Abstract
(1) There are limited clinical trials to support the effectiveness of mouth rinses when used as a preprocedural rinse against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This systematic review aims to evaluate the efficacy of antiseptic mouth rinses as a preprocedural rinse in reducing SARS-CoV-2 oral viral load in-vivo. (2) Methods: A literature search was conducted through November 2022 for the following databases: PubMed, Web of Science, Cochrane Library, and Google Scholar. The evaluated outcomes were quantitative changes in viral load and the statistical significance of that change after using antiseptic mouth rinses. (3) Results: 14 randomized controlled trials (RCT) were selected for risk of bias assessment and data extraction. (4) Conclusion: Within the limits of this systematic review, preprocedural mouth rinses may significantly reduce SARS-CoV-2 in the mouth, thus, reducing the viral particles available for airborne dispersion. Preprocedural mouth rinses may be an effective strategy for reducing airborne SARS-CoV-2 dispersion in the environment. Their use may be a preventive strategy to reduce the spread of COVID-19 in selected medical and healthcare facilities, including dental clinics. Potential preprocedural mouth rinses are identified for use as an integral part of safe practice for healthcare protocols. This systematic review was registered with the National Institute for Health Research, international prospective register of systematic reviews (PROSPERO): CRD42022315177.
Keywords:
SARS-CoV; COVID; mouthwash; antiseptics; cetylpyridinium; chlorhexidine; iodine; peroxide; antiviral; rinses 1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission has been linked to exhaled aerosols [1]. Viral load generating this mode of transmission of SARS-CoV-2 is highest in the oropharynx, nasopharynx, and nasal cavity [2]. Aerosols can be generated during speech, breathing, coughing, and sneezing. Patients that are pre-symptomatic, symptomatic or asymptomatic are all potential sources of transmission [3,4]. Infection control practices have been critical in controlling the infection and the spread of the virus [5]. Vaccination has been a key role in reducing SARS-CoV-2 spread [6]; however, the vaccines have failed to effectively exclude infectious virus in the upper respiratory tract [7]. Oral antiseptics have been reported to reduce the risk of disease transmission and viral infectivity from aerosol-generating procedures.
Saliva has been used for detecting SARS-CoV-2 in patients with COVID-19, reporting a detection rate of up to 91.7% [8]. Patients with COVID-19 had the highest salivary viral load during the first week after symptom onset when evaluating endotracheal aspirate and saliva samples [9]. This may explain the rapid spread of the pandemic and warrant the use of mouth rinses as a preventive health measure [10].
This suggested that saliva from the oral cavity can be a potential high-risk route of infection for SARS-CoV-2 [11]. The involvement of saliva in SARS-CoV-2 spread suggested that antimicrobial mouthwashes containing substances with virucidal activity can help reduce viral transmission in high-risk environments [12]. This includes household and healthcare settings that perform aerosol-generating procedures. Virucidal effects have been seen in substances contained in oral antiseptics [13,14,15]. Interestingly, animal models observing SARS-CoV-1, a virus similar in genomics to SARS-CoV-2, showed that the virus persisted in oral mucous membranes for up to 2 days before infecting the lower respiratory tract [16]. This may present an opportunity to control disease progression with a potential therapeutic antiseptic rinse applicable to the oral cavity.
Promising data in both in vitro and clinical studies have shown the virucidal effects of antimicrobial mouthwashes or oral antiseptics against viruses such as influenza [17]. In vitro, contact of 15–60 s with different oral antiseptics resulted in solid virucidal effects and reduced viral infectivity [18,19,20]. Specifically relating to coronaviruses, evidence of virucidal effects against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS-CoV-1 has been seen with povidone-iodine (PVP), an active ingredient in several oral antiseptics [21,22]. Another substance, cetylpyridinium chloride (CPC), was reported to decrease more than a thousand times the infectivity of SARS-CoV-2 measured by the tissue culture [23]. This activity was confirmed in distinct variants of SARS-CoV- 2, suggesting a broad antiviral efficacy. In addition, chlorhexidine (CHX) and hydrogen peroxide (H2O2) have in vitro virucidal effects against coronaviruses and SARS-CoV-2 [19,20,24,25,26,27].
Antiseptic mouth rinses have been adopted into the pre-appointment protocols of many dental offices due to the COVID-19 pandemic. This is based empirically on the available outcome of in vitro studies at the start of the pandemic to reduce the oral viral load and the possibility of transmission [27,28]. However, extremely limited clinical trials were available then to support the effectiveness of the mouth rinses on SARS-CoV-2. This systematic review aims to evaluate which mouth rinses effectively reduce oral SARS-CoV-2 shedding in the oral cavity and assess the substantivity of the mouth rinses to suppress the viral load.
2. Materials and Methods
2.1. Focus Question
The focus question is, “What are the clinical effects of oral antiseptics on SARS-CoV-2 in patients with COVID-19?”. The PICOS parameters are Participants: patients infected with SARS-CoV-2; Interventions: oral antiseptic mouthwashes; Comparisons: use of saline or water or compared to baseline; Outcomes: antiviral effects; Study Design: clinical studies.
2.2. Literature Search and Study Design
PubMed, Web of Science, Cochrane Library, and Google Scholar were searched until November 2022. Google Scholar was used for the grey literature search. The following keywords (Table 1) were used to search each database: “SARS-CoV-2”, “COVID-19”, “coronavirus”, “oral antiseptics”, “mouthwash”, “chlorhexidine”, “iodine”, “peroxide”, “cetylpyridinium”, “alcohol”, “chlorine dioxide”, “povidone”, “octenidine dihydrochloride”, “polyaminopropyl biguanide”, “chloride”. The reference list of the selected articles was further hand-searched for any articles not included in the initial search. Authors of the selected articles were contacted to request additional quantitative data or information regarding their studies. The corresponding authors of the selected studies were given one week to respond with any additional information.

Table 1.
Keyword Search.
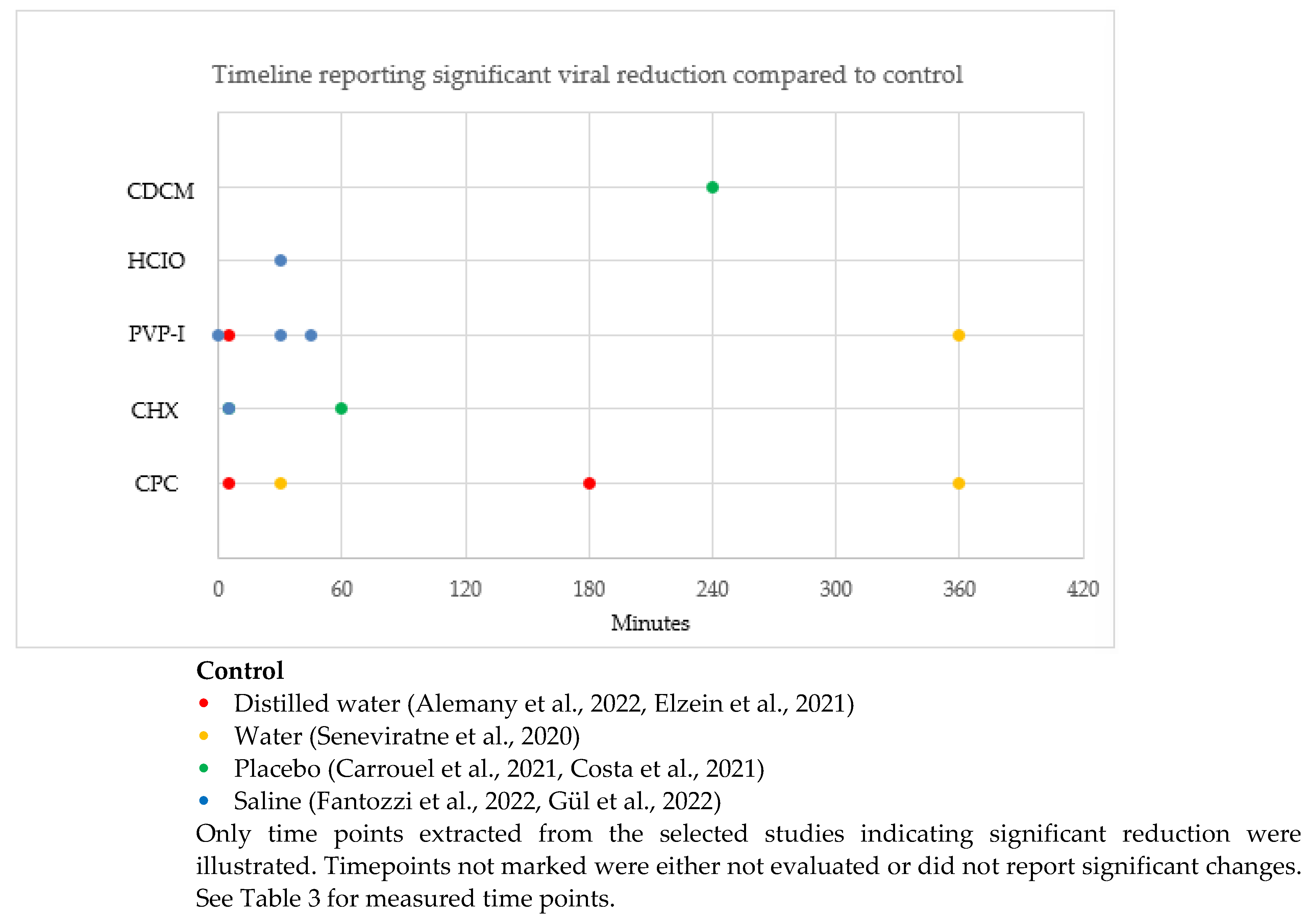
This systematic review was registered with the National Institute for Health Research, international prospective register of systematic reviews (PROSPERO): CRD42022315177. There were no amendments to the initial PROSPERO registered information. This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [29]. The flowchart (Figure 1) illustrates the systematic literature search conducted according to the PRISMA guidelines [29].
