Update on Classic and Novel Approaches in Metastatic Triple-Negative Breast Cancer Treatment: A Comprehensive Review
Abstract
1. Introduction
2. Data Analysis
2.1. Study Selection
2.2. Data Collection Process
2.3. Risk of Bias Assessment
2.4. Data Source
3. Antineoplastic Agents
3.1. Chemotherapy
3.1.1. Platinum-Based Chemotherapy
3.1.2. Taxanes
3.1.3. Anthracyclines
3.1.4. Anti-Metabolites
3.1.5. Microtubule Inhibitors
3.1.6. Alkylating Agents
3.1.7. Antibody-Drug Conjugates
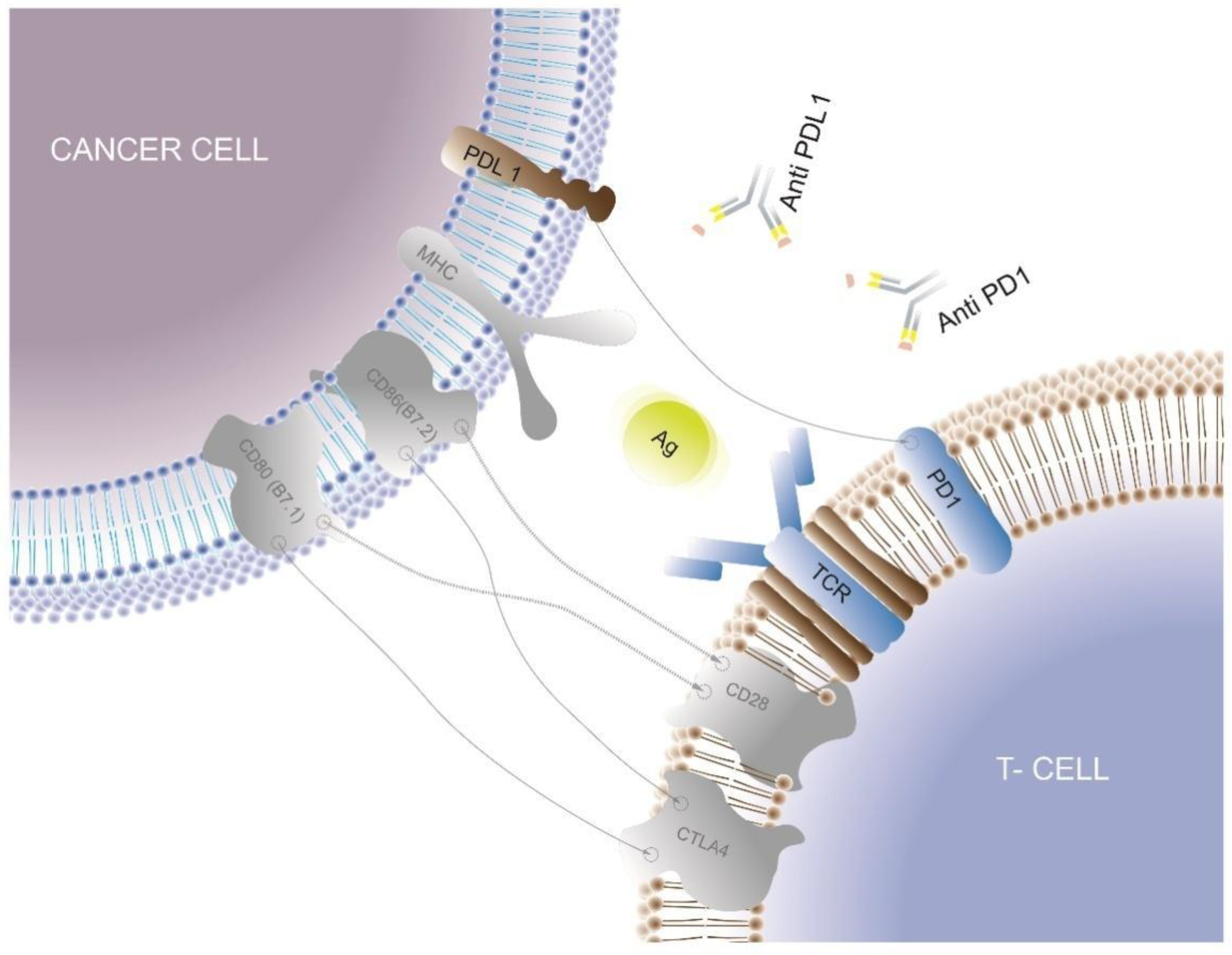
3.2. Immune Checkpoint Inhibitors
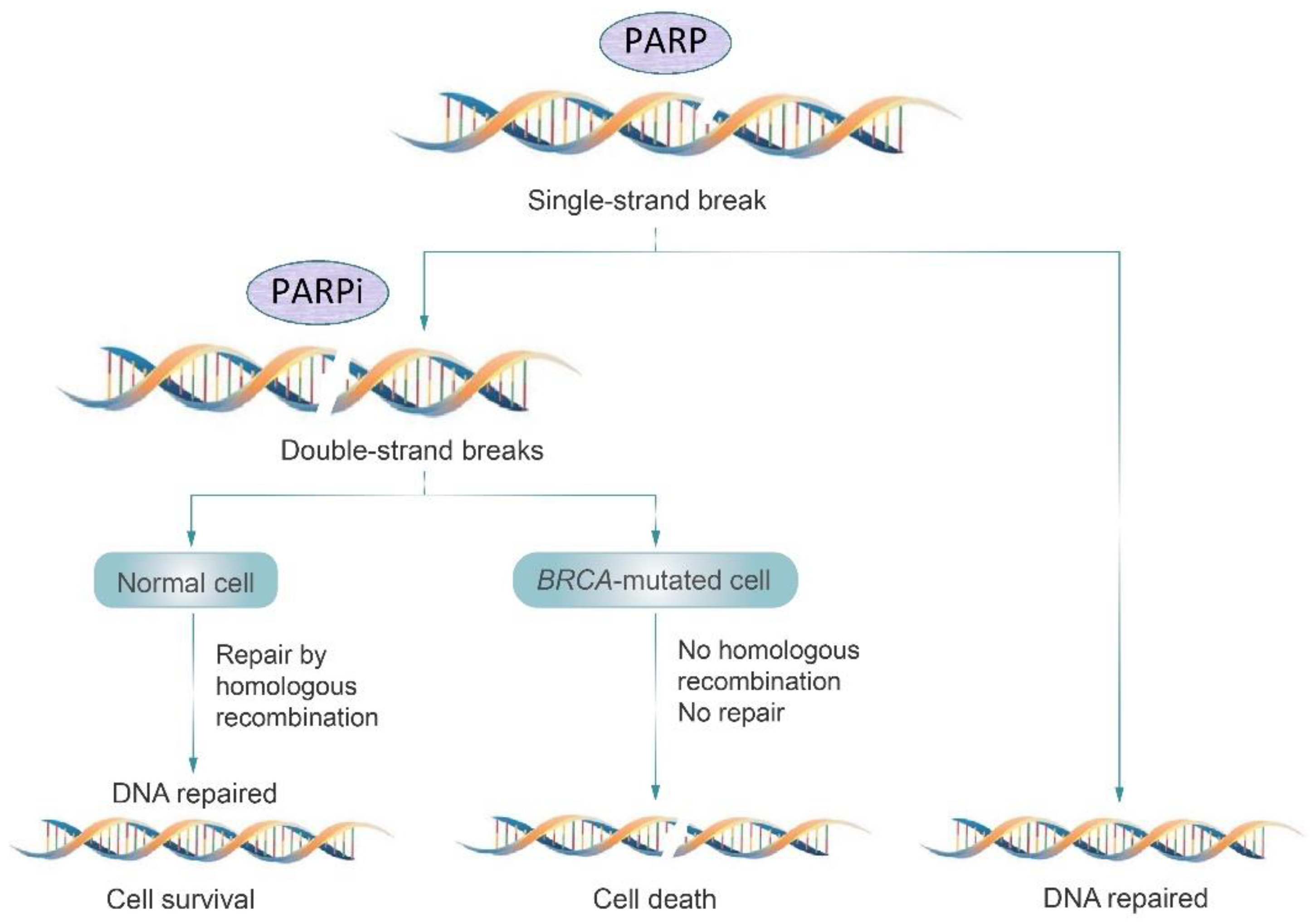
3.3. PARP Inhibitors
3.4. Tyrosine Kinase Inhibitors
3.5. Alternative Therapies
4. Focus on the New NCCN Clinical Practice Guidelines
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492. [Google Scholar] [CrossRef] [PubMed]
- Dameri, M.; Ferrando, L.; Cirmena, G.; Vernieri, C.; Pruneri, G.; Ballestrero, A.; Zoppoli, G. Multi-Gene Testing Overview with a Clinical Perspective in Metastatic Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7154. [Google Scholar] [CrossRef]
- Weigelt, B.; Reis-Filho, J.S. Histological and molecular types of breast cancer: Is there a unifying taxonomy? Nat. Rev. Clin. Oncol. 2009, 6, 718–730. [Google Scholar] [CrossRef]
- Prat, A.; Adamo, B.; Cheang, M.C.U.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular Characterization of Basal-Like and Non-Basal-Like Triple-Negative Breast Cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Prat, A.; Lluch, A.; Albanell, J.; Barry, W.T.; Fan, C.; Chacón, J.I.; Parker, J.S.; Calvo, L.; Plazaola, A.; Arcusa, A.; et al. Predicting response and survival in chemotherapy-treated triple-negative breast cancer. Br. J. Cancer 2014, 111, 1532–1541. [Google Scholar] [CrossRef]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N.; et al. Differential Response to Neoadjuvant Chemotherapy Among 7 Triple-Negative Breast Cancer Molecular Subtypes. Clin. Cancer