymorphism with Hepatocellular Carcinoma Susceptibility in a Chinese Population. DNA Cell Biol. 2011, 30, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Amiot, L.; Vu, N.; Samson, M. Biology of the immunomodulatory molecule HLA-G in human liver diseases. J. Hepatol. 2015, 62, 1430–1437. [Google Scholar] [CrossRef]
- Amiot, L.; Vu, N.; Rauch, M.; L’helgoualc’h, A.; Chalmel, F.; Gascan, H.; Turlin, B.; Guyader, D.; Samson, M. Expression of HLA-G by mast cells is associated with hepatitis C virus-induced liver fibrosis. J. Hepatol. 2014, 60, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Lim, H.S.; Kim, Y.S.; Hong, D.J.; Kim, H.S. Soluble human leukocyte antigen-G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue Antigens 2012, 79, 97–103. [Google Scholar] [CrossRef]
- Lin, A.; Chen, H.-X.; Zhu, C.-C.; Zhang, X.; Xu, H.-H.; Zhang, J.-G.; Wang, Q.; Zhou, W.-J.; Yan, W.-H. Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J. Cell. Mol. Med. 2010, 14, 2162–2171. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.F.; Willcox, B.E.; Wyer, J.R.; Boulter, J.M.; O’Callaghan, C.A.; Maenaka, K.; Stuart, D.I.; Jones, E.Y.; Van Der Merwe, P.A.; Bell, J.I.; et al. Classical and Nonclassical Class I Major Histocompatibility Complex Molecules Exhibit Subtle Conformational Differences That Affect Binding to CD8αα*. J. Biol. Chem. 2000, 275, 15232–15238. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, B.; Shang, J.; Yao, Y. HLA-G: An Important Mediator of Maternal-Fetal Immune-Tolerance. Front. Immunol. 2021, 12, 744324. [Google Scholar] [CrossRef]
- Sanders, S.K.; Giblin, P.A.; Kavathas, P.B. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class 1 molecule on cytotrophoblasts. J. Exp. Med. 1991, 174, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Attia, J.V.D.; Dessens, C.E.; van de Water, R.; Houvast, R.D.; Kuppen, P.J.K.; Krijgsman, D. The Molecular and Functional Characteristics of HLA-G and the Interaction with Its Receptors: Where to Intervene for Cancer Immunotherapy? Int. J. Mol. Sci. 2020, 21, 8678. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Shen, Y.; Xia, M.; Xu, L.; Pan, N.; Yin, Y.; Miao, F.; Shen, C.; Xie, W.; Zhang, J. Expression of the nonclassical HLA class I and MICA/B molecules in human hepatocellular carcinoma. Neoplasma 2011, 58, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.; Luo, W.; Zhu, Q.; Huang, H.; Peng, H.; Liu, R.; Xie, M.; Li, S.; Li, M.; Hu, X.; et al. Tumor-Derived Soluble MICA Obstructs the NKG2D Pathway to Restrain NK Cytotoxicity. Aging Dis. 2020, 11, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cariani, E.; Pilli, M.; Zerbini, A.; Rota, C.; Olivani, A.; Zanelli, P.; Zanetti, A.; Trenti, T.; Ferrari, C.; Missale, G. HLA and Killer Immunoglobulin-like Receptor Genes as Outcome Predictors of Hepatitis C Virus–Related Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 5465–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, M.J.W.; Rajagopalan, S.; Altmann, D.M.; Boyton, R.J.; Sun, P.D.; Long, E.O. Human NK cell receptor KIR2DS4 detects a conserved bacterial epitope presented by HLA-C. Proc. Natl. Acad. Sci. USA 2019, 116, 12964–12973. [Google Scholar] [CrossRef] [Green Version]
- Graef, T.; Moesta, A.K.; Norman, P.J.; Abi-Rached, L.; Vago, L.; Aguilar, A.M.O.; Gleimer, M.; Hammond, J.A.; Guethlein, L.A.; Bushnell, D.A.; et al. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J. Exp. Med. 2009, 206, 2557–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Pan, L.; Chen, L.; Feng, X.; Zhou, L.; Zheng, S. Non-classical MHC-Ι genes in chronic hepatitis B and hepatocellular carcinoma. Immunogenetics 2012, 64, 251–258. [Google Scholar] [CrossRef]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Cheung, P.F.Y.; Yip, C.W.; Wong, N.C.L.; Fong, D.Y.T.; Ng, L.W.C.; Wan, A.M.Y.; Wong, C.K.; Cheung, T.T.; Ng, I.O.L.; Poon, R.T.P.; et al. Granulin-Epithelin Precursor Renders Hepatocellular Carcinoma Cells Resistant to Natural Killer Cytotoxicity. Cancer Immunol. Res. 2014, 2, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Lei, J.; Chen, L.; Deng, H.; Dong, D.; Jin, T.; Liu, X.; Yuan, R.; Qiu, Y.; Ge, J.; et al. Human Leukocyte Antigen F Locus Adjacent Transcript 10 Overexpression Disturbs WISP1 Protein and mRNA Expression to Promote Hepatocellular Carcinoma Progression. Hepatology 2018, 68, 2268–2284. [Google Scholar] [CrossRef] [Green Version]