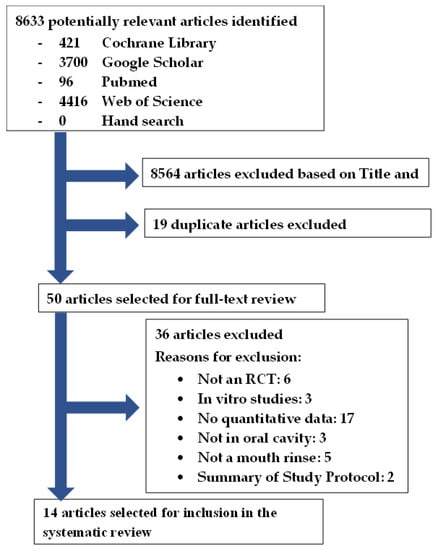
Figure 1.
Article selection flowchart in accordance with the PRISMA guidelines [26].
2.3. Inclusion Criteria
- Randomized controlled trials
- Clinical studies on SARS-CoV-2 only
- Only studies reporting quantitative data on viral load
- Only mouthwashes are used in the oral cavity
2.4. Exclusion Criteria
- In-vitro studies were excluded
- Descriptive studies were excluded
- Case reports were excluded
- Nasal irrigation/sprays were excluded
- Studies reporting qualitative data were excluded
- Studies reporting data as % of SAR-CoV-2 positive patients were excluded
2.5. Screening and Data Extraction
The “Title and Abstract” were independently screened by two reviewers (M.T. and M.W.); articles were excluded if they did not meet the inclusion criteria. The full text was independently analyzed by two reviewers (M.T. and M.W.) and verified by another two reviewers (J.J.S. and A.D.). A previously pilot-tested data extraction sheet was used by one reviewer (M.T.) for data extraction and independently verified by two other reviewers (J.J.S. and A.D.).
2.6. Risk of Bias Assessment
The risk of bias (Table 2) was assessed using the revised tool for assessing the risk of bias in randomized trials (RoB 2) [30]. Two reviewers (J.J.S. and A.D.) independently scored the risk of bias for the selected studies. Any disagreements were resolved with a discussion with a third reviewer (M.T.).

Table 2.
Risk of Bias Assessment 2 (ROB2).
3. Results
3.1. Search Results
The search yielded 8633 studies: 421 in Cochrane Library, 3700 in Google Scholar, 96 in PubMed, 4416 in Web of Science, and no additional articles from hand searching of the reference list of the selected articles (Figure 1). After the title and abstract screening, the duplicates were removed, and 50 articles remained for full-text analysis. After full-text analysis, 36 were eliminated: 6 for not being a randomized trial [44,45,46,47], 3 for being an in vitro study [48,49,50], 17 for not having quantitative data of viral load [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67], 3 for not being in the oral cavity [68,69,70], 5 for not being a mouth rinse [71,72,73,74,75], and 2 for being a summary protocol [76,77]. Finally, 14 articles were selected for data extraction [12,31,32,33,34,35,36,37,38,39,40,41,42,43].
3.2. Quality of Evidence
The risk of bias (Table 2) of the selected 14 randomized controlled trials reported mostly low risk of bias. Ten of the 14 studies have a low risk of bias [12,31,32,33,34,35,38,40,41,43], three studies have one domain with unclear risk of bias [36,37,42], and 1 study has two domains with unclear risk of bias or high risk of bias [39]. All selected studies utilized baseline or control or both for comparison. However, selected studies with controls were too heterogenous to enable a meaningful meta-analysis. The quantitative data reported utilized different units of measure, monitored different sampling time points, different rinse protocols, different follow-up periods, different mouth rinses and different combinations of mouth rinses.
3.3. Study Characteristics
All 14 selected studies were randomized controlled trials (Table 3). Many of these studies assessed viral load in saliva by measuring viral RNA by means of reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The included studies conducted randomized controlled trials of patients infected with SARS-CoV-2. The outcomes reported were viral load pre-intervention and viral load at designated time intervals post-intervention.

Table 3.
Selected studies.
Mouth rinses evaluated in the selected studies were PVP-I (0.5%, 1%, 2%), H2O2 (1%, 1.5%), CPC (0.7%, 0.75%, CHX (0.12%, 0.2%, beta-cyclodextrin and citrox (CDCM), CPC + Zn, H2O2 + CHX, HCIO (0.02%), BAC. The controls used in the studies were either distilled water, water, saline or an unreported placebo. Patients in the selected studies rinsed 10–20 mL of an antiseptic mouth rinse for 30–60 s. Three studies did an additional rinse again some minutes later [33,34,41]. The follow-up duration ranges from immediate to 6 h after rinsing. Six of the 14 studies reported no adverse reaction from the mouth rinses [12,33,34,35,39,42]. The other eight studies did not report adverse reactions [31,32,36,37,38,40,41,43].
3.4. Outcomes
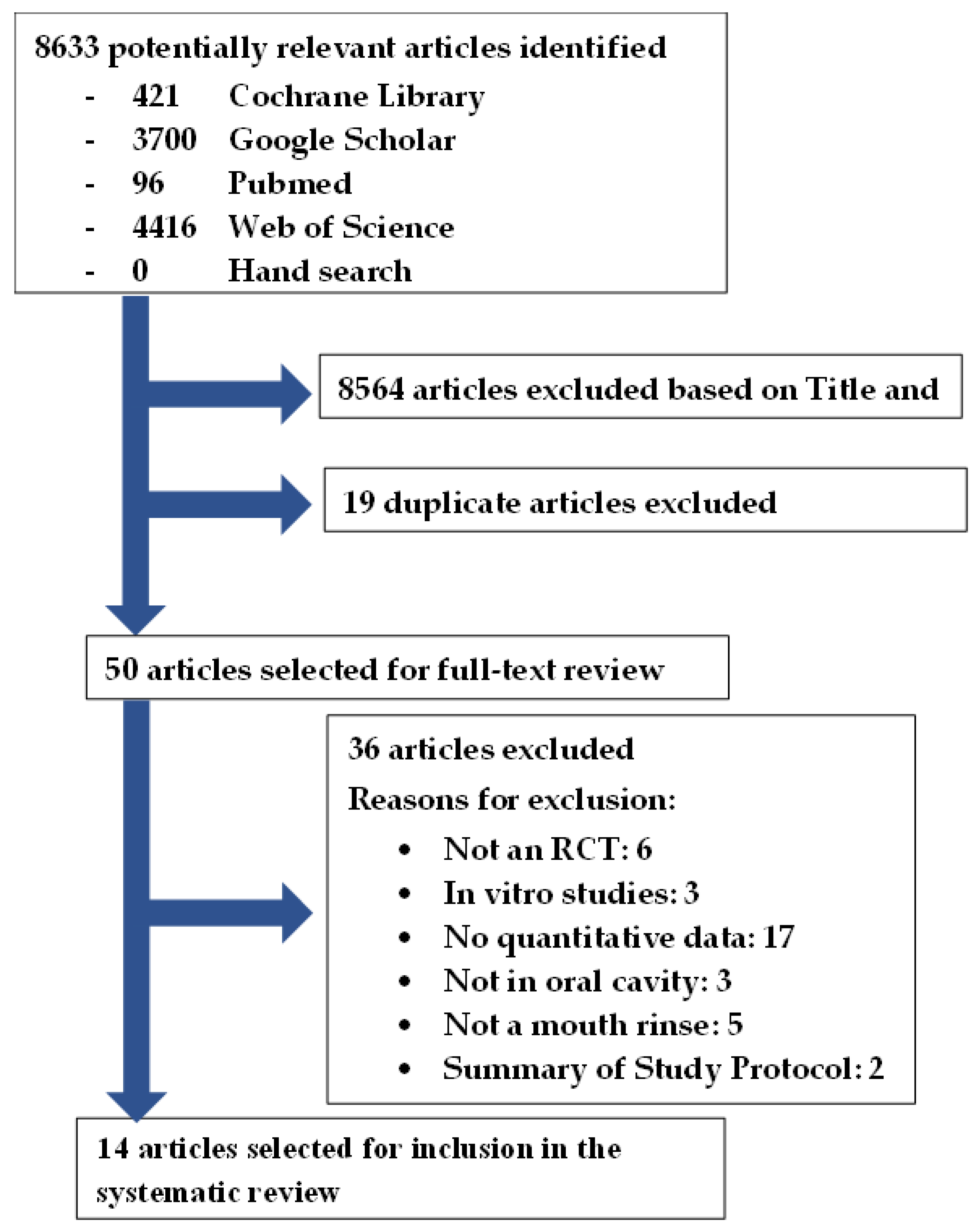
The timeline reporting a significant difference in SARS-CoV-2 reduction compared to the baseline in the selected studies is illustrated in Figure 2. Timepoints unmarked in Figure 2 were either not evaluated or did not report significant changes.
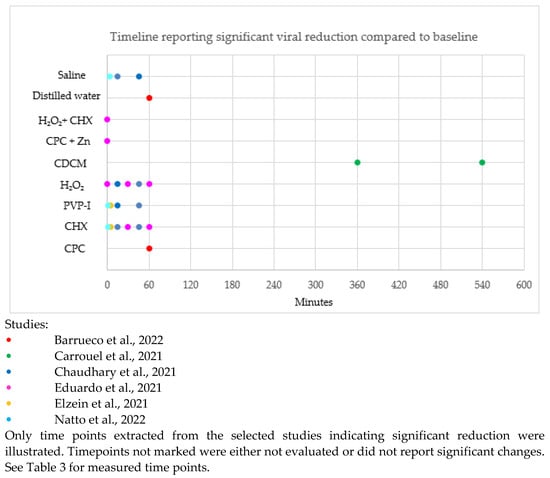
Figure 2.
Timeline of significant difference in SARS-CoV-2 reduction after rinsing with antiseptic compared to baseline [31,32,33,35,36,41].
CPC, CHX, H2O2 and distilled water showed a significant reduction of SARS-CoV-2 up to 60 min compared to baseline. PVP-I and saline showed a significant reduction of SARS-CoV-2 up to 45 min compared to baseline. CDCM showed a significant reduction of SARS-CoV-2 up to 540 min compared to baseline. H2O2 + CHX and CPC + Zn only reported a significant reduction of SARS-CoV-2 immediately post-rinse.
Thus, CPC, CHX, H2O2, PVP-I, CDCM, saline and distilled water can significantly affect viral reduction up to 45–60 min compared to baseline. However, further evaluation beyond 60 min is needed to report on the long-term efficacy.
