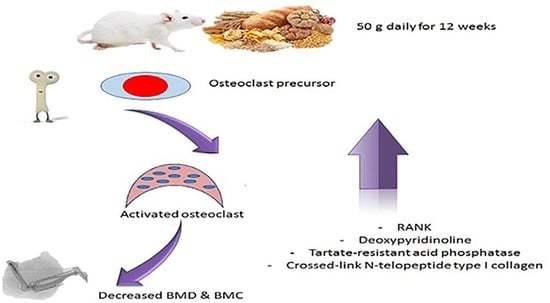
In Rats, Whole and Refined Grains Decrease Bone Mineral Density and Content through Modulating Osteoprotegerin and Receptor Activator of Nuclear Factor Kappa B
Abstract
:1. Practical Application
2. Introduction
3. Material and Methods
3.1. Animals
3.2. Experimental Design
3.3. Blood Collection and Biochemical Analysis
3.4. Measurement of BMD (Bone Mineral Density) and BMC (Bone Mineral Content) of Right Tibia
3.5. Estimation of Tibial Bone Oxidative and Anti-Oxidative Parameters
3.6. Statistical Analysis
4. Results
4.1. Grain Consumption Decreased OPG and Increased RANK
4.2. Grain Consumption Increased Bone Resorption Markers
4.3. Grain Consumption Reduced OC and ALP
4.4. Effect of Grain on Bone Mineral Density (BMD) and Bone Mineral Content (BMC)
5. Effect of Grains on Body Weight
5.1. Grains Increased MDA and Decreased GSH, SOD, and CAT
5.2. Correlations between Different Parameters Studied
6. Discussion
Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, U.H. Bone remodeling in post-menopausal osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; de Laet, C.E.; van Daele, P.L.; Weel, A.E.; Witteman, J.C.; Hofman, A.; Pols, H.A. Risk factors for increased bone loss in an elderly population: The rotterdam study. Am. J. Epidemiol. 1998, 147, 871–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type i and type ii osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kwan Tat, S.; Padrines, M.; Théoleyre, S.; Heymann, D.; Fortun, Y. Il-6, rankl, tnf-alpha/il-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar]
- Elesawy, B.H.; Alsanie, W.F.; Algahtany, M.A.; Al-Ashkhari, J.M.; Alyarobi, A.K.; Sakr, H.F. Whole and refined grains change behavior and reduce brain derived neurotrophic factor and neurotrophin-3 in rats. J. Food Biochem. 2021, 45, e13867. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Duerksen, D.; Pinto-Sanchez, M.I.; Anca, A.; Schnetzler, J.; Case, S.; Zelin, J.; Smallwood, A.; Turner, J.; Verdú, E.; Butzner, J.D.; et al. Management of bone health in patients with celiac disease: Practical guide for clinicians. Can. Fam. Physician 2018, 64, 433–438. [Google Scholar]
- van Staa, T.P.; Dennison, E.M.; Leufkens, H.G.; Cooper, C. Epidemiology of fractures in england and wales. Bone 2001, 29, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Kavuncu, V.; Dundar, U.; Ciftci, I.H.; Evcik, D.; Yigit, I. Is there any requirement for celiac disease screening routinely in postmenapausal women with osteoporosis? Rheumatol. Int. 2009, 29, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Stenson, W.F.; Newberry, R.; Lorenz, R.; Baldus, C.; Civitelli, R. Increased prevalence of celiac disease and need for routine screening among patients with osteoporosis. Arch. Intern. Med. 2005, 165, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtaugh, M.A.; Jacobs, D.R., Jr.; Jacob, B.; Steffen, L.M.; Marquart, L. Epidemiological support for the protection of whole grains against diabetes. Proc. Nutr. Soc. 2003, 62, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.B.; Pozniak, C.; Fahima, T.; Krugman, T. Grain protein content and thousand kernel weight qtls identified in a durum × wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2020, 133, 119–131. [Google Scholar] [CrossRef]
