Current State and Prospectives for Proton Boron Capture Therapy
Abstract
1. Introduction
2. Main Texts
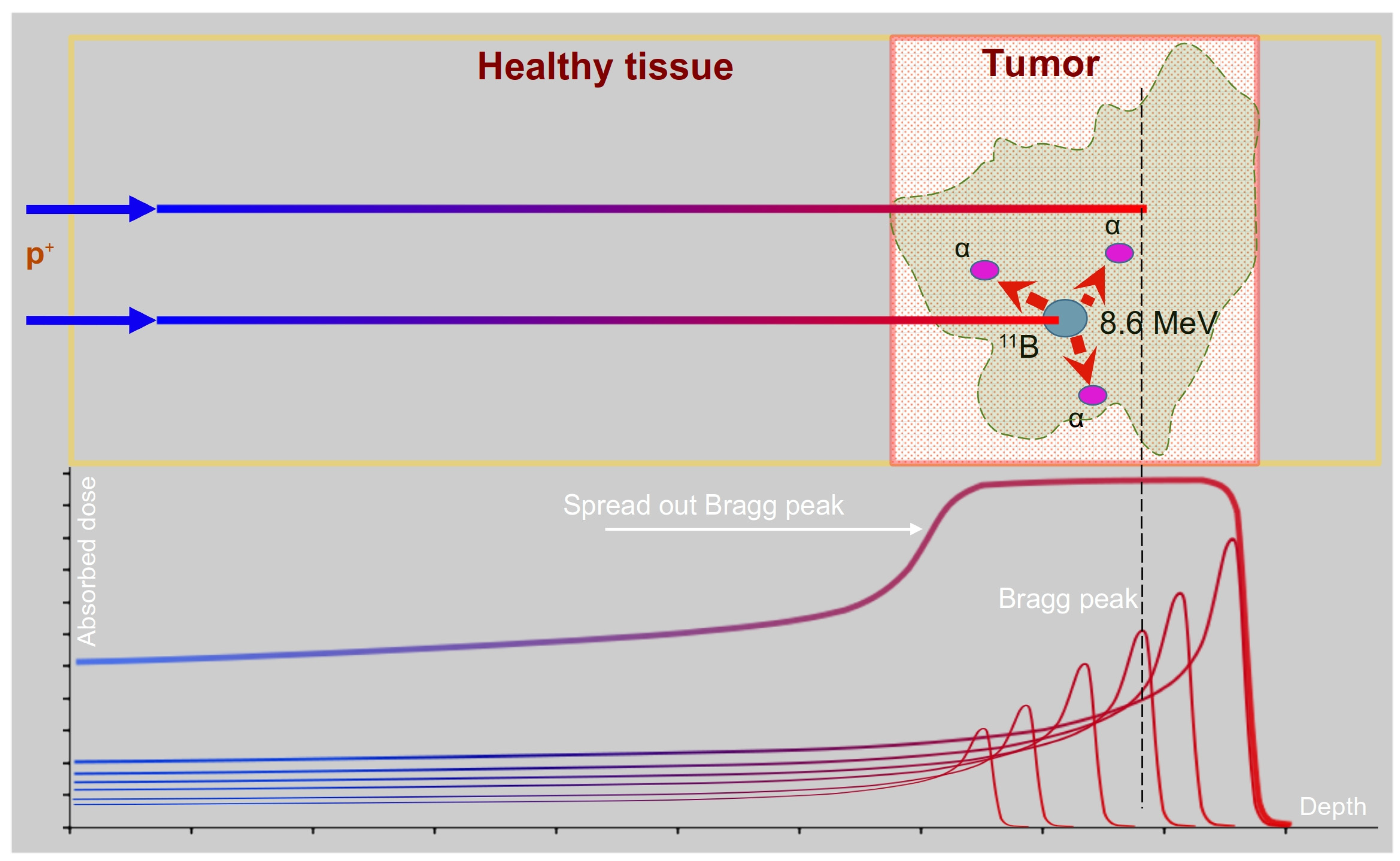
2.1. Principles and Advantages of PBCT
2.2. Simulation Studies
2.3. In Vitro Studies
2.3.1. Biological Effects of Boron during Proton Irradiation
2.3.2. Dependency of Enhancement Effects of Boron on the Proton Energy
2.3.3. Radiosensitizing Effects of Boron Compounds during Gamma and Proton Irradiation
2.3.4. Possible Mechanisms of Radiosensitizing Effects of Boron
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, K.; Gulidov, I.; Fatkhudinov, T.; Koryakin, S.; Kaprin, A. Fast and Furious: Fast Neutron Therapy in Cancer Treatment. Int. J. Part. Ther. 2022, 9, 59–69. [Google Scholar] [CrossRef]
- Ryzhikova, O.A. Proton and hadron therapy in the treatment of oncological diseases. Oncosurgery 2011, 3, 89. [Google Scholar]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef]
- Mohan, R. A review of proton therapy—Current status and future directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Lane, S.A.; Slater, J.M.; Yang, G.Y. Image-Guided Proton Therapy: A Comprehensive Review. Cancers 2023, 15, 2555. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Das, I.J.; Ling, C.C. Empowering Intensity Modulated Proton Therapy Through Physics and Technology: An Overview. Int. J. Radiat. Oncol. 2017, 99, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Hyer, D.E.; Hill, P.M.; Wang, D.; Smith, B.R.; Flynn, R.T. A dynamic collimation system for penumbra reduction in spot-scanning proton therapy: Proof of concept. Med. Phys. 2014, 41, 091701. [Google Scholar] [CrossRef] [PubMed]
- Matney, J.; Park, P.C.; Bluett, J.; Chen, Y.P.; Liu, W.; Court, L.E.; Liao, Z.; Li, H.; Mohan, R. Effects of Respiratory Motion on Passively Scattered Proton Therapy Versus Intensity Modulated Photon Therapy for Stage III Lung Cancer: Are Proton Plans More Sensitive to Breathing Motion? Int. J. Radiat. Oncol. 2013, 87, 576–582. [Google Scholar] [CrossRef]
- Sheino, I.N.; Izhevskij, P.V.; Lipengolts, A.A.; Kulakov, V.N.; Wagner, A.A.; Sukhikh, E.S.; Varlachev, V.A. Development of binary technologies of radiotherapy of malignant neoplasms: Condition and problems. Bull. Sib. Med. 2017, 16, 192–209. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Usami, N.; Porcel, E.; Lacombe, S.; Le Sech, C. Enhancement of radiation effect by heavy elements. Mutat. Res. Mol. Mech. Mutagen. 2010, 704, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Kageji, T.; Nagahiro, S.; Mizobuchi, Y.; Matsuzaki, K.; Nakagawa, Y.; Kumada, H. Boron neutron capture therapy (BNCT) for newly-diagnosed glioblastoma: Comparison of clinical results obtained with BNCT and conventional treatment. J. Med. Investig. 2014, 61, 254–263. [Google Scholar] [CrossRef]