Saline and water appear to have a significant effect in the reduction of SARS-CoV-2 compared to baseline. It is important to distinguish if mouth rinses are significantly better than water and saline alone.
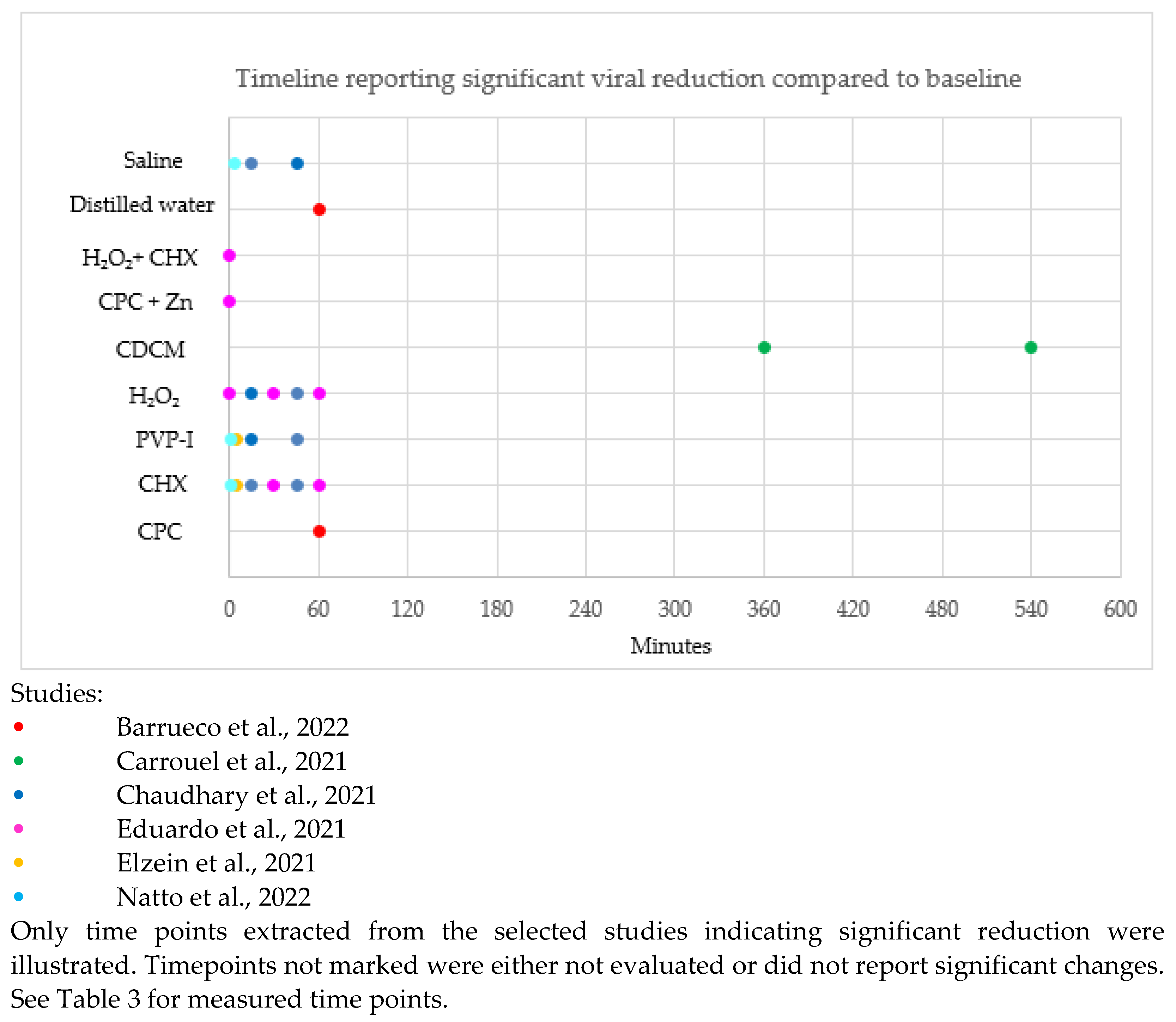
The timeline reporting a significant difference in SARS-CoV-2 reduction compared to control in the selected studies is illustrated in Figure 3. Timepoints unmarked in Figure 3 were either not evaluated or did not report significant changes.
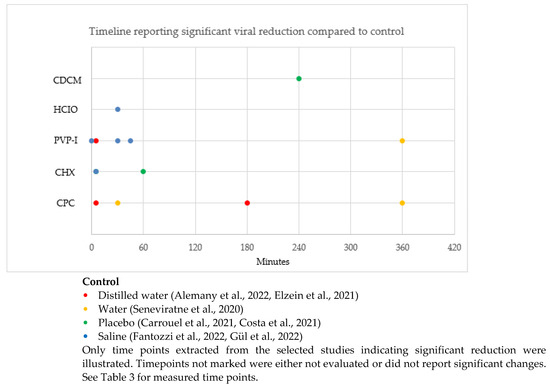
Figure 3.
Timeline of significant difference in SAR-CoV-2 reduction after rinsing with antiseptic compared to control [12,32,34,36,37,39,43].
CPC showed a significant reduction of SARS-CoV-2 at 5 min, 30 min, 180 min and 360 min compared to controls (distilled water and water). PVP-I showed a significant reduction of SARS-CoV-2 at 5 min, 30 min, 45 min and 360 min compared to controls (distilled water and saline). CHX showed a significant reduction of SARS-CoV-2 at 5 min and 60 min compared to controls (placebo and saline). HCIO showed a significant reduction of SARS-CoV-2 at 30 min compared to the control (saline). CDCM showed a significant reduction of SARS-CoV-2 at 240 min compared to the control (placebo).
Our analyses demonstrated that PVP-I and CPC are effective for up to 360 min, CDCM for up to 240 min, CHX for up to 60 min, and HCIO for up to 30 min. Data for significant viral reduction for CHX beyond 60 min and HCIO beyond 30 min has not been reported.
4. Discussion
Preprocedural antiseptic rinses are suggested for COVID-19 management and prevention. Public health officials generally agree that one of the primary vehicles of SARS-CoV-2 transmission and infection is human aerosol particles containing SARS-CoV-2 particles.
These guidelines were encouraged to be followed, especially by medical and dental offices seeing potential COVID-19 patients. In addition to these guidelines, strategies to minimize the risk of COVID-19 transmission from patient to healthcare provider need to be explored. Potentially cost-effective and efficient methods to reduce oral SARS-CoV-2 are preprocedural mouth rinses for all patients seen by medical (e.g., ENT, anesthesiologists, audiologists, speech therapists) and dental healthcare providers.
This paper identifies antimicrobial mouth rinses which have been reported to have significant effects on the reduction of airborne SARS-CoV-2 particles.
4.1. Effect and Substantivity of Oral Antiseptics
Mouth rinses have historically been used to reduce cross-contamination from aerosolized bacteria [78]. They have been reported effective against herpes, influenza, parainfluenza, and hepatitis B [55]. This suggests mouth rinses could significantly mitigate SAR-CoV-2 in the oral cavity [55].
The use of mouth rinses to remove SARS-CoV-2 in the oral cavity does not equate to treatment for COVID-19, nor does it provide a permanent reduction in oral viral load. SARS-CoV-2 can replicate in the respiratory system, and virus-infected secretions from the upper respiratory tract can reinfect the oral cavity during speech or coughing.
Most mouth rinses reduce SARS-CoV-2 viral load at 15 min post-rinse, with the persistence of viral load reduction extending to 45 min [33]. Mouth rinses reduce the viral load by disrupting the viral envelope and by mechanical flushing of the virus. The SARS-CoV-2 envelope consists of lipids and glycosylated proteins, which can be disrupted by cationic and amphiphilic compounds in mouth rinses [17]. The spike glycoprotein of SAR-CoV-2 is inserted in the viral envelope. Disruption of the viral envelope causes increased permeability and neutralization of SARS-CoV-2 [79]. Antiseptic mouth rinses physicochemically disrupt and destroy the SARS-CoV-2 viral lipid envelope [80]. Experimental evidence using density gradient ultracentrifugation followed by RT-qPCR showed that antiviral activity against coronaviruses is mostly caused by disruption of the viral envelope [81]. Thus, mouth rinses may be a safe and effective option to reduce SARS-CoV-2 transmission from the mouth.
However, the exposure time of SARS-CoV-2 to the mouth rinses in vivo depends on the salivary clearance and the substantivity of the mouth rinse. The antiviral effects of the mouth rinses can be diluted by the patient’s salivary flow in vivo [82].
PVP-I was proposed as a preoperative gargle in COVID-19 cases to mitigate the spread of SARS-CoV-2 among healthcare workers [83]. PVP-I application was reported to suppress SARS-CoV-2 RNA in saliva in the short-term [33,84]. It was reported to inactivate up to 99.99% of SARS-CoV-2 in 30 s [35]. Other concentrations of PVP-I (0.5%, 1%, and 1.5%) also reported similar inactivation of SARS-CoV-2 even at 15 s [85]. This effect could extend up to 3 h [36,86]. However, PVP-I did not sustain a prolonged oral and nasal SARS-CoV-2 RNA reduction 8 h after application [68]. It is reported that PVP-I can be used safely for up to 6 months in the mouth [36,85,86,87,88,89].
The half-life of CPC in the mouth ranged from 3–5 h after rinsing [90]. This finding appears consistent with prior investigations showing a potential persistence of activity between 180 and 300 min [91]. CPC can adhere to the oral mucosa for several hours. However, CPC failed to show substantivity and duration of activity to the level of CHX [92].
CPC showed antiviral activity and the potential to inactivate the COVID-19 coronavirus intraorally [93]. It continues to be active against viral particles increasing the viral nucleocapsid levels between 1–3 h. CPC can have synergistic effects and increase substantivity with other active ingredients. CPC has less side effects than rinses containing CHX, such as tooth staining, taste disruption, burning sensation, and mouth ulcers though mostly aesthetic effects [94,95].
CPC combined with O-cymen-5-ol extended the substantivity by more than 1 h and significantly improved antibacterial effects compared to CPC alone [96]. Because CPC shows synergistic potential on substantivity with other active ingredients, it may be a focus of future studies to assess the effects of CPC and CHX in combination and the resultant substantivity on SARS-CoV-2.