- Trinchieri, G. Interleukin-12: A cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 1994, 84, 4008–4027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, E.G.; Mariani, L.; Radbruch, A.; Höfer, T. Sequential Polarization and Imprinting of Type 1 T Helper Lymphocytes by Interferon-γ and Interleukin-12. Immunity 2009, 30, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; He, X.-L.; Qi, L.; Shi, W.; Yuan, L.-W.; Huang, M.-Y.; Xu, Y.-L.; Chen, X.; Gu, L.; Zhang, L.-L.; et al. Myricetin inhibits interferon-γ-induced PD-L1 and IDO1 expression in lung cancer cells. Biochem. Pharmacol. 2022, 197, 114940. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Jaw, J.J.; Stutzman, N.C.; Zou, Z.; Sun, P.D. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2011, 91, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Amadei, B.; Urbani, S.; Cazaly, A.; Fisicaro, P.; Zerbini, A.; Ahmed, P.; Missale, G.; Ferrari, C.; Khakoo, S.I. Activation of Natural Killer Cells During Acute Infection With Hepatitis C Virus. Gastroenterology 2010, 138, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; Zhou, C. Killer immunoglobulin-like receptors/human leukocyte antigen class-I, a crucial immune pathway in cancer. Ann. Transl. Med. 2020, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Ino, Y.; Hori, S.; Yamazaki-Itoh, R.; Naito, C.; Shimasaki, M.; Esaki, M.; Nara, S.; Kishi, Y.; Shimada, K.; et al. Expression of classical human leukocyte antigen class I antigens, HLA-E and HLA-G, is adversely prognostic in pancreatic cancer patients. Cancer Sci. 2020, 111, 3057–3070. [Google Scholar] [CrossRef]
- Wei, Y.; Bingyu, W.; Lei, Y.; Xingxing, Y. The antifibrotic role of natural killer cells in liver fibrosis. Exp. Biol. Med. 2022, 247, 1235–1243. [Google Scholar] [CrossRef]
- Wijaya, R.S.; Read, S.A.; Schibeci, S.; Eslam, M.; Azardaryany, M.K.; El-Khobar, K.; van der Poorten, D.; Lin, R.; Yuen, L.; Lam, V.; et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J. Hepatol. 2019, 71, 252–264. [Google Scholar] [CrossRef]
- Rodgers, J.R.; Cook, R.G. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 2005, 5, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Rascle, P.; Woolley, G.; Jost, S.; Manickam, C.; Reeves, R.K. NK cell education: Physiological and pathological influences. Front. Immunol. 2023, 14, 1087155. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.L.; Hviid, T.V.F. HLA Class Ib-receptor interactions during embryo implantation and early pregnancy. Hum. Reprod. Updat. 2022, 28, 435–454. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; Gonçalves, R.M.-B.; Menier, C.; Dausset, J.; Carosella, E.D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 11520–11525. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ikizawa, K.; Hu, D.; Werneck, M.B.; Wucherpfennig, K.W.; Cantor, H. Regulation of Activated CD4+ T Cells by NK Cells via the Qa-1–NKG2A Inhibitory Pathway. Immunity 2007, 26, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soloski, M.J.; Decloux, A.; Aldrich, C.J.; Forman, J. Structural and Functional Characteristics of the Class Ib Molecule, Qa-1. Immunol. Rev. 1995, 147, 67–89. [Google Scholar] [CrossRef]
- Borst, L.; van der Burg, S.H.; van Hall, T. The NKG2A–HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 5549–5556. [Google Scholar] [CrossRef]
- Hosseini, E.; Schwarer, A.P.; Ghasemzadeh, M. Do human leukocyte antigen E polymorphisms influence graft-versus-leukemia after allogeneic hematopoietic stem cell transplantation? Exp. Hematol. 2015, 43, 149–157. [Google Scholar] [CrossRef]
- Michaëlsson, J.; De Matos, C.T.; Achour, A.; Lanier, L.L.; Kärre, K.; Söderström, K. A Signal Peptide Derived from hsp60 Binds HLA-E and Interferes with CD94/NKG2A Recognition. J. Exp. Med. 2002, 196, 1403–1414. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.G.; Doyle, A.D.P.; Graham, L.V.; Khakoo, S.I.; Blunt, M.D. Disruption of the NKG2A:HLA-E Immune Checkpoint Axis to Enhance NK Cell Activation against Cancer. Vaccines 2022, 10, 1993. [Google Scholar] [CrossRef]
- Nguyen, S.; Beziat, V.; Dhedin, N.; Kuentz, M.; Vernant, J.P.; Debre, P.; Vieillard, V. HLA-E upregulation on IFN-γ-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2008, 43, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, T.G.; Crispim, J.C.O.; Miranda, F.A.; Hassumi, M.K.; de Mello, J.M.Y.; Simões, R.T.; Souto, F.; Soares, E.G.; Donadi, E.A.; Soares, C.P. Expression of the Nonclassical HLA-G and HLA-E Molecules in Laryngeal Lesions as Biomarkers of Tumor Invasiveness. Histol. Histopathol. 2011, 26, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.M.; Bianchini, M.; Von Euw, E.M.; Barrio, M.M.; Bravo, A.I.; Furman, D.; Domenichini, E.; Macagno, C.; Pinsky, V.; Zucchini, C.; et al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int. J. Oncol. 2008, 32, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, E.; Poschke, I.; Villabona, L.; Carlson, J.W.; Lundqvist, A.; Kiessling, R.; Seliger, B.; Masucci, G.V. Non-classical HLA-class I expression in serous ovarian carcinoma: Correlation with the HLA-genotype, tumor infiltrating immune cells and prognosis. Oncoimmunology 2016, 5, e1052213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Kruijf, E.M.; Sajet, A.; van Nes, J.G.H.; Natanov, R.; Putter, H.; Smit, V.T.H.B.M.; Liefers, G.J.; van den Elsen, P.J.; van de Velde, C.J.H.; Kuppen, P.J.K. HLA-E and HLA-G Expression in Classical HLA Class I-Negative Tumors Is of Prognostic Value for Clinical Outcome of Early Breast Cancer Patients. J. Immunol. 2010, 185, 7452–7459. [Google Scholar] [CrossRef] [Green Version]