- Miyatake, S.-I.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Ono, K. Boron Neutron Capture Therapy of Malignant Gliomas. Intracranial Gliomas Part III-Innov. Treat. Modalities 2018, 32, 48–56. [Google Scholar] [CrossRef]
- Bikchurina, M.; Bykov, T.; Kasatov, D.; Kolesnikov, I.; Makarov, A.; Shchudlo, I.; Sokolova, E.; Taskaev, S. The Measurement of the Neutron Yield of the 7Li(p,n)7Be Reaction in Lithium Targets. Biology 2021, 10, 824. [Google Scholar] [CrossRef]
- Chandra, S.; Lorey, I.I.D.R.; Smith, D.R. Quantitative subcellular secondary ion mass spectrometry (SIMS) imaging of boron-10 and boron-11 isotopes in the same cell delivered by two combined BNCT drugs: In vitro studies on human glioblastoma T98G cells. Radiat. Res. 2002, 157, 700–710. [Google Scholar] [CrossRef]
- Kabalka, G.; Yao, M.-L.; Marepally, S.; Chandra, S. Biological evaluation of boronated unnatural amino acids as new boron carriers. Appl. Radiat. Isot. 2009, 67, S374–S379. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. 2018, 38, 36–37. [Google Scholar] [CrossRef]
- Liu, N.; Lee, S.L.; Wo, J.-K. Evaluation of Proton-Boron Fusion-Enhanced Proton Therapy (PBFEPT) by Using a Simulation Method. New Phys. Sae Mulli 2019, 69, 215–220. [Google Scholar] [CrossRef]
- Yoon, D.-K.; Jung, J.-Y.; Suh, T.S. Application of proton boron fusion reaction to radiation therapy: A Monte Carlo simulation study. Appl. Phys. Lett. 2014, 105, 223507. [Google Scholar] [CrossRef]
- Oliphant, M.L.E.; Rutherford, L. Experiments on the transmutation of elements by protons. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1933, 141, 259–281. [Google Scholar] [CrossRef]
- Moreau, D. Potentiality of the proton-boron fuel for controlled thermonuclear fusion. Nucl. Fusion 1977, 17, 13–20. [Google Scholar] [CrossRef]
- Picciotto, A.; Margarone, D.; Velyhan, A.; Bellutti, P.; Krasa, J.; Szydlowsky, A.; Bertuccio, G.; Shi, Y.; Mangione, A.; Prokůpek, J.; et al. Boron-Proton Nuclear-Fusion Enhancement Induced in Boron-Doped Silicon Targets by Low-Contrast Pulsed Laser. Phys. Rev. X 2014, 4, 031030. [Google Scholar] [CrossRef]
- Sikora, M.H.; Weller, H.R. A New Evaluation of the 11B(p,𝛼)𝛼𝛼 Reaction Rates. J. Fusion Energy 2016, 35, 538–543. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Yoon, D.-K.; Barraclough, B.; Lee, H.C.; Suh, T.S.; Lu, B. Comparison between proton boron fusion therapy (PBFT) and boron neutron capture therapy (BNCT): A Monte Carlo study. Oncotarget 2017, 8, 39774–39781. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Yoon, D.-K.; Lee, H.C.; Lu, B.; Suh, T.S. The investigation of physical conditions of boron uptake region in proton boron fusion therapy (PBFT). AIP Adv. 2016, 6, 095119. [Google Scholar] [CrossRef]