Antimicrobial effects associated with CPC and CHX are linked to the cationic state of the substance that allows binding to negatively charged oral proteins and surface proteins of antigens [97,98]. However, it should be noted that the inactivation of these cationic substances can be brought about by anionic compounds such as the detergents used in oral hygiene products.
CHX-containing mouth rinses showed short-term reductions in SARS-CoV-2, which returned to baseline levels in 2 h [99]. Significant reductions of viral load by CHX were reported at timelines up to 60 min [35]. Thus, CHX is substantive for at least 60 min [34]. Conflicting reports suggest that CHX weakly inactivates coronaviruses [81]. And may indicate that saliva interactions and mouth rinse concentration may influence the efficacy of the mouth rinse. A reduction of salivary pH has been reported to decrease the effects of CHX [100]. Reduced pH has electrostatic drug-protein effects that decrease the protein binding ability of CHX [101]. The concentration of the CHX solution may affect the effectiveness pharmacologically. Dental plaque can act as a reservoir for CHX and prolong its effects in biofilm [102]. The ability of saliva to function as a reservoir for CHX can be a factor for antimicrobial effects [103,104]. The concentration of CHX may influence the magnitude of such effects. It has been demonstrated that a 0.2% concentration of CHX has more antimicrobial activity than 0.12% concentrations [105,106].
Moreover, CHX shows greater potential for reservoirs at higher concentrations. There was a significant difference in plaque inhibition of 0.2% CHX concentration compared to 0.12% concentration [107]. Based on CHX’s ability with respect to concentration as a reservoir for plaque, one may theorize that CHX concentration may have comparative effects as a reservoir in salvia and produce antimicrobial effects. CHX lozenges showed some beneficial effects, and the higher CHX concentration as it dissolves in saliva may produce greater antiviral activity [108].
H2O2 disrupts the viral envelope and degrades the viral RNA via oxidative properties [109]. H2O2 effectively reduces SARS-CoV-2 titers in saliva immediately after rinsing [35]. However, the viral load returned to baseline values within 60 min after rinsing [35]. Thus, the lack of substantivity is a disadvantage of H2O2 as a mouth rinse.
In adults with asymptomatic or mild COVID-19, CDCM had a significant effect on SARS-CoV-2 salivary viral load reduction 4 h after the initial dose [32]. However, the data on the long-term benefits of CDCM and its effects on patients with high salivary load is limited.
Using a combination of 2 or more antiseptic agents may improve the antiviral capacity. However, antagonistic effects leading to decreased antiviral impact cannot be excluded. COVID-19 patients rinsing with H2O2 followed by CHX resulted in a minimal reduction in salivary viral load; the compaction was minimal even immediately post-rinse [35]. The secondary CHX rinse may have washed out the H2O2 before there was sufficient contact time for antiviral activity. It is possible the outcomes can be improved by rinsing with CHX first, and then H2O2 since CHX has greater substantivity than H2O2. There are potential benefits to the sequential use of different types of mouth rinses. However, further research is needed to evaluate synergistic mouth rinse combinations that produce maximal antiviral effects.
4.2. Clinical Outcomes Compared to In Vitro
The reported in vitro virucidal effects of mouth rinses against various coronaviruses resulted in mouth rinses being recommended during the pandemic to curb the transmission of SARS-CoV-2 [81,110]. Based on in vitro studies, PVP-I 0.5–1.5% and CPC were more effective mouth rinses in reducing viral load and inactivating COVID-19 than CHX and H2O2 [93].
In vivo, PVP-I was effective against SARS-CoV-2 at the lowest concentration of 0.5% PVP-I and at the lowest contact time of 15 s. These effects were like those seen in vitro, where similar concentrations (PVP-I of 0.5%, 1.25%, and 1.5%) were seen to inactivate SARS-CoV-2 at both 15 and 30 s. Other in vitro studies have shown a concentration of PVP-I as low as 0.23% was effective at inactivating SARS-CoV-2 in 15 s.
CPC at a concentration as low as 0.5% in vitro can completely inactivate the SARS-CoV-2 virus in as little as 15 s. In vivo, CPC was shown to reduce viral load, remain in saliva, and retain its effectiveness due to its high substantivity [93]. CPC has been effective both in the presence and absence of saliva [111].
CHX-containing mouth rinses and H2O2 demonstrated lower virucidal properties than CPC and PVP-I [112]. CHX concentration ranging from 0.12–2% was effective in vivo and in vitro against SARS-CoV-2. However, conflicting reports showed CHX and H2O2 independently had no virucidal effects against SARS-CoV-2.
Furthermore, H2O2 was less effective as a preprocedural mouth rinse due to its inability to remain active in saliva for an effective period. At concentrations of 1.5% and 3.0% H2O2 had minimal in vitro antiviral effects after 15 or 30 s. Thus, 0.5–1.5% PVP-I may be preferred over H2O2 for SARS-CoV-2 inactivation during the pandemic [85].
However, in vivo, results reported in the selected studies were modest compared to the in vitro results [23,113,114,115,116,117,118]. In addition, in vitro, results were not consistently reproduced in vivo. CHX did not significantly reduce SARS-CoV-2 viral load in vitro [25] but showed some effectiveness in vivo. Thus, oral rinses should be used in tandem with strict preventive measures.
Some factors contribute to the lack of consistency between in vitro and in vivo data. Rinsing in vivo generates a shearing effect leading to the rupture of viruses and viral cell junctions. This mechanical effect inherent in rinsing is absent in vitro studies. Furthermore, saliva in vivo contains multiple proteins and glycoproteins, which may modify the effect of mouth rinses. Mouth rinses like H2O2 can be inactivated by catalase enzymes in saliva. Saliva also contains bacteria which may bind to the active ingredients in the mouth rinses, limiting the concentration needed for virucidal effects.
Salivary flow can also dilute mouth rinses in vivo [119]. The salivary flow rate of 5 mL/min can dilute mouthwash concentration. The intraoral substantivity of mouthwashes may explain differences reported between in vivo results compared to in vitro. Furthermore, in vitro studies use standardized virucidal efficacy tests that do not represent in vivo oral cavity antiviral effects.
In vitro, studies are mostly conducted at room temperature. This may affect the mouth rinses’ viral activity and virucidal properties compared to body temperature. The oral cavity has a higher mean temperature of 36.6 °C. SARS-CoV-2 may be more stable at room temperature than at the elevated temperature of the oral cavity, which may contribute to some errors in the outcome.
Many in vitro studies utilize distilled water as a control. Distilled water in vitro did not produce a virucidal effect [120]. Distilled water may reduce the viral load by reducing viral lipid attachments, but it does not affect viral protein stability or viability.
In vivo, studies utilizing distilled water reported a low but significant decrease in viral load [120]. Mechanical forces during rinsing may release viral particles more effectively and affect viral viability by osmotic pressures.
4.3. Limitations
4.3.1. Patient Selection and Sample Size
Limitations to the selected studies include small cohort sizes, unreported baseline oral conditions, and a limited range of patients (e.g., only patients with high viral load or limiting to inpatients or severe COVID-19 and asymptomatic patients were not delineated for inclusion). Most of the studies were conducted on symptomatic hospitalized patients. Asymptomatic patients may not be included. Therefore, outcomes may not represent the whole spectrum of COVID-19 patients. The small cohort sizes may be due to the drastic decreases in COVID-19 cases as the community recovers from the infection. In addition, inter-patient variability can also contribute to data variations.
The limited sample size should be expanded to a larger study population for future studies to increase the statistical power. The baseline data should also include periodontal status, oral hygiene, plaque index, gingival index and quantification of salivary flow. In addition, both positive and negative controls should be included for comparison.
4.3.2. Sampling Methods
Sampling methods for the diagnosis of COVID-19 include saliva samples, throat gargle samples, nasopharyngeal swabs, and oropharyngeal swabs. The selected studies utilized the saliva sampling method (self-collected or investigator supervised) to evaluate the effectiveness of the oral rinses.
Saliva sampling methods have the advantage of avoiding invasive procedures, decreasing the risk of nosocomial transmission, screening large populations quickly, and providing an alternative in situations where nasopharyngeal swabs are contraindicated [8]. Saliva samples are self-collected and reliable [121,122,123]. However, patient training, including supervised collection of the first samples, may be needed to avoid interpatient variability. Since unsupervised self-collection is challenging to standardize; therefore, there may be some discrepancies due to the loss of samples and lower viral loads reported. Studies that utilize saliva samples collected by trained investigators may reduce patient bias and variability [37].
Some limitations of the saliva collection technique relate to the time delay in which saliva samples were collected to the time of symptom onset in asymptomatic patients. It is estimated that 30% of patients do not develop symptoms [124,125]. And SARS-CoV-2 infectivity peaks at or before symptom onset [126]. The delay in saliva collection after symptoms onset may present with lower viral salivary concentration to evaluate further reduction affected by the mouth rinses. Salivary viral load decreased, corresponding to the number of days from infection. In substantivity studies of mouth rinses, it may be difficult to confirm if the viral load decrease is due to the mouth rinse or due to the progression of the infection. In previous studies comparing saliva samples and nasopharyngeal samples, when nasopharyngeal samples still reported a low positive for SARS-CoV-2, a negative was reported for the virus in saliva [116].
Furthermore, other methods, like the throat gargle method, may be better than saliva sampling. The saline mouth and throat gargle method is reported to be easily tolerated by patients and a more sensitive test than saliva collection [127]. Throat gargle samples also showed higher viral load values than nasopharyngeal and oropharyngeal swabs [128,129]. Further studies required comparing the SARS-CoV-2 viral load between saliva collection and other sampling methods collected simultaneously in each patient to better assess the use of saliva as a diagnostic tool for SARS-CoV-2 viral load.