- Jinushi, M.; Takehara, T.; Tatsumi, T.; Kanto, T.; Miyagi, T.; Suzuki, T.; Kanazawa, Y.; Hiramatsu, N.; Hayashi, N. Negative Regulation of NK Cell Activities by Inhibitory Receptor CD94/NKG2A Leads to Altered NK Cell-Induced Modulation of Dendritic Cell Functions in Chronic Hepatitis C Virus Infection. J. Immunol. 2004, 173, 6072–6081. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-K.; Liao, X.-W.; Yang, C.-K.; Yu, T.-D.; Liu, Z.-Q.; Gong, Y.-Z.; Huang, K.-T.; Zeng, X.-M.; Han, C.-Y.; Zhu, G.-Z.; et al. Diagnostic and prognostic biomarkers of Human Leukocyte Antigen complex for hepatitis B virus-related hepatocellular carcinoma. J. Cancer 2019, 10, 5173–5190. [Google Scholar] [CrossRef]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef] [Green Version]
- Jacquelot, N.; Seillet, C.; Souza-Fonseca-Guimaraes, F.; Sacher, A.G.; Belz, G.T.; Ohashi, P.S. Natural Killer Cells and Type 1 Innate Lymphoid Cells in Hepatocellular Carcinoma: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9044. [Google Scholar] [CrossRef]
- Borst, L.; Sluijter, M.; Sturm, G.; Charoentong, P.; Santegoets, S.J.; van Gulijk, M.; van Elsas, M.J.; Groeneveldt, C.; van Montfoort, N.; Finotello, F.; et al. NKG2A Is a Late Immune Checkpoint on CD8 T Cells and Marks Repeated Stimulation and Cell Division. Int. J. Cancer 2022, 150, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Highton, A.J.; Diercks, B.-P.; Möckl, F.; Martrus, G.; Sauter, J.; Schmidt, A.H.; Bunders, M.J.; Körner, C.; Guse, A.H.; Altfeld, M. High Metabolic Function and Resilience of NKG2A-Educated NK Cells. Front. Immunol. 2020, 11, 559576. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, L.; Liu, X.; Wang, X.; Yan, H.; Pu, Q.; Xie, Y.; Jiang, Y.; Du, J.; Yang, Z. The imbalance between NKG2A and NKG2D expression is involved in NK cell immunosuppression and tumor progression of patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol. Res. 2023, 53, 417–431. [Google Scholar] [CrossRef]
- Tesi, B.; Schlums, H.; Cichocki, F.; Bryceson, Y.T. Epigenetic Regulation of Adaptive NK Cell Diversification. Trends Immunol. 2016, 37, 451–461. [Google Scholar] [CrossRef]
- Rölle, A.; Meyer, M.; Calderazzo, S.; Jäger, D.; Momburg, F. Distinct HLA-E Peptide Complexes Modify Antibody-Driven Effector Functions of Adaptive NK Cells. Cell Rep. 2018, 24, 1967–1976.e4. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, S.; Oliviero, B.; Lombardi, A.; Varchetta, S.; Mele, D.; Sangiovanni, A.; Rossi, G.; Donadon, M.; Torzilli, G.; Soldani, C.; et al. Deficient Natural Killer Cell NKp30-Mediated Function and Altered NCR3 Splice Variants in Hepatocellular Carcinoma. Hepatology 2019, 69, 1165–1179. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, E.; Vaquero, J.; Férnandez-Barrena, M.G.; Lasarte, J.J.; Avila, M.A.; Sarobe, P.; Reig, M.; Calvo, M.; Fabregat, I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers 2021, 13, 3248. [Google Scholar] [CrossRef]
- Lassen, M.G.; Lukens, J.R.; Dolina, J.S.; Brown, M.G.; Hahn, Y.S. Intrahepatic IL-10 Maintains NKG2A+Ly49− Liver NK Cells in a Functionally Hyporesponsive State. J. Immunol. 2010, 184, 2693–2701. [Google Scholar] [CrossRef] [Green Version]
- Gunturi, A.; Berg, R.E.; Crossley, E.; Murray, S.; Forman, J. The role of TCR stimulation and TGF-Beta? in controlling the expression of CD94/NKG2A receptors on CD8 T?cells. Eur. J. Immunol. 2005, 35, 766–775. [Google Scholar] [CrossRef]
- Wu, M.; Mei, F.; Liu, W.; Jiang, J. Comprehensive characterization of tumor infiltrating natural killer cells and clinical significance in hepatocellular carcinoma based on gene expression profiles. Biomed. Pharmacother. 2020, 121, 109637. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.J.; Nguyen, A.T.; Lobos, C.A.; Szeto, C.; Chatzileontiadou, D.S.M.; Gras, S. The Unconventional Role of HLA-E: The Road Less Traveled. Mol. Immunol. 2020, 120, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Goodlett, D.R.; Ishitani, A.; Marquardt, H.; Geraghty, D.E. HLA-E Surface Expression Depends on Binding of TAP-Dependent Peptides Derived from Certain HLA Class I Signal Sequences. J. Immunol. 1998, 160, 4951–4960. [Google Scholar] [CrossRef] [PubMed]
- Pump, W.C.; Kraemer, T.; Huyton, T.; Hò, G.-G.T.; Blasczyk, R.; Bade-Doeding, C. Between Innate and Adaptive Immune Responses: NKG2A, NKG2C, and CD8+ T Cell Recognition of HLA-E Restricted Self-Peptides Acquired in the Absence of HLA-Ia. Int. J. Mol. Sci. 2019, 20, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braud, V.; Jones, E.Y.; McMichael, A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur. J. Immunol. 1997, 27, 1164–1169. [Google Scholar] [CrossRef]