- Prasad, K.; Dhar, I. Oxidative stress as a mechanism of added sugar-induced cardiovascular disease. Int. J. Angiol. 2014, 23, 217–226. [Google Scholar]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [Green Version]
- Huh, Y.J.; Kim, J.M.; Kim, H.; Song, H.; So, H.; Lee, S.Y.; Kwon, S.B.; Kim, H.J.; Kim, H.H.; Lee, S.H.; et al. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2006, 13, 1138–1146. [Google Scholar] [CrossRef]
- Huffman, F.G.; Vaccaro, J.A.; Zarini, G.G.; Vieira, E.R. Osteoporosis, activities of daily living skills, quality of life, and dietary adequacy of congregate meal participants. Geriatrics 2018, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Worldwide incidence of hip fracture in elderly women: Relation to consumption of animal and vegetable foods. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M585–M592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, D.L.; Knutsen, S.F.; Beeson, W.L.; Rajaram, S.; Fraser, G.E. Effects of meat consumption and vegetarian diet on risk of wrist fracture over 25 years in a cohort of peri- and postmenopausal women. Public Health Nutr. 2008, 11, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, L.K. Dietary animal and plant protein and human bone health: A whole foods approach. J. Nutr. 2003, 133, 187–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, I.; Seneweera, S.; Hirotsu, N. Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice 2018, 11, 018–0200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef]
- Romas, E.; Gillespie, M.T.; Martin, T.J. Involvement of receptor activator of nfkappab ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone 2002, 30, 340–346. [Google Scholar]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 1742–4933. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am. J. Med. 2008, 121, 014. [Google Scholar] [CrossRef]
- Koh, J.M.; Khang, Y.H.; Jung, C.H.; Bae, S.; Kim, D.J.; Chung, Y.E.; Kim, G.S. Higher circulating hscrp levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: Evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005, 16, 1263–1271. [Google Scholar] [CrossRef]
- Ganesan, K.; Teklehaimanot, S.; Tran, T.H.; Asuncion, M.; Norris, K. Relationship of c-reactive protein and bone mineral density in community-dwelling elderly females. J. Natl. Med. Assoc. 2005, 97, 329–333. [Google Scholar]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. Allergens to wheat and related cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar] [PubMed]
- Kotze, L.M.; Skare, T.; Vinholi, A.; Jurkonis, L.; Nisihara, R. Impact of a gluten-free diet on bone mineral density in celiac patients. Rev. Esp. Enferm. Dig. 2016, 108, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.T.; Nisihara, R.M.; Kotze, L.R.; Olandoski, M.; Kotze, L.M. Low bone mineral density in brazilian patients at diagnosis of celiac disease. Arq. Gastroenterol. 2015, 52, 176–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, H.F.; Hussein, A.M.; Eid, E.A.; Boudaka, A.; Lashin, L.S. Impact of dehydroepiandrosterone (dhea) on bone mineral density and bone mineral content in a rat model of male hypogonadism. Vet. Sci. 2020, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Ylipahkala, H.; Alatalo, S.L.; Janckila, A.J.; Heikkinen, J.E.; Suominen, H.; Cheng, S.; Vaananen, H.K. Serum tartrate-resistant acid phosphatase 5b, but not 5a, correlates with other markers of bone turnover and bone mineral density. Calcif. Tissue Int. 2002, 71, 20–25. [Google Scholar] [CrossRef]