4.3.3. Diagnostics
Assessing the viral load alone may not be the most efficient method for assessing the effectiveness of mouth rinses, especially mouth rinses that primarily target the viral envelope and not the viral RNA. Viral RNA may persist in saliva after the disruption of the virus particle due to the protective effects of protein complexes [130]. Viral RNA, embedded in intact viral particles or released from disrupted ones, can be recovered by RNA extraction methods. Thus, viruses detected via real-time PCR (RT-PCR) do not correlate with the presence of complete viral particles [55]. The RT-PCR detection of the SARS-CoV-2 viral load includes both live and non-infective viral particles and does not assess the viability of the virus. Therefore, even though the data may report that mouth rinses have no significant effect on SARS-CoV-2 viral load as measured by quantitative PCR, there is a possibility that the viral particles may be non-viable. RT-PCR technique can only detect RNA copies and not the infectivity of the detected virus fragments. Therefore, the reported viral load did not correspond to the infectious virus.
To assess for SARS-CoV-2 infectivity would require viral isolation assays and cell culture. Without viral cultures, we cannot exclude the possibility that mouth rinses suppressed viable SARS-CoV-2 and not viral RNA. Viral cell culture is the gold standard for assessing viral infectivity. However, viral cell culture cannot only be performed when there is insufficient sample volume or low viral load. Virus recovery is usually possible from samples with high viral loads greater than 106 copies/mL [131,132]. Samples with lower viral loads or limited volume may limit the viability of the culture.
Monkey Vero-E6 lines are widely used to assess SARS-CoV-2 infectivity in viral culture [113,114,133,134]. Since new protocols utilizing human lung cells are being developed [135]; therefore, future studies utilizing human cell lines would be a more realistic infection model. However, culturing SARS-CoV-2 in a cell culture to evaluate active virus replication requires special laboratory conditions such as biosafety level 4. Due to this limitation in culturing SARS-CoV-2, many studies still utilized viral RNA load as a reliable surrogate marker [136].
The increase in nucleocapsid protein levels indicates an increase in the disruption of viral particles with no infectious capacity. However, the possibility of saliva’s natural disruption of the viral particles cannot be excluded [12]. Furthermore, the high variability in the nucleocapsid protein levels may limit the applicability of the results reported.
Mouth rinses alter viral proteins and membranes [113] and may also be cytotoxic to cultured cells used in infection studies. This can limit the evaluation of antiseptics to biocompatible concentrations. Other limitations of the included studies were the lack of analysis on the infectivity of viruses left in the mouth after rinsing and the viruses bound to soft tissue [35].
4.4. Biocide Resistance
Most selected studies reported no adverse reaction from the mouth rinses evaluated. The rest of the selected studies did not report on adverse reactions, and any adverse reactions arising from mouth rinse use was unknown. Long-term use of mouth rinses may pose some risks. Minor side effects of antiseptic mouth rinses may include stained teeth, transient loss of taste, or black hairy tongue. Other risks of using mouth rinses are the development of biocide resistance. Mouth rinses containing alcohol should be avoided by patients with alcohol addictions, reformed alcoholics, alcohol allergies, and religious or cultural convictions against alcohol.
The prolonged use of a sublethal dose of mouth rinses may increase the risk of gram-negative bacterial overgrowth and biocide resistance [137,138,139,140,141]. Gram-positive bacteria have a lower minimum inhibitory concentration (MIC) than gram-negative bacteria; the bacterial cell wall of gram-positive bacteria has a higher affinity to antiseptic mouth rinses. Development of biocide resistance was reported for prolonged low-level CHX exposure [142]. Possible mechanisms of biocide resistance include efflux pump dysfunction and mutation of the cell membrane [142].
Biocide resistance has been linked to bacterial cross-resistance to antibiotics; drug resistance was reported after frequent CPC use [143]. Biocide resistance was mostly reported for non-oral bacteria. There were limited studies reported for oral bacteria. Short-term use of mouth rinses did not report non-native bacteria or gram-negative bacterial overgrowth [144]. Long-term use of mouth rinses may increase the risk of biocide resistance, antibiotic cross-resistance, and phenotypic adaptation.
5. Conclusions
In addition to the implementation of improved personal protection and engineering enhanced air filtration, preprocedural mouth rinses may be an effective strategy and cost-reduction solution for reducing airborne SARS-CoV-2 dispersion in the environment and be an integral part of safe practice for healthcare protocols. Within the limits of this systematic review, preprocedural rinses may reduce SARS-CoV-2 particles in the mouth of COVID-19 patients, thus, reducing the number of viral particles available for airborne dispersion. Furthermore, the viral neutralization properties and the mechanisms of action of the mouth rinses were poorly understood. Future research on laboratory analysis assays for capsid disassembly and viral uncoating of SARS-CoV-2 exposed to these mouth rinses is needed.
Antiseptic mouth rinses may be a preventive strategy to reduce the spread of COVID-19 in selected medical and healthcare facilities, including dental clinics. Further studies are needed to investigate the clinical effectiveness of mouth rinses in reducing SARS-CoV-2 transmission in the household, medical facilities, and dental clinics.
This paper identifies potential antimicrobial mouth rinses which have significant effects on the reduction of SARS-CoV-2 particles in the oral cavity.
Author Contributions
Conceptualization, M.T. and J.B.S.; resources, A.D., J.J.S., M.W., M.T. and J.B.S.; data curation, M.T.; writing—original draft preparation, A.D., J.J.S. and M.T.; writing—review and editing, A.D., J.J.S., M.W., M.T. and J.B.S.; visualization, M.W. and M.T.; supervision, J.B.S. and M.T.; project administration, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meselson, M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2063. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Wu, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.; Suzuki, J.B. SARS-CoV-2: Overview and Its Impact on Oral Health. Biomedicines 2021, 9, 1690. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.; Suzuki, J.B. COVID-19: Current Overview on SARS-CoV-2 and the Dental Implications. Oral Health 2022. Available online: https://www.oralhealthgroup.com/features/covid-19-current-overview-on-sars-cov-2-and-the-dental-implications/ (accessed on 14 May 2023).
- Ting, M.; Molinari, J.A.; Suzuki, J.B. Current SARS-CoV-2 Protective Strategies for Healthcare Professionals. Biomedicines 2023, 11, 808. [Google Scholar] [CrossRef]
- Ting, M.; Suzuki, J.B. Is the COVID-19 Pandemic Over? The Current Status of Boosters, Immunosenescence, Long Haul COVID, and Systemic Complications. Int. J. Transl. Med. 2022, 2, 230–241. [Google Scholar] [CrossRef]
- Buttenheim, A.M. SARS-CoV-2 Vaccine Acceptance: We May Need to Choose Our Battles. Ann. Intern. Med. 2020, 173, 1018–1019. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Yip, C.C.; Chan, K.H.; Wu, T.C.; Chan, J.M.; Leung, W.S.; Chik, T.S.H.; Choi, C.Y.C.; Kandamby, D.H.; et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.Y.; Cai, J.P.; Chan, J.M.C.; Chik, T.S.H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Deana, N.F.; Seiffert, A.; Aravena-Rivas, Y.; Alonso-Coello, P.; Munoz-Millan, P.; Espinoza-Espinoza, G.; Pineda, P.; Zaror, C. Recommendations for Safe Dental Care: A Systematic Review of Clinical Practice Guidelines in the First Year of the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10059. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J. Dent. Res. 2020, 99, 989. [Google Scholar] [CrossRef]
- Alemany, A.; Perez-Zsolt, D.; Raich-Regue, D.; Munoz-Basagoiti, J.; Ouchi, D.; Laporte-Villar, C.; Baro, B.; Henríquez, N.; Prat, N.; Gianinetto, M.O.; et al. Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: A Double-Blind Randomized Clinical Trial. J. Dent. Res. 2022, 101, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Bernal, C.G.; Reyes Uribe, E.; Salazar Flores, J.; Varela Hernández, J.J.; Gómez-Sandoval, J.R.; Martínez Salazar, S.Y.; Gutiérrez Maldonado, A.F.; Aguilar Martínez, J.; Lomelí Martínez, S.M. Oral Antiseptics against SARS-CoV-2: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8768. [Google Scholar] [CrossRef]
- Chumpitaz-Cerrate, V.; Chávez-Rimache, L.; Ruiz-Ramirez, E.; Franco-Quino, C.; Erazo-Paredes, C. Evaluation of Current Evidence on the Use of Oral Antiseptics Against SARS-CoV-2: A Narrative Review. J. Int. Soc. Prev. Community Dent. 2022, 12, 488–499. [Google Scholar] [CrossRef]