- Jucaud, V.; Ravindranath, M.H.; Terasaki, P.I. Immunobiology of HLA Class-Ib Molecules in Transplantation. SOJ Immunol. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Hosseini, E.; Minagar, A.; Ghasemzadeh, M.; Arabkhazaeli, A.; Ghasemzadeh, A. HLA-E*01:01 + HLA-E*01:01 genotype confers less susceptibility to COVID-19, while HLA-E*01:03 + HLA-E*01:03 genotype is associated with more severe disease. Hum. Immunol. 2023, 84, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-Y.; Rha, M.-S.; Choi, S.J.; Lee, H.S.; Han, J.W.; Nam, H.; Kim, D.-U.; Lee, J.G.; Kim, M.S.; Park, J.Y.; et al. Identification of a distinct NK-like hepatic T-cell population activated by NKG2C in a TCR-independent manner. J. Hepatol. 2022, 77, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.; Ruiz-Cabello, F.; Pedrinaci, S.; Méndez, R.; Jiménez, P.; Geraghty, D.E.; Garrido, F. Analysis of HLA-E expression in human tumors. Immunogenetics 2003, 54, 767–775. [Google Scholar] [CrossRef]
- Cazzetta, V.; Bruni, E.; Terzoli, S.; Carenza, C.; Franzese, S.; Piazza, R.; Marzano, P.; Donadon, M.; Torzilli, G.; Cimino, M.; et al. NKG2A expression identifies a subset of human Vδ2 T cells exerting the highest antitumor effector functions. Cell Rep. 2021, 37, 109871. [Google Scholar] [CrossRef]
- Chang, K.-M.; Traum, D.; Park, J.-J.; Ho, S.; Ojiro, K.; Wong, D.K.; Wahed, A.S.; Terrault, N.A.; Khalili, M.; Sterling, R.K.; et al. Distinct phenotype and function of circulating Vδ1+ and Vδ2+ γδT-cells in acute and chronic hepatitis B. PLoS Pathog. 2019, 15, e1007715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A Class I Antigen, HLA-G, Expressed in Human Trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Fons, P.; Chabot, S.; Cartwright, J.E.; Lenfant, F.; L’Faqihi, F.; Giustiniani, J.; Herault, J.-P.; Gueguen, G.; Bono, F.; Savi, P.; et al. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 receptor expressed by endothelial cells. Blood 2006, 108, 2608–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwich, E.; Hò, G.-G.T.; LeMaoult, J.; Bade-Döding, C.; Carosella, E.D.; Horn, P.A.; Rebmann, V. Soluble HLA-G and HLA-G Bearing Extracellular Vesicles Affect ILT-2 Positive and ILT-2 Negative CD8 T Cells Complementary. Front. Immunol. 2020, 11, 2046. [Google Scholar] [CrossRef]
- Dumont, C.; Jacquier, A.; Verine, J.; Noel, F.; Goujon, A.; Wu, C.-L.; Hung, T.-M.; Desgrandchamps, F.; Culine, S.; Carosella, E.D.; et al. CD8+PD-1–ILT2+ T Cells Are an Intratumoral Cytotoxic Population Selectively Inhibited by the Immune-Checkpoint HLA-G. Cancer Immunol. Res. 2019, 7, 1619–1632. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef] [Green Version]
- Shiroishi, M.; Tsumoto, K.; Amano, K.; Shirakihara, Y.; Colonna, M.; Braud, V.M.; Allan, D.S.J.; Makadzange, A.; Rowland-Jones, S.; Willcox, B.; et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. USA 2003, 100, 8856–8861. [Google Scholar] [CrossRef] [Green Version]
- Adolf, I.C.; Almars, A.; Dharsee, N.; Mselle, T.; Akan, G.; Nguma, I.J.; Nateri, A.S.; Atalar, F. HLA-G and single nucleotide polymorphism (SNP) associations with cancer in African populations: Implications in personal medicine. Genes Dis. 2021, 9, 1220–1233. [Google Scholar] [CrossRef]
- Krijgsman, D.; Roelands, J.; Hendrickx, W.; Bedognetti, D.; Kuppen, P.J.K. HLA-G: A New Immune Checkpoint in Cancer? Int. J. Mol. Sci. 2020, 21, 4528. [Google Scholar] [CrossRef]
- Bian, X.; Si, Y.; Zhang, M.; Wei, R.; Yang, X.; Ren, H.; Zheng, G.; Wang, C.; Zhang, Y. Down-expression of miR-152 lead to impaired anti-tumor effect of NK via upregulation of HLA-G. Tumor Biol. 2015, 37, 3749–3756. [Google Scholar] [CrossRef]
- Cai, M.-Y.; Xu, Y.-F.; Qiu, S.-J.; Ju, M.-J.; Gao, Q.; Li, Y.-W.; Zhang, B.-H.; Zhou, J.; Fan, J. Human Leukocyte Antigen-G Protein Expression Is an Unfavorable Prognostic Predictor of Hepatocellular Carcinoma following Curative Resection. Clin. Cancer Res. 2009, 15, 4686–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ye, Z.; Meng, X.-Q.; Zheng, S.-S. Expression of HLA-G in patients with hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Catamo, E.; Zupin, L.; Crovella, S.; Celsi, F.; Segat, L. Non-classical MHC-I human leukocyte antigen (HLA-G) in hepatotropic viral infections and in hepatocellular carcinoma. Hum. Immunol. 2014, 75, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Catamo, E.; Zupin, L.; Freato, N.; Polesello, V.; Celsi, F.; Crocè, S.L.; Masutti, F.; Pozzato, G.; Segat, L.; Crovella, S. HLA-G regulatory polymorphisms are associated with susceptibility to HCV infection. HLA 2017, 89, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Martelli-Palomino, G.; Pancotto, J.A.; Muniz, Y.C.; Mendes-Junior, C.T.; Castelli, E.C.; Massaro, J.D.; Krawice-Radanne, I.; Poras, I.; Rebmann, V.; Carosella, E.D.; et al. Polymorphic Sites at the 3′ Untranslated Region of the HLA-G Gene Are Associated with Differential hla-g Soluble Levels in the Brazilian and French Population. PLoS ONE 2013, 8, e71742. [Google Scholar] [CrossRef] [PubMed]