- Shimizu, S.; Matsuura, T.; Umezawa, M.; Hiramoto, K.; Miyamoto, N.; Umegaki, K.; Shirato, H. Preliminary analysis for integration of spot-scanning proton beam therapy and real-time imaging and gating. Phys. Med. 2014, 30, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.; Finocchiaro, P.; Meo, S.L.; Colonna, N. On the (un)effectiveness of proton boron capture in proton therapy. Eur. Phys. J. Plus 2019, 134, 361. [Google Scholar] [CrossRef]
- Tabbakh, F.; Hosmane, N.S. Enhancement of Radiation Effectiveness in Proton Therapy: Comparison Between Fusion and Fission Methods and Further Approaches. Sci. Rep. 2020, 10, 5466. [Google Scholar] [CrossRef]
- Chiniforoush, T.A.; Hadadi, A.; Kasesaz, Y.; Sardjono, Y. Evaluation of effectiveness of equivalent dose during proton boron fusion therapy (PBFT) for brain cancer: A Monte Carlo study. Appl. Radiat. Isot. 2021, 170, 109596. [Google Scholar] [CrossRef]
- Meyer, H.J.; Titt, U.; Mohan, R. Technical note: Monte Carlo study of the mechanism of proton–boron fusion therapy. Med. Phys. 2022, 49, 579–582. [Google Scholar] [CrossRef]
- Adam, D.; Bednarz, B. SU-F-T-140: Assessment of the Proton Boron Fusion Reaction for Practical Radiation Therapy Applications Using MCNP6. Med. Phys. 2016, 43, 3494. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Manti, L.; Margarone, D.; Petringa, G.; Giuffrida, L.; Minopoli, A.; Picciotto, A.; Russo, G.; Cammarata, F.; Pisciotta, P.; et al. First experimental proof of Proton Boron Capture Therapy (PBCT) to enhance protontherapy effectiveness. Sci. Rep. 2018, 8, 1141. [Google Scholar] [CrossRef]
- Bláha, P.; Feoli, C.; Agosteo, S.; Calvaruso, M.; Cammarata, F.P.; Catalano, R.; Ciocca, M.; Cirrone, G.A.P.; Conte, V.; Cuttone, G.; et al. The Proton-Boron Reaction Increases the Radiobiological Effectiveness of Clinical Low- and High-Energy Proton Beams: Novel Experimental Evidence and Perspectives. Front. Oncol. 2021, 11, 682647. [Google Scholar] [CrossRef]
- Tomimatsu, N.; Tahimic, C.G.T.; Otsuki, A.; Burma, S.; Fukuhara, A.; Sato, K.; Shiota, G.; Oshimura, M.; Chen, D.J.; Kurimasa, A. Ku70/80 Modulates ATM and ATR Signaling Pathways in Response to DNA Double Strand Breaks. J. Biol. Chem. 2007, 282, 10138–10145. [Google Scholar] [CrossRef] [PubMed]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Shtam, T.; Burdakov, V.; Garina, A.; Garaeva, L.; Tran, N.H.; Volnitskiy, A.; Kuus, E.; Amerkanov, D.; Pack, F.; Andreev, G.; et al. Experimental validation of proton boron capture therapy for glioma cells. Sci. Rep. 2023, 13, 1341. [Google Scholar] [CrossRef]