- Weber, J.; Bonn, E.L.; Auer, D.L.; Kirschneck, C.; Buchalla, W.; Scholz, K.J.; Cieplik, F. Preprocedural mouthwashes for infection control in dentistry-an update. Clin. Oral Investig. 2023, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Silva, R.; Jardim, A.C.G.; Siqueira, W.L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Investig. 2020, 24, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Popkin, D.L.; Zilka, S.; Dimaano, M.; Fujioka, H.; Rackley, C.; Salata, R.; Griffith, A.; Mukherjee, P.K.; Ghannoum, M.A.; Esper, F. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog. Immun. 2017, 2, 252–269. [Google Scholar] [CrossRef]
- Kariwa, H.; Fujii, N.; Takashima, I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 2006, 212 (Suppl. S1), 119–123. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.; Robison, R.; Milici, J.; Alam, S.; Quillen, D.; Goldenberg, D.; Kass, R. Lowering the transmission and spread of human coronavirus. J. Med. Virol. 2021, 93, 1605–1612. [Google Scholar] [CrossRef]
- Steinhauer, K.; Meister, T.L.; Todt, D.; Krawczyk, A.; Passvogel, L.; Becker, B.; Paulmann, D.; Bischoff, B.; Pfaender, S.; Brill, F.H.; et al. Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J. Hosp. Infect. 2021, 111, 180–183. [Google Scholar] [CrossRef]
- Wood, A.; Payne, D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J. Hosp. Infect. 1998, 38, 283–295. [Google Scholar] [CrossRef]
- Eggers, M.; Koburger-Janssen, T.; Eickmann, M.; Zorn, J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash Against Respiratory and Oral Tract Pathogens. Infect. Dis. Ther. 2018, 7, 249–259. [Google Scholar] [CrossRef]
- Munoz-Basagoiti, J.; Perez-Zsolt, D.; Leon, R.; Blanc, V.; Raich-Regue, D.; Cano-Sarabia, M.; Trinité, B.; Pradenas, E.; Blanco, J.; Gispert, J.; et al. Mouthwashes with CPC Reduce the Infectivity of SARS-CoV-2 Variants In Vitro. J. Dent. Res. 2021, 100, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Bruggemann, Y.; Todt, D.; Conzelmann, C.; Muller, J.A.; Gross, R.; Münch, J.; Krawczyk, A.; Steinmann, J.; Steinmann, J.; et al. Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2. J. Infect. Dis. 2020, 222, 1289–1292. [Google Scholar] [CrossRef]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Sanabria, A.; Ridge, J.A.; Ng, W.T.; Bree, R.; Rinaldo, A.; Takes, R.P.; Mäkitie, A.A.; Carvalho, A.L.; Bradford, C.R.; et al. COVID-19 pandemic: Effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck 2020, 42, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 88, 18. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sanchez Barrueco, A.; Mateos-Moreno, M.V.; Martinez-Beneyto, Y.; Garcia-Vazquez, E.; Campos Gonzalez, A.; Zapardiel Ferrero, J.; Castaño, A.B.; Rueda, I.A.; Aubá, J.M.V.; Español, C.C.; et al. Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: Evidence from a randomized double-blind clinical trial. Emerg. Microbes Infect. 2022, 11, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Valette, M.; Gadea, E.; Esparcieux, A.; Illes, G.; Langlois, M.E.; Perrier, H.; Dussart, C.; Tramini, P.; Ribaud, M.; et al. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: A multicentre, randomized, double-blind controlled trial. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1494–1501. [Google Scholar] [CrossRef]
- Chaudhary, P.; Melkonyan, A.; Meethil, A.; Saraswat, S.; Hall, D.L.; Cottle, J.; Wenzel, M.; Ayouty, N.; Bense, S.; Casanova, F.; et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial. J. Am. Dent. Assoc. 2021, 152, 903–908. [Google Scholar] [CrossRef]
- Costa, D.D.; Brites, C.; Vaz, S.N.; de Santana, D.S.; Dos Santos, J.N.; Cury, P.R. Chlorhexidine mouthwash reduces the salivary viral load of SARS-CoV-2: A randomized clinical trial. Oral Dis. 2022, 28 (Suppl. S2), 2500–2508. [Google Scholar] [CrossRef]
- Eduardo, F.P.; Correa, L.; Heller, D.; Daep, C.A.; Benitez, C.; Malheiros, Z.; Stewart, B.; Ryan, M.; Machado, C.M.; Hamerschlak, N.; et al. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 2021, 7, e07346. [Google Scholar] [CrossRef] [PubMed]
- Elzein, R.; Abdel-Sater, F.; Fakhreddine, S.; Hanna, P.A.; Feghali, R.; Hamad, H.; Ayoubf, F. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J. Evid. Based Dent. Pract. 2021, 21, 101584. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, P.J.; Pampena, E.; Pierangeli, A.; Oliveto, G.; Sorrentino, L.; Di Vanna, D.; Pampena, R.; Lazzaro, A.; Gentilini, E.; Mastroianni, C.M.; et al. Efficacy of antiseptic mouthrinses against SARS-CoV-2: A prospective randomized placebo-controlled pilot study. Am. J. Otolaryngol. 2022, 43, 103549. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Barrueco, Á.S.; Martinez-Beneyto, Y.; Mateos-Moreno, M.V.; Ausina-Márquez, V.; García-Vázquez, E.; Puche-Torres, M.; Giner, M.J.F.; González, A.C.; Coello, J.M.S.; et al. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Sci. Rep. 2021, 11, 24392. [Google Scholar] [CrossRef] [PubMed]
- Sevinc Gul, S.N.; Dilsiz, A.; Saglik, I.; Aydin, N.N. Effect of oral antiseptics on the viral load of SARS-CoV-2: A randomized controlled trial. Dent. Med. Probl. 2022, 59, 357–363. [Google Scholar] [CrossRef]
- Meister, T.L.; Gottsauner, J.M.; Schmidt, B.; Heinen, N.; Todt, D.; Audebert, F.; Buder, F.; Lang, H.; Gessner, A.; Steinmann, E.; et al. Mouthrinses against SARS-CoV-2—High antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial. Virus Res. 2022, 316, 198791. [Google Scholar] [CrossRef]
- Natto, Z.S.; Bakhrebah, M.A.; Afeef, M.; Al-Harbi, S.; Nassar, M.S.; Alhetheel, A.F.; Ashi, H. The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study. Medicine 2022, 101, e28925. [Google Scholar] [CrossRef]
- Redmond, S.N.; Li, D.F.; Ghaddara, H.A.; Haq, M.F.; Jones, L.D.; Nguyen, A.M.; Tiktin, M.; Cadnum, J.L.; Navas, M.E.; Bingham, J.; et al. A pilot randomized trial to evaluate the efficacy of oral and nasal povidone iodine in reducing the burden of severe acute respiratory syndrome coronavirus 2 RNA in patients with coronavirus disease 2019. Infect. Control. Hosp. Epidemiol. 2023, 44, 679–681. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Balan, P.; Ko, K.K.K.; Udawatte, N.S.; Lai, D.; Ng, D.H.L.; Venkatachalam, I.; Lim, K.S.; Ling, M.L.; Oon, L.; et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 2021, 49, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Martínez Lamas, L.; Diz Dios, P.; Pérez Rodríguez, M.T.; Del Campo Pérez, V.; Cabrera Alvargonzalez, J.J.; López Domínguez, A.M.; Feijoo, J.F.; Freitas, M.D.; Posse, J.L. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2022, 28 (Suppl. S1), 908–911. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Pfefferle, S.; Buttner, H.; Knobloch, J.K.; Lutgehetmann, M. Impact of Oral Rinsing with Octenidine Based Solution on SARS-CoV-2 Loads in Saliva of Infected Patients an Exploratory Study. Int. J. Environ. Res. Public Health 2022, 19, 5582. [Google Scholar] [CrossRef] [PubMed]
- Biber, A.; Lev, D.; Mandelboim, M.; Lustig, Y.; Harmelin, G.; Shaham, A.; Erster, O. The role of mouthwash sampling in SARS-CoV-2 diagnosis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Friedland, P.; Tucker, S.; Goodall, S.; Julander, J.; Mendenhall, M.; Molloy, P.; de Jong, D.M.; Musungaie, D.B.; Sikazwe, C.; Panta, K.; et al. In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray. Aust. J. Otolaryngol. 2022, 2022, 5. [Google Scholar] [CrossRef]
- Saud, Z.; Tyrrell, V.J.; Zaragkoulias, A.; Protty, M.B.; Statkute, E.; Rubina, A.; Bentley, K.; White, D.A.; Rodrigues, P.D.S.; Murphy, R.C.; et al. The SARS-CoV2 envelope differs from host cells, exposes procoagulant lipids, and is disrupted in vivo by oral rinses. J. Lipid Res. 2022, 63, 100208. [Google Scholar] [CrossRef]
- Schürmann, M.; Aljubeh, M.; Tiemann, C.; Sudhoff, H. Mouthrinses against SARS-CoV-2: Anti-inflammatory effectivity and a clinical pilot study. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 5059–5067. [Google Scholar] [CrossRef]
- Ozduman, Z.C.; Oglakci, B.; Dogan, M.; Deger, C.; Eliguzeloglu Dalkilic, E. How does antiseptic mouthwashes against SARS-COV-2 affect the bond strength of universal adhesive to enamel? Microsc. Res. Tech. 2022, 85, 1199–1208. [Google Scholar] [CrossRef]
- Avhad, S.K.; Bhanushali, M.; Sachdev, S.S.; Save, S.S.; Kalra, D.; Dn, K. Comparison of Effectiveness of Chlorine Dioxide Mouthwash and Chlorhexidine Gluconate Mouthwash in Reduction of Oral Viral Load in Patients with COVID-19. Indian J. Public Health Res. Dev. 2020, 11, 27–32. [Google Scholar] [CrossRef]
- Belcaro, G.; Corsi, M.; Agus, G.B.; Cesarone, M.R.; Cornelli, U.; Cotellese, R.; Feragalli, B.; Hu, S. Thrombo-prophylaxis prevents thrombotic events in home-managed COVID patients. A registry study. Minerva Med. 2020, 111, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Di Domênico, M.B.; Cesca, H.; Ponciano, T.H.J.; dos Santos, R.B.; Lenz, U.; Antunes, V.P.; Godinho, V.W.; Collares, K.; Corazza, P.H. Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: Preliminary results of a randomized double-blind clinical trial. Epidemiol. Health 2021, 43, e2021032. [Google Scholar] [CrossRef] [PubMed]
- Di Domênico, M.B.; Collares, K.; dos Santos, R.B.; Lenz, U.; Antunes, V.P.; Godinho, V.W.; Cesca, H.; Ponciano, T.H.J.; Corazza, P.H. Hydrogen peroxide as an auxiliary treatment for COVID-19 in Brazil: A randomized double-blind clinical trial. Epidemiol. Health 2021, 43, e2021051. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Huang, J.T. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J. Med. Virol. 2021, 93, 4370–4373. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Baharom, N.; Sulaiman, W.S.W.; Rashid, Z.Z.; Ken, W.K.; Ali, U.K.; Othman, S.N.; Samat, M.N.; Kori, N.; Periyasamy, P.; et al. Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils—A clinical trial. medRxiv 2020, 2020, 20180448. [Google Scholar]