- Souto, F.J.D.; Crispim, J.C.O.; Ferreira, S.C.; da Silva, A.S.M.; Bassi, C.L.; Soares, C.P.; Zucoloto, S.; Rouas-Freiss, N.; Moreau, P.; Martinelli, A.L.C.; et al. Liver HLA-G expression is associated with multiple clinical and histopathological forms of chronic hepatitis B virus infection. J. Viral Hepat. 2011, 18, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tong, S.; Li, S.; Wang, X.; Ren, H.; Yin, W. Increased ILT2 expression contributes to dysfunction of CD56dimCD16+NK cells in chronic hepatitis B virus infection. Antivir. Res. 2022, 205, 105385. [Google Scholar] [CrossRef]
- Liu, L.; Wang, L.; Zhao, L.; He, C.; Wang, G. The Role of HLA-G in Tumor Escape: Manipulating the Phenotype and Function of Immune Cells. Front. Oncol. 2020, 10, 597468. [Google Scholar] [CrossRef]
- Littera, R.; Perra, A.; Miglianti, M.; Piras, I.S.; Mocci, S.; Lai, S.; Melis, M.; Zolfino, T.; Balestrieri, C.; Conti, M.; et al. The double-sided of human leukocyte antigen-G molecules in type 1 autoimmune hepatitis. Front. Immunol. 2022, 13, 1007647. [Google Scholar] [CrossRef]
- Hò, G.-G.T.; Celik, A.A.; Huyton, T.; Hiemisch, W.; Blasczyk, R.; Simper, G.S.; Bade-Doeding, C. NKG2A/CD94 Is a New Immune Receptor for HLA-G and Distinguishes Amino Acid Differences in the HLA-G Heavy Chain. Int. J. Mol. Sci. 2020, 21, 4362. [Google Scholar] [CrossRef]
- Carosella, E.D.; Gregori, S.; Tronik-Le Roux, D. HLA-G/LILRBs: A Cancer Immunotherapy Challenge. Trends Cancer 2021, 7, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Airoldi, I. HLA-G and Other Immune Checkpoint Molecules as Targets for Novel Combined Immunotherapies. Int. J. Mol. Sci. 2022, 23, 2925. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, S.D.; Biro, P.A.; Holmes, C.H. HLA-F Is a Predominantly Empty, Intracellular, TAP-Associated MHC Class Ib Protein with a Restricted Expression Pattern. J. Immunol. 2000, 164, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodridge, J.P.; Lee, N.; Burian, A.; Pyo, C.-W.; Tykodi, S.S.; Warren, E.H.; Yee, C.; Riddell, S.R.; Geraghty, D.E. HLA-F and MHC-I Open Conformers Cooperate in a MHC-I Antigen Cross-Presentation Pathway. J. Immunol. 2013, 191, 1567–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F Complex without Peptide Binds to MHC Class I Protein in the Open Conformer Form. J. Immunol. 2010, 184, 6199–6208. [Google Scholar] [CrossRef] [Green Version]
- Arosa, F.A.; Esgalhado, A.J.; Reste-Ferreira, D.; Cardoso, E.M. Open MHC Class I Conformers: A Look through the Looking Glass. Int. J. Mol. Sci. 2021, 22, 9738. [Google Scholar] [CrossRef]
- Schulte, D.; Vogel, M.; Langhans, B.; Krämer, B.; Körner, C.; Nischalke, H.D.; Steinberg, V.; Michalk, M.; Berg, T.; Rockstroh, J.K.; et al. The HLA-E(R)/HLA-E(R) Genotype Affects the Natural Course of Hepatitis C Virus (HCV) Infection and Is Associated with HLA-E-Restricted Recognition of an HCV-Derived Peptide by Interferon-Gamma-Secreting Human CD8(+) T Cells. J. Infect. Dis. 2009, 200, 1397–1401. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, M.H.; Selvan, S.R.; Terasaki, P.I. Augmentation of anti-HLA-E antibodies with concomitant HLA-Ia reactivity in IFNγ-treated autologous melanoma cell vaccine recipients. J. Immunotoxicol. 2012, 9, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Morinaga, T.; Iwatsuki, M.; Yamashita, K.; Yasuda-Yoshihara, N.; Yamane, T.; Matsumoto, C.; Harada, K.; Eto, K.; Kurashige, J.; Ishimoto, T.; et al. Dynamic Alteration in HLA-E Expression and Soluble HLA-E via Interaction with Natural Killer Cells in Gastric Cancer. Ann. Surg. Oncol. 2023, 30, 1240–1252. [Google Scholar] [CrossRef]
- Barrett, D.M.; Gustafson, K.S.; Wang, J.; Wang, S.Z.; Ginder, G.D. A GATA Factor Mediates Cell Type-Restricted Induction of HLA-E Gene Transcription by Gamma Interferon. Mol. Cell. Biol. 2004, 24, 6194–6204. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, N.; Sayed, A.; Jeppesen, H.B.; Persson, G.; Weisdorf, I.; Funck, T.; Hviid, T.V.F. Characterization of HLA-G Regulation and HLA Expression in Breast Cancer and Malignant Melanoma Cell Lines upon IFN-γ Stimulation and Inhibition of DNA Methylation. Int. J. Mol. Sci. 2020, 21, 4307. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Boyle, L.H.; Gillingham, A.K.; Munro, S.; Trowsdale, J. Selective Export of HLA-F by Its Cytoplasmic Tail. J. Immunol. 2006, 176, 6464–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Ishitani, A.; Geraghty, D.E. HLA-F is a surface marker on activated lymphocytes. Eur. J. Immunol. 2010, 40, 2308–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burian, A.; Wang, K.L.; Finton, K.A.K.; Lee, N.; Ishitani, A.; Strong, R.K.; Geraghty, D.E. HLA-F and MHC-I Open Conformers Bind Natural Killer Cell Ig-Like Receptor KIR3DS1. PLoS ONE 2016, 11, e0163297. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Hölzemer, A.; Martrus, G.; Chung, A.W.; Pacheco, Y.; Simoneau, C.R.; Rucevic, M.; Lamothe-Molina, P.A.; Pertel, T.; Kim, T.-E.; et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat. Immunol. 2016, 17, 1067–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunemann, S.; Schöbel, A.; Kah, J.; Fittje, P.; Hölzemer, A.; Langeneckert, A.E.; Hess, L.U.; Poch, T.; Martrus, G.; Garcia-Beltran, W.F.; et al. Interactions Between KIR3DS1 and HLA-F Activate Natural Killer Cells to Control HCV Replication in Cell Culture. Gastroenterology 2018, 155, 1366–1371.e3. [Google Scholar] [CrossRef]