- Seibel, M.J. Biochemical markers of bone turnover: Part i: Biochemistry and variability. Clin. Biochem. Rev. 2005, 26, 97–122. [Google Scholar]
- Christgau, S.; Bitsch-Jensen, O.; Hanover Bjarnason, N.; Gamwell Henriksen, E.; Qvist, P.; Alexandersen, P.; Bang Henriksen, D. Serum crosslaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone 2000, 26, 505–511. [Google Scholar] [CrossRef]
- Francis, R.M. The effects of testosterone on osteoporosis in men. Clin. Endocrinol. 1999, 50, 411–414. [Google Scholar] [CrossRef]





| Chow Diet (Con Group) | Whole Grain | Refined Grain | |
|---|---|---|---|
| Carbohydrate % | 63 | 68 | 71 |
| Fat % | 13 | 12 | 9 |
| Protein % | 24 | 20 | 20 |
| Nutrients | Whole Grain | Refined Grain |
|---|---|---|
| Calories from Protein % | 20 | 20 |
| Calories from Total lipids % | 12 | 9 |
| Calories from Carbohydrates % | 68 | 71 |
| Dietary fiber (g/kg) | 115 | 19 |
| Starch and sugar(g/kg) | 700 | 830 |
| Zn (µg/g) | 29 | 8 |
| Fe (µg/g) | 35 | 13 |
| Magnesium (mg/g) | 1.38 | 0.22 |
| Vitamin B6 (mg/g) | 7.5 | 1.4 |
| Folate (mg/g) | 0.57 | 0.11 |
| Ferulic acid (mg/g) | 0.5 | 0.04 |
| Vitamin A (µg/g) | 32.8 | 5.7 |
| Control | Whole Grain | Refined Grain | |
|---|---|---|---|
| MDA (nmol/g tissue) | 8.21 ± 0.14 | 11.34 ± 0.16 * | 12.61 ± 0.18 * # |
| GSH (mg/g tissue) | 6.42 ± 0.12 | 4.15 ± 0.11 * | 4.75 ± 0.23 * |
| SOD (U/g tissue) | 54.14 ± 3.14 | 27.14 ± 4.51 * | 32.14 ± 5.61 * |
| CAT (U/g tissue) | 0.58 ± 0.02 | 0.27 ± 0.08 * | 0.39 ± 0.05 * |
| Control | Whole Grain | Refined Grain | |
|---|---|---|---|
| RANK (ng/mL) | 5.287 ± 1.134 | 8.78 ± 0.90 * | 8.95 ± 0.88 * |
| OPG (pg/mL) | 220.1 ± 12.18 | 151.7 ± 15.86 * | 161.9 ± 22.94 * |
| TRAP 5b (ng/mL) | 15.19 ± 3.381 | 29.46 ± 6.575 * | 31.53 ± 6.625 * |
| DPD (ng/mL) | 96.66 ± 14.33 | 128.1 ± 8.104 * | 134.9 ± 9.980 * |
| NTXI (ng/mL) | 4.923 ± 0.5537 | 6.739 ± 1.292 * | 6.496 ± 0.7727 * |
| Osteocalcin (pg/mL) | 12.24 ± 1.199 | 7.106 ± 1.054 * | 7.719 ± 1.47 * |
| ALP (ng/mL) | 513.1 ± 29.48 | 412.3 ± 40.70 * | 269.6 ± 49.37 * # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, H.; Khired, Z.; Moghadas, M. In Rats, Whole and Refined Grains Decrease Bone Mineral Density and Content through Modulating Osteoprotegerin and Receptor Activator of Nuclear Factor Kappa B. Biomedicines 2023, 11, 1686. https://doi.org/10.3390/biomedicines11061686
Sakr H, Khired Z, Moghadas M. In Rats, Whole and Refined Grains Decrease Bone Mineral Density and Content through Modulating Osteoprotegerin and Receptor Activator of Nuclear Factor Kappa B. Biomedicines. 2023; 11(6):1686. https://doi.org/10.3390/biomedicines11061686
Chicago/Turabian StyleSakr, Hussein, Zenat Khired, and Marzieh Moghadas. 2023. "In Rats, Whole and Refined Grains Decrease Bone Mineral Density and Content through Modulating Osteoprotegerin and Receptor Activator of Nuclear Factor Kappa B" Biomedicines 11, no. 6: 1686. https://doi.org/10.3390/biomedicines11061686
APA StyleSakr, H., Khired, Z., & Moghadas, M. (2023). In Rats, Whole and Refined Grains Decrease Bone Mineral Density and Content through Modulating Osteoprotegerin and Receptor Activator of Nuclear Factor Kappa B. Biomedicines, 11(6), 1686. https://doi.org/10.3390/biomedicines11061686