- Mukhtar, K.; Qassim, S.; Al Qahtani, S.A.; Danjuma, M.I.-M.; Mohamedali, M.; Farhan, H.A.; Khudair, M.F.; El Tayeh, A.R.; Al-Dosari, M.; Babiker, M.E.; et al. A randomized trial on the regular use of potent mouthwash in COVID-19 treatment. MedRxiv 2021, 2020, 20234997. [Google Scholar]
- Ogun, S.A.; Erinoso, O.; Aina, O.O.; Ojo, O.I.; Adejumo, O.; Adeniran, A.; Bowale, A.; Olaniyi, C.A.; Adedoyin, B.M.; Mutiu, B.; et al. Efficacy of Hexetidine, Thymol and Hydrogen Peroxide-Containing Oral Antiseptics in Reducing SARS-CoV-2 Virus in the Oral Cavity: A Pilot Study. West Afr. J. Med. 2022, 39, 83–89. [Google Scholar] [CrossRef]
- Poleti, M.L.; Gregório, D.; Bistaffa, A.G.I.; Fernandes, K.B.P.; Vilhena, F.V.; Santos, P.S.D.S.; Simão, A.N.C.; Lozovoy, M.A.B.; Tatibana, B.T.; Fernandes, T.M.F. Use of mouthwash and dentifrice containing an antimicrobial phthalocyanine derivative for the reduction of clinical symptoms of COVID-19: A randomized triple-blind clinical trial. J. Evid. Based Dent. Pract. 2022, 22, 101777. [Google Scholar] [CrossRef]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef]
- Cyril Vitug, L.; Santiaguel, J.; Angelica Exconde, M.A.; Ma Teresa Tricia, B. Oral Gargle of Ethanol-Based Mouthwash Solution and Its Effects on Adult Patients with Mild COVID-19 Infection: An Open Label-Randomized Controlled Trial. Chest 2021, 160, A2504. [Google Scholar] [CrossRef]
- Santos, P.S.D.S.; Orcina, B.D.F.; Machado, R.R.G.; Vilhena, F.V.; Alves, L.M.D.C.; Zangrando, M.S.R.; de Oliveira, R.C.; Soares, M.Q.S.; Simão, A.N.C.; Pietro, E.C.I.N.; et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: Randomised trial. Sci. Rep. 2021, 11, 19937. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Saha, A.; Jamir, L.; Kakkar, R. Protection at Portal of Entry (PPE) with Povidone Iodine for COVID-19. Int. J. Med. Public Health 2020, 10, 166–168. [Google Scholar] [CrossRef]
- Warabi, Y.; Tobisawa, S.; Kawazoe, T.; Murayama, A.; Norioka, R.; Morishima, R.; Inoue, T.; Shimizu, T.; Takahashi, K. Effects of oral care on prolonged viral shedding in coronavirus disease 2019 (COVID-19). Spec. Care Dent. 2020, 40, 470–474. [Google Scholar] [CrossRef]
- Saraswathi, S.; Chalageri, V.H.; Bhushan, S.; Ranganath, T.; Rani, V.D.; Majgi, S.M.; Vijay, K.; Hema, M.S.; Sanadi, S.L.; Nasreen, P.; et al. Impact of Steam Inhalation, Saline Gargling, and Povidone-Iodine Gargling on Clinical Outcome of COVID-19 Patients in Bengaluru, Karnataka: A Randomized Control Trial. Indian. J. Community Med. 2022, 47, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Choudhury MI, M.; Shabnam, N.; Ahsan, T.; Kabir, M.S.; Md Khan, R.; Ahsan, S.A. Effect of 1% Povidone Iodine Mouthwash/Gargle, Nasal and Eye Drop in COVID-19 patient. Bioresearch Commun. 2021, 7, 919–923. [Google Scholar] [CrossRef]
- Brito-Reia, V.C.; da Silva Bastos, R.; Vilhena, F.V.; Honório, H.M.; da Costa Alves, L.M.; Frazão, P.; da Silva Santos, P.S. Population-based virucidal phthalocyanine gargling/rinsing protocol to reduce the risk of coronavirus disease-2019: A community trial. GMS Hyg. Infect. Control 2022, 17, Doc23. [Google Scholar]
- Guenezan, J.; Garcia, M.; Strasters, D.; Jousselin, C.; Lévêque, N.; Frasca, D.; Mimoz, O. Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients with COVID-19: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 400–401. [Google Scholar] [CrossRef]
- Keitel, V.; Jensen, B.; Feldt, T.; Fischer, J.C.; Bode, J.G.; Matuschek, C.; Bölke, E.; Budach, W.; Plettenberg, C.; Scheckenbach, K.; et al. Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): A structured summary of a study protocol for a randomized controlled trial. Trials 2021, 22, 343. [Google Scholar] [CrossRef]
- Khan, M.M.; Parab, S.R.; Paranjape, M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in COVID-19 pandemic. Am. J. Otolaryngol. 2020, 41, 102618. [Google Scholar] [CrossRef]
- Arefin, M.K.; Rumi, S.K.N.F.; Uddin, A.K.M.N.; Banu, S.S.; Khan, M.; Kaiser, A.; Chowdhury, J.A.; Khan, A.S.; Hasan, M.J. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: An open-label randomized clinical trial. Indian J. Otolaryngol. Head Neck Surg. Off. Publ. Assoc. Otolaryngol. India 2022, 74 (Suppl. S2), 2963–2967. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Esper, F.; Buchheit, K.; Arters, K.; Adkins, I.; Ghannoum, M.A.; Salata, R.A. Randomized, double-blind, placebo-controlled clinical trial to assess the safety and effectiveness of a novel dual-action oral topical formulation against upper respiratory infections. BMC Infect. Dis. 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Quek, A.M.L.; Ooi, D.S.Q.; Sengupta, S.; Lakshminarasappa, S.R.; Koo, C.Y.; So, J.B.Y.; Goh, B.C.; Loh, K.S.; Fisher, D.; et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int. J. Infect. Dis. 2021, 106, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Siami, Z.; Aghajanian, S.; Mansouri, S.; Mokhames, Z.; Pakzad, R.; Kabir, K.; Norouzi, M.; Soleimani, A.; Yaghoobi, M.H.; Shadabi, S.; et al. Effect of Ammonium Chloride in addition to standard of care in outpatients and hospitalized COVID-19 patients: A randomized clinical trial. Int. J. Infect. Dis. 2021, 108, 306–308. [Google Scholar] [CrossRef]
- Wright, J.K.; Tan, D.H.S.; Walmsley, S.L.; Hulme, J.; O’connor, E.; Snider, C.; Cheng, I.; Chan, A.K.; Borgundvaag, B.; McLeod, S.; et al. Protecting Frontline Health Care Workers from COVID-19 with Hydroxychloroquine Pre-exposure Prophylaxis: A structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada. Trials 2020, 21, 647. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.J.; Rumi, S.; Banu, S.S.; Uddin, A.; Islam, M.S.; Arefin, M.K. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: A structured summary of a study protocol for an open-label randomized clinical trial. Trials 2021, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Kazmi, S.M.R.; Iqbal, N.T.; Iqbal, J.; Ali, S.T.; Abbas, S.A. A quadruple blind, randomised controlled trial of gargling agents in reducing intraoral viral load among hospitalised COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 785. [Google Scholar] [CrossRef]
- Koletsi, D.; Belibasakis, G.N.; Eliades, T. Interventions to Reduce Aerosolized Microbes in Dental Practice: A Systematic Review with Network Meta-analysis of Randomized Controlled Trials. J. Dent. Res. 2020, 99, 1228–1238. [Google Scholar] [CrossRef]
- Eggers, M. Infectious Disease Management and Control with Povidone Iodine. Infect. Dis. Ther. 2019, 8, 581–593. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- O’Donnell, V.B.; Thomas, D.; Stanton, R.; Maillard, J.Y.; Murphy, R.C.; Jones, S.A.; Humphreys, I.; Wakelam, M.J.O.; Fegan, C.; Wise, M.P.; et al. Potential Role of Oral Rinses Targeting the Viral Lipid Envelope in SARS-CoV-2 Infection. Function 2020, 1, zqaa002. [Google Scholar] [CrossRef]
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Sharma, S.; Aggarwal, A. Preanesthetic Povidone-Iodine gargles for patients with COVID-19. J. Clin. Anesth. 2020, 67, 110035. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, A.; Peña-Cardelles, J.F.; Ordonez-Fernandez, E.; Montero-Alonso, M.; Kewalramani, N.; Salgado-Peralvo, A.O.; Végh, D.; Gargano, A.; Parra, G.; Guerra-Guajardo, L.I.; et al. Povidone-Iodine as a Pre-Procedural Mouthwash to Reduce the Salivary Viral Load of SARS-CoV-2: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 2877. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse. J. Prosthodont. 2020, 29, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, S.J.; Kirk-Bayley, J.; Sunkaraneni, V.S.; Combes, J. Povidone iodine. Br. Dent. J. 2020, 228, 656–657. [Google Scholar] [CrossRef] [PubMed]
- Alshaeri, H.K.; Natto, Z.S. A contemporary look at COVID-19 medications: Available and potentially effective drugs. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9188–9195. [Google Scholar]
- Natto, Z.S.; Alshaeri, H.K. Characteristics of First Cases of Coronavirus Disease 2019 and the Effort to Prevent the Early Spread of COVID-19 in Saudi Arabia. Risk Manag. Healthc. Policy 2021, 14, 315–321. [Google Scholar] [CrossRef]
- Natto, Z.S.; Alshehri, M.M.; Alghamdi, F.K. Infection Control Practices at the Dental Clinics in Jeddah, Saudi Arabia. J. Multidiscip. Healthc. 2021, 14, 2951–2957. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef]
- Roberts, W.R.; Addy, M. Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. I. Effect on plaque accumulation and salivary bacteria. J. Clin. Periodontol. 1981, 8, 213–219. [Google Scholar] [CrossRef]
- Elworthy, A.; Greenman, J.; Doherty, F.M.; Newcombe, R.G.; Addy, M. The substantivity of a number of oral hygiene products determined by the duration of effects on salivary bacteria. J. Periodontol. 1996, 67, 572–576. [Google Scholar] [CrossRef]