- Oliva, A.; Kinter, A.L.; Vaccarezza, M.; Rubbert, A.; Catanzaro, A.; Moir, S.; Monaco, J.; Ehler, L.; Mizell, S.; Jackson, R.; et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Investig. 1998, 102, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Juárez, A.; Gonzalez, R.; Camacho, A.; Manzanares-Martin, B.; Caruz, A.; Martínez-Peinado, A.; Torre-Cisneros, J.; Pineda, J.A.; Peña, J.; Rivero, A. Natural Killer KIR3DS1 Is Closely Associated with HCV Viral Clearance and Sustained Virological Response in HIV/HCV Patients. PLoS ONE 2013, 8, e61992. [Google Scholar] [CrossRef] [Green Version]
- Hò, G.-G.T.; Hiemisch, W.; Pich, A.; Behrens, G.M.N.; Blasczyk, R.; Bade-Doeding, C. The Loss of HLA-F/KIR3DS1 Ligation Is Mediated by Hemoglobin Peptides. Int. J. Mol. Sci. 2020, 21, 8012. [Google Scholar] [CrossRef] [PubMed]
- Mele, D.; Pasi, A.; Cacciatore, R.; Mantovani, S.; Oliviero, B.; Mondelli, M.U.; Varchetta, S. Decreased interferon-γ production by NK cells from KIR haplotype B carriers in hepatitis C virus infection. Liver Int. 2019, 39, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Frias, M.; Rivero-Juárez, A.; Rodriguez-Cano, D.; Camacho, Á.; López-López, P.; Risalde, M..; Manzanares-Martín, B.; Brieva, T.; Machuca, I.; Rivero, A. HLA-B, HLA-C and KIR improve the predictive value of IFNL3 for Hepatitis C spontaneous clearance. Sci. Rep. 2018, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Han, H.; Zhang, F.; Lv, S.; Li, Z.; Fang, Z. Lesion human leukocyte antigen-F expression is associated with a poor prognosis in patients with hepatocellular carcinoma. Oncol. Lett. 2015, 9, 300–304. [Google Scholar] [CrossRef] [Green Version]
- Wimalarathne, M.M.; Wilkerson-Vidal, Q.C.; Hunt, E.C.; Love-Rutledge, S.T. The case for FAT10 as a novel target in fatty liver diseases. Front. Pharmacol. 2022, 13, 972320. [Google Scholar] [CrossRef]
- Middleton, P.; Vergis, N. Mitochondrial dysfunction and liver disease: Role, relevance, and potential for therapeutic modulation. Ther. Adv. Gastroenterol. 2021, 14, 17562848211031394. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Buerger, S.; Groettrup, M. The ubiquitin-like modifier FAT10 in antigen processing and antimicrobial defense. Mol. Immunol. 2015, 68, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; French, B.; Tillman, B.; French, S. Different roles of FAT10, FOXO1, and ADRA2A in hepatocellular carcinoma tumorigenesis in patients with alcoholic steatohepatitis (ASH) vs non-alcoholic steatohepatitis (NASH). Exp. Mol. Pathol. 2018, 105, 144–149. [Google Scholar] [CrossRef]
- Jia, Y.; Ji, P.; French, S.W. The Role of FAT10 in Alcoholic Hepatitis Pathogenesis. Biomedicines 2020, 8, 189. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, K.; Hu, J.; Yan, C.; Li, M.; Yu, X.; Liu, X.; Lei, J.; Guo, W.; Wu, L.; et al. Ubiquitin-like Protein FAT10 Promotes the Invasion and Metastasis of Hepatocellular Carcinoma by Modifying β-Catenin Degradation. Cancer Res. 2014, 74, 5287–5300. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gong, M.; French, B.A.; Li, J.; Tillman, B.; French, S.W. Mallory–Denk Body (MDB) formation modulates ufmylation expression epigenetically in alcoholic hepatitis (AH) and non-alcoholic steatohepatitis (NASH). Exp. Mol. Pathol. 2014, 97, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, B.; Lee, A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Re, V.; Simula, M.P.; Cannizzaro, R.; Sansonno, D.E.; Canzonieri, V.; Gloghini, A.; Carbone, A.; Colombatti, A.; Marin, M.D.; De Zorzi, M.; et al. HCV inhibits antigen processing and presentation and induces oxidative stress response in gastric mucosa. Proteom. Clin. Appl. 2008, 2, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Mah, M.M.; Roverato, N.; Groettrup, M. Regulation of Interferon Induction by the Ubiquitin-Like Modifier FAT10. Biomolecules 2020, 10, 951. [Google Scholar] [CrossRef]
- Arshad, M.; Abdul Hamid, N.; Chan, M.C.; Ismail, F.; Tan, G.C.; Pezzella, F.; Tan, K.-L. NUB1 and FAT10 Proteins as Potential Novel Biomarkers in Cancer: A Translational Perspective. Cells 2021, 10, 2176. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, Z.; Liu, B.; Yang, P.; Wu, J.; Han, L.; Han, T.; Chen, T. FAT10 promotes hepatocellular carcinoma (HCC) carcinogenesis by mediating P53 degradation and acts as a prognostic indicator of HCC. J. Gastrointest. Oncol. 2021, 12, 1823–1837. [Google Scholar] [CrossRef]
- Sadagopan, A.; Michelakos, T.; Boyiadzis, G.; Ferrone, C.; Ferrone, S. Human Leukocyte Antigen Class I Antigen-Processing Machinery Upregulation by Anticancer Therapies in the Era of Checkpoint Inhibitors. JAMA Oncol. 2022, 8, 462. [Google Scholar] [CrossRef]