- Manandhar, M.; Bright, S.J.; Flint, D.B.; Martinus, D.K.J.; Kolachina, R.V.; Kacem, M.B.; Titt, U.; Martin, T.J.; Lee, C.L.; Morrison, K.; et al. Effect of boron compounds on the biological effectiveness of proton therapy. Med. Phys. 2022, 49, 6098–6109. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, D.; Garaeva, L.; Burdakov, V.; Volnitskiy, A.; Razgildina, N.; Garina, A.; Amerkanov, D.; Pack, F.; Shabalin, K.; Ivanov, E.; et al. Radiosensitizing effect of boron enhances the effectiveness of proton therapy in vitro. RAD Conf. Proc. 2020, 4, 60–65. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Hill, M.A. Non-targeted effects of radiation exposure: Recent advances and implications: Figure 1. Radiat. Prot. Dosim. 2015, 166, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Little, J.B. Unexpected Sensitivity to the Induction of Mutations by Very Low Doses of Alpha-Particle Radiation: Evidence for a Bystander Effect. Radiat. Res. 1999, 152, 552–557. [Google Scholar] [CrossRef]
- Nagasawa, H.; Huo, L.; Little, J.B. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int. J. Radiat. Biol. 2003, 79, 35–41. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Kadhim, M.A.; Pocock, D.A.; Papworth, D.; Stevens, D.L.; Goodhead, D.T.; Wright, E.G. Chromosomal instability in the descendants of unirradiated surviving cells after α-particle irradiation. Proc. Natl. Acad. Sci. USA 1998, 95, 5730–5733. [Google Scholar] [CrossRef]
- Yin, X.; Tian, W.; Wang, L.; Wang, J.; Zhang, S.; Cao, J.; Yang, H. Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway and miR-21 in irradiated keratinocytes. Sci. Rep. 2015, 5, 11373. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.E.; Multhoff, G. Non-targeted effects of photon and particle irradiation and the interaction with the immune system. Front. Oncol. 2012, 2, 80. [Google Scholar] [CrossRef] [PubMed]
| Source | Software | Dose Enhancement per 100 ppm 11B |
|---|---|---|
| Jung et al., 2017 [26] | MCNPX | 5–9% |
| Yoon et al., 2014 [21], Adam et al., 2016 [33], Liu et al., 2019 [20] | MCNPX, GEANT | 0.01–0.03% |
| Mazzone et al., 2019 [29], Tabbakh and Hosmane, 2020 [30], Chiniforoush et al., 2021 [31], Meyer et al., 2022 [32] | MCNPX, GEANT | 0.00005–0.0004% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.H.; Shtam, T.; Marchenko, Y.Y.; Konevega, A.L.; Lebedev, D. Current State and Prospectives for Proton Boron Capture Therapy. Biomedicines 2023, 11, 1727. https://doi.org/10.3390/biomedicines11061727
Tran NH, Shtam T, Marchenko YY, Konevega AL, Lebedev D. Current State and Prospectives for Proton Boron Capture Therapy. Biomedicines. 2023; 11(6):1727. https://doi.org/10.3390/biomedicines11061727
Chicago/Turabian StyleTran, Nhan Hau, Tatiana Shtam, Yaroslav Yu Marchenko, Andrey L. Konevega, and Dmitry Lebedev. 2023. "Current State and Prospectives for Proton Boron Capture Therapy" Biomedicines 11, no. 6: 1727. https://doi.org/10.3390/biomedicines11061727
APA StyleTran, N. H., Shtam, T., Marchenko, Y. Y., Konevega, A. L., & Lebedev, D. (2023). Current State and Prospectives for Proton Boron Capture Therapy. Biomedicines, 11(6), 1727. https://doi.org/10.3390/biomedicines11061727