- Anderson, E.R.; Patterson, E.I.; Richards, S.; Pitol, A.K.; Edwards, T.; Wooding, D.; Buist, K.; Green, A.; Mukherjee, S.; Hoptroff, M.; et al. CPC-containing oral rinses inactivate SARS-CoV-2 variants and are active in the presence of human saliva. J. Med. Microbiol. 2022, 71, 001508. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Wang, G.; Panigrahy, A.; Zhang, G. Triclosan and triclocarban as potential risk factors of colitis and colon cancer: Roles of gut microbiota involved. Sci. Total Environ. 2022, 842, 156776. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 2019, 10, 2042098619854881. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, F.-R.; Viñas, M.; Sierra, J.M.; Vinuesa, T.; de Henestrosa, A.R.F.; Furmanczyk, M.; Trullàs, C.; Jourdan, E.; López-López, J.; Jorba, M. Substantivity of mouth-rinse formulations containing cetylpyridinium chloride and O-cymen-5-ol: A randomized-crossover trial. BMC Oral Health 2022, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- McCoy, L.C.; Wehler, C.J.; Rich, S.E.; Garcia, R.I.; Miller, D.R.; Jones, J.A. Adverse events associated with chlorhexidine use: Results from the Department of Veterans Affairs Dental Diabetes Study. J. Am. Dent. Assoc. 2008, 139, 178–183. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, J.-M.; Lee, Y.; Park, J.-W.; Suh, J.-Y.; Um, H.-S.; Kim, Y.-G. The antiplaque and bleeding control effects of a cetylpyridinium chloride and tranexamic acid mouth rinse in patients with gingivitis. J. Periodontal Implant. Sci. 2017, 47, 134–142. [Google Scholar] [CrossRef]
- Yoon, J.G.; Yoon, J.; Song, J.Y.; Yoon, S.Y.; Lim, C.S.; Seong, H.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Clinical Significance of a High SARS-CoV-2 Viral Load in the Saliva. J. Korean Med. Sci. 2020, 35, e195. [Google Scholar] [CrossRef]
- Bonesvoll, P.; Lokken, P.; Rolla, G. Influence of concentration, time, temperature and pH on the retention of chlorhexidine in the human oral cavity after mouth rinses. Arch. Oral Biol. 1974, 19, 1025–1029. [Google Scholar] [CrossRef]
- Gjermo, P.; Bonesvoll, P.; Rolla, G. Relationship between plaque-inhibiting effect and retention of chlorhexidine in the human oral cavity. Arch. Oral Biol. 1974, 19, 1031–1034. [Google Scholar] [CrossRef]
- Otten, M.P.; Busscher, H.J.; van der Mei, H.C.; Abbas, F.; van Hoogmoed, C.G. Retention of antimicrobial activity in plaque and saliva following mouthrinse use in vivo. Caries Res. 2010, 44, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Tomas, I.; Garcia-Caballero, L.; Lopez-Alvar, E.; Suarez-Cunqueiro, M.; Diz, P.; Seoane, J. In situ chlorhexidine substantivity on saliva and plaque-like biofilm: Influence of circadian rhythm. J. Periodontol. 2013, 84, 1662–1672. [Google Scholar] [CrossRef]
- Garcia-Caballero, L.; Quintas, V.; Prada-Lopez, I.; Seoane, J.; Donos, N.; Tomas, I. Chlorhexidine substantivity on salivary flora and plaque-like biofilm: An in situ model. PLoS ONE 2013, 8, e83522. [Google Scholar] [CrossRef] [PubMed]
- Cousido, M.C.; Tomas Carmona, I.; Garcia-Caballero, L.; Limeres, J.; Alvarez, M.; Diz, P. In vivo substantivity of 0.12% and 0.2% chlorhexidine mouthrinses on salivary bacteria. Clin. Oral Investig. 2010, 14, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Tomas, I.; Cousido, M.C.; Tomas, M.; Limeres, J.; Garcia-Caballero, L.; Diz, P. In vivo bactericidal effect of 0.2% chlorhexidine but not 0.12% on salivary obligate anaerobes. Arch. Oral Biol. 2008, 53, 1186–1191. [Google Scholar] [CrossRef]
- Berchier, C.E.; Slot, D.E.; Van der Weijden, G.A. The efficacy of 0.12% chlorhexidine mouthrinse compared with 0.2% on plaque accumulation and periodontal parameters: A systematic review. J. Clin. Periodontol. 2010, 37, 829–839. [Google Scholar] [CrossRef]
- Supranoto, S.C.; Slot, D.E.; Addy, M.; Van der Weijden, G.A. The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: A systematic review. Int. J. Dent. Hyg. 2015, 13, 83–92. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Barkvoll, P.; Rølla, G.; Svendsen, K. Interaction between chlorhexidine digluconate and sodium lauryl sulfate in vivo. J. Clin. Periodontol. 1989, 16, 593–595. [Google Scholar] [CrossRef]
- Schiott, C.R.; Löe, H.; Jensen, S.B.; Kilian, M.; Davies, R.M.; Glavind, K. The effect of chlorhexidine mouthrinses on the human oral flora. J. Periodontal Res. 1970, 5, 84–89. [Google Scholar] [CrossRef]
- Xu, C.; Wang, A.; Hoskin, E.R.; Cugini, C.; Markowitz, K.; Chang, T.L.; Fine, D.H. Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro. Pathogens 2021, 10, 272. [Google Scholar] [CrossRef]
- Koch-Heier, J.; Hoffmann, H.; Schindler, M.; Lussi, A.; Planz, O. Inactivation of SARS-CoV-2 through Treatment with the Mouth Rinsing Solutions ViruProX((R)) and BacterX((R)) Pro. Microorganisms 2021, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Tadakamadla, J.; Boccalari, E.; Rathore, V.; Dolci, C.; Tartaglia, G.M.; Tadakamadla, S.K. In vitro studies evaluating the efficacy of mouth rinses on SARS-CoV-2: A systematic review. J. Infect. Public Health 2021, 14, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.S.; Tessema, B.; Frank, S.; Westover, J.B.; Brown, S.M.; Capriotti, J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. 2021, 100 (Suppl. S2), 192S–196S. [Google Scholar] [CrossRef]
- Ting, M.; Suzuki, J.B. The In Vitro Virucidal Effects of Mouthwashes on SARS-CoV-2. Int. J. Transl. Med. 2022, 2, 387–397. [Google Scholar] [CrossRef]
- Dawes, C. Circadian rhythms in human salivary flow rate and composition. J. Physiol. 1972, 220, 529–545. [Google Scholar] [CrossRef]
- Mendoza, J.P.I.M.; Ubillús, B.P.T.; Bolívar, G.T.S.; Palacios, R.D.P.C.; Lopez, P.S.G.H.; Rodríguez, D.A.P.; Koecklin, K.H.U. Antiviral effect of mouthwashes against SARS-CoV-2: A systematic review. Saudi Dent. J. 2022, 34, 167–193. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef]
- Alemany, A.; Millat-Martinez, P.; Ouchi, D.; Corbacho-Monné, M.; Bordoy, A.E.; Esteban, C.; Hernández, Á.; Casañ, C.; Gonzalez, V.; Costes, G.; et al. Self-collected mid-nasal swabs and saliva specimens, compared with nasopharyngeal swabs, for SARS-CoV-2 detection in mild COVID-19 patients. J. Infect. 2021, 83, 709–737. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Collins, K.; Corbit, M.; Ramirez, M.; Winters, C.R.; Katz, L.; Ross, M.; Relkin, N.; Zhou, W. Comparison of saliva and nasopharyngeal swab SARS-CoV-2 RT-qPCR testing in a community setting. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef]
- Pullano, G.; Di Domenico, L.; Sabbatini, C.E.; Valdano, E.; Turbelin, C.; Debin, M.; Guerrisi, C.; Kengne-Kuetche, C.; Souty, C.; Hanslik, T.; et al. Underdetection of cases of COVID-19 in France threatens epidemic control. Nature 2021, 590, 134–139. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D.M.; Tilley, P.; Al-Rawahi, G.N.; Srigley, J.A.; Ford, G.; Pedersen, H.; Pabbi, A.; Hannam-Clark, S.; Charles, M.; Dittrick, M.; et al. Self-Collected Saline Gargle Samples as an Alternative to Health Care Worker-Collected Nasopharyngeal Swabs for COVID-19 Diagnosis in Outpatients. J. Clin. Microbiol. 2021, 59, e02427-20. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Saito, M.; Adachi, E.; Yamayoshi, S.; Koga, M.; Iwatsuki-Horimoto, K.; Kawaoka, Y.; Yotsuyanagi, H. Gargle Lavage as a Safe and Sensitive Alternative to Swab Samples to Diagnose COVID-19: A Case Report in Japan. Clin. Infect. Dis. 2020, 71, 893–894. [Google Scholar] [CrossRef]
- Park, N.J.; Li, Y.; Yu, T.; Brinkman, B.M.; Wong, D.T. Characterization of RNA in saliva. Clin. Chem. 2006, 52, 988–994. [Google Scholar] [CrossRef]
- Gottsauner, M.J.; Michaelides, I.; Schmidt, B.; Scholz, K.J.; Buchalla, W.; Widbiller, M.; Hitzenbichler, F.; Ettl, T.; Reichert, T.E.; Bohr, C.; et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral Investig. 2020, 24, 3707–3713. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Komine, A.; Yamaguchi, E.; Okamoto, N.; Yamamoto, K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Q.; Zhu, J.; Shi, W.; Han, Z.; Zhang, Y.; Chen, K. Virucidal effect of povidone-iodine against SARS-CoV-2 in vitro. J. Int. Med. Res. 2021, 49, 3000605211063695. [Google Scholar] [CrossRef] [PubMed]
- Schutz, D.; Conzelmann, C.; Fois, G.; Gross, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef]
- Joynt, G.M.; Wu, W.K. Understanding COVID-19: What does viral RNA load really mean? Lancet Infect. Dis. 2020, 20, 635–636. [Google Scholar] [CrossRef]
- Loe, H.; Schiott, C.R. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J. Periodontal Res. 1970, 5, 79–83. [Google Scholar] [CrossRef]
- Emilson, C.G.; Fornell, J. Effect of toothbrushing with chlorhexidine gel on salivary microflora, oral hygiene, and caries. Scand. J. Dent. Res. 1976, 84, 308–319. [Google Scholar] [CrossRef]
- Maynard, J.H.; Jenkins, S.M.; Moran, J.; Addy, M.; Newcombe, R.G.; Wade, W.G. A 6-month home usage trial of a 1% chlorhexidine toothpaste (II). Effects on the oral microflora. J. Clin. Periodontol. 1993, 20, 207–211. [Google Scholar] [CrossRef]
- Kampf, G. Acquired resistance to chlorhexidine—Is it time to establish an ‘antiseptic stewardship’ initiative? J. Hosp. Infect. 2016, 94, 213–227. [Google Scholar] [CrossRef]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral. Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Biocide use and antibiotic resistance: The relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 2003, 3, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.R.; Beighton, D.; Nugent, Z.; Jackson, R.J. Effect of use of 0.05% cetylpyridinium chloride mouthwash on normal oral flora. J. Dent. 1997, 25, 35–40. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).