- Takahashi, A.; Umemura, A.; Yano, K.; Okishio, S.; Kataoka, S.; Okuda, K.; Seko, Y.; Yamaguchi, K.; Moriguchi, M.; Okanoue, T.; et al. Tyrosine Kinase Inhibitors Stimulate HLA Class I Expression by Augmenting the IFNγ/STAT1 Signaling in Hepatocellular Carcinoma Cells. Front. Oncol. 2021, 11, 707473. [Google Scholar] [CrossRef]
- Sung, P.S.; Racanelli, V.; Shin, E.-C. CD8+ T-Cell Responses in Acute Hepatitis C Virus Infection. Front. Immunol. 2014, 5, 266. [Google Scholar] [CrossRef] [Green Version]
- Crispe, I.N. Hepatocytes as Immunological Agents. J. Immunol. 2016, 196, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2013, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-Z.; Wang, T.; Wang, X.-F.; Zhang, Y.-Q.; Song, S.-X.; Ma, C.-Q. Improving the ability of CAR-T cells to hit solid tumors: Challenges and strategies. Pharmacol. Res. 2021, 175, 106036. [Google Scholar] [CrossRef] [PubMed]
- Khatwani, N.; Romee, R.; Pillai, A.B. Editorial: Innate immune cell therapy of cancer. Front. Immunol. 2022, 13, 4415. [Google Scholar] [CrossRef]
- Shen, J.; Yang, D.; Ding, Y. Advances in Promoting the Efficacy of Chimeric Antigen Receptor T Cells in the Treatment of Hepatocellular Carcinoma. Cancers 2022, 14, 5018. [Google Scholar] [CrossRef]
- Ragoonanan, D.; Khazal, S.J.; Abdel-Azim, H.; McCall, D.; Cuglievan, B.; Tambaro, F.P.; Ahmad, A.H.; Rowan, C.M.; Gutierrez, C.; Schadler, K.; et al. Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nat. Rev. Clin. Oncol. 2021, 18, 435–453. [Google Scholar] [CrossRef]
- Valiullina, A.K.; Zmievskaya, E.A.; Ganeeva, I.A.; Zhuravleva, M.N.; Garanina, E.E.; Rizvanov, A.A.; Petukhov, A.V.; Bulatov, E.R. Evaluation of CAR-T Cells’ Cytotoxicity against Modified Solid Tumor Cell Lines. Biomedicines 2023, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Brown, J.; Yu, Y.; Lee, D.; Zhou, K.; Dunn, Z.S.; Hon, R.; Wilson, M.; Kramer, A.; Zhu, Y.; et al. Targeting Immunosuppressive Tumor-Associated Macrophages Using Innate T Cells for Enhanced Antitumor Reactivity. Cancers 2022, 14, 2749. [Google Scholar] [CrossRef]
- Johanna, I.; Hernández-López, P.; Heijhuurs, S.; Scheper, W.; Bongiovanni, L.; de Bruin, A.; Beringer, D.X.; Oostvogels, R.; Straetemans, T.; Sebestyen, Z.; et al. Adding Help to an HLA-A*24:02 Tumor-Reactive γδTCR Increases Tumor Control. Front. Immunol. 2021, 12, 752699. [Google Scholar] [CrossRef]
- Rossi, J.-F.; Lu, Z.Y.; Massart, C.; Levon, K. Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer. Front. Immunol. 2021, 12, 595722. [Google Scholar] [CrossRef]
- Huang, M.; Cai, H.; Han, B.; Xia, Y.; Kong, X.; Gu, J. Natural Killer Cells in Hepatic Ischemia-Reperfusion Injury. Front. Immunol. 2022, 13, 870038. [Google Scholar] [CrossRef]
- Boudreau, J.E.; Hsu, K.C. Natural Killer Cell Education and the Response to Infection and Cancer Therapy: Stay Tuned. Trends Immunol. 2018, 39, 222–239. [Google Scholar] [CrossRef]
- Ding, W.; Xu, X.; Qian, Y.; Xue, W.; Wang, Y.; Du, J.; Jin, L.; Tan, Y. Prognostic Value of Tumor-Infiltrating Lymphocytes in Hepatocellular Carcinoma: A Meta-Analysis. Medicine 2018, 97, e13301. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Q.; Li, X.; Guo, Y.; Zhang, B.; Jin, Y.; Zhu, C.; Shen, Y.; Yang, P.; Shi, Y.; et al. Heterogeneity induced GZMA-F2R communication inefficient impairs antitumor immunotherapy of PD-1 mAb through JAK2/STAT1 signal suppression in hepatocellular carcinoma. Cell Death Dis. 2022, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liang, X.; Wang, P.; Hu, Y.; Qi, Y.; Jin, Y.; Du, Y.; Fang, C.; Tian, J. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano 2020, 14, 12679–12696. [Google Scholar] [CrossRef]
- Li, Z.; Han, N.; Ren, X.; Zhang, Y.; Chu, X. Effectiveness of TKI Inhibitors Combined With PD-1 in Patients With Postoperative Early Recurrence of HCC: A Real-World Study. Front. Oncol. 2022, 12, 833884. [Google Scholar] [CrossRef]
- Haber, P.K.; Castet, F.; Torres-Martin, M.; Andreu-Oller, C.; Puigvehí, M.; Miho, M.; Radu, P.; Dufour, J.-F.; Verslype, C.; Zimpel, C.; et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. Gastroenterology 2023, 164, 72–88.e18. [Google Scholar] [CrossRef]
- Zhang, Y.; Schmidt-Wolf, I.G.H. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020, 235, 9291–9303. [Google Scholar] [CrossRef]
- Yang, C.-K.; Huang, C.-H.; Hu, C.-H.; Fang, J.-H.; Chen, T.-C.; Lin, Y.-C.; Lin, C.-Y. Immunophenotype and antitumor activity of cytokine-induced killer cells from patients with hepatocellular carcinoma. PLoS ONE 2023, 18, e0280023. [Google Scholar] [CrossRef]
- Kim, N.J.; Yoon, J.H.; Tuomi, A.C.; Lee, J.; Kim, D. In-situ tumor vaccination by percutaneous ablative therapy and its synergy with immunotherapeutics: An update on combination therapy. Front. Immunol. 2023, 14, 1118845. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.-S.; Zhou, J.; Zhou, Q.-M.; Zhao, M.; Wang, Q.-J.; Huang, L.-X.; Li, Y.-Q.; Chen, S.-P.; Wu, P.-H.; Xia, J.-C. Minimally Invasive Treatment Combined With Cytokine-induced Killer Cells Therapy Lower the Short-term Recurrence Rates of Hepatocellular Carcinomas. J. Immunother. 2008, 31, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, J.; Zhang, H.; Liu, X.; Zuo, F.; Zhao, Y.; Zhao, Y.; Yin, X.; Guo, X.; Wu, X.; et al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell 2023, 41, 272–287.e9. [Google Scholar] [CrossRef]
- Tinker, A.V.; Hirte, H.W.; Provencher, D.; Butler, M.; Ritter, H.; Tu, D.; Azim, H.A.; Paralejas, P.; Grenier, N.; Hahn, S.-A.; et al. Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221. Clin. Cancer Res. 2019, 25, 6052–6060. [Google Scholar] [CrossRef] [Green Version]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Shi, J.; Zhang, Y.; Liu, J.; An, C.; Zhu, H.; Wu, P.; Hu, W.; Qin, R.; Yao, D.; et al. NKG2D discriminates diverse ligands through selectively mechano-regulated ligand conformational changes. EMBO J. 2022, 41, e107739. [Google Scholar] [CrossRef]
- Han, Y.; Sun, F.; Zhang, X.; Wang, T.; Jiang, J.; Cai, J.; Gao, Q.; Hezam, K.; Liu, Y.; Xie, J.; et al. CD24 targeting bi-specific antibody that simultaneously stimulates NKG2D enhances the efficacy of cancer immunotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1179–1190. [Google Scholar] [CrossRef]
- Jeong, W.-I.; Park, O.; Gao, B. Abrogation of the Antifibrotic Effects of Natural Killer Cells/Interferon-Gamma Contributes to Alcohol Acceleration of Liver Fibrosis. Gastroenterology 2008, 134, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Pant, A.; Lim, M. CAR-T Therapy in GBM: Current Challenges and Avenues for Improvement. Cancers 2023, 15, 1249. [Google Scholar] [CrossRef]
- Titov, A.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Petukhov, A.; Rakhmatullina, A.; Miftakhova, R.; Fainshtein, M.; Rizvanov, A.; Bulatov, E. Adoptive Immunotherapy beyond CAR T-Cells. Cancers 2021, 13, 743. [Google Scholar] [CrossRef]
- Haanen, J.B.; Cerundolo, V. NKG2A, a New Kid on the Immune Checkpoint Block. Cell 2018, 175, 1720–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzetta, V.; Depierreux, D.; Colucci, F.; Mikulak, J.; Mavilio, D. NKG2A Immune Checkpoint in Vδ2 T Cells: Emerging Application in Cancer Immunotherapy. Cancers 2023, 15, 1264. [Google Scholar] [CrossRef]
- Wu, S.-J.; Lin, C.-T.; Liao, C.H.; Lin, C.-M. Immunotherapeutic potential of blinatumomab-secreting γ9δ2 T Cells. Transl. Oncol. 2023, 31, 101650. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Bai, Y.; Lin, A.; Jiang, A.; Zhou, C.; Cheng, Q.; Liu, Z.; Chen, X.; Zhang, J.; Luo, P. Gamma delta T-cell-based immune checkpoint therapy: Attractive candidate for antitumor treatment. Mol. Cancer 2023, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. γδ T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Zumwalde, N.A.; Sharma, A.; Xu, X.; Ma, S.; Schneider, C.L.; Romero-Masters, J.C.; Hudson, A.W.; Gendron-Fitzpatrick, A.; Kenney, S.C.; Gumperz, J.E. Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. J. Clin. Investig. 2017, 2, e93179. [Google Scholar] [CrossRef] [Green Version]
- Lonez, C.; Hendlisz, A.; Shaza, L.; Aftimos, P.; Vouche, M.; Donckier, V.; Machiels, J.-P.H.; Van Den Eynde, M.; Canon, J.-L.; Carrasco, J.; et al. Celyad’s Novel CAR T-Cell Therapy for Solid Malignancies. Curr. Res. Transl. Med. 2018, 66, 53–56. [Google Scholar] [CrossRef]


| Receptors | Ligands | Reference | |
|---|---|---|---|
| Inhibitory receptors | KIR2DL1 | HLA-C2 | [26] |
| KIR2DL2 | HLA-C1 | [27] | |
| KIR2DL3 | HLA-C1 | [21] | |
| KIR3DL1 | HLA-Bw4 | [19,28] | |
| KIR3DL2 | HLA-A3, HLA-A11, HLA-B27 | [29,30] | |
| KIR2DL4 | HLA-G, soluble HLA-G | [31,32,33,34,35,36,37] | |
| CD94/NKG2A | HLA-E, HLA-G | [1,2,3,4,5,6] | |
| ILT2, ILT4, CD8 | HLA-A, B, C, G, soluble HLA-G | [38,39,40,41] | |
| Activating receptors | CD94/NKG2D | HLA-E, HLA-G, MIC-A/B | [2,3,12,33] |
| CD94/NKG2C | HLA-E, HLA-G | [31,35,42,43] | |
| KIR2DS1 | HLA-C2 | [44] | |
| KIR2DS2 | HLA-C1 | [44] | |
| KIR2DS4full isotype | HLA-A11, HLA-C05/epitope specific | [45,46] | |
| KIR3DS1 | HLA-F open form, HLA-F/HLA-1 heterodimer open form | [47] | |
| NKp30 | HLA-B7 | [48] | |
| Proteasome | Target proteins for degradation mRNA protein | HLA FAT10 (HLA-F-adjacent transcript 10) | [49,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Re, V.; Tornesello, M.L.; Racanelli, V.; Prete, M.; Steffan, A. Non-Classical HLA Class 1b and Hepatocellular Carcinoma. Biomedicines 2023, 11, 1672. https://doi.org/10.3390/biomedicines11061672
De Re V, Tornesello ML, Racanelli V, Prete M, Steffan A. Non-Classical HLA Class 1b and Hepatocellular Carcinoma. Biomedicines. 2023; 11(6):1672. https://doi.org/10.3390/biomedicines11061672
Chicago/Turabian StyleDe Re, Valli, Maria Lina Tornesello, Vito Racanelli, Marcella Prete, and Agostino Steffan. 2023. "Non-Classical HLA Class 1b and Hepatocellular Carcinoma" Biomedicines 11, no. 6: 1672. https://doi.org/10.3390/biomedicines11061672
APA StyleDe Re, V., Tornesello, M. L., Racanelli, V., Prete, M., & Steffan, A. (2023). Non-Classical HLA Class 1b and Hepatocellular Carcinoma. Biomedicines, 11(6), 1672. https://doi.org/10.3390/biomedicines11061672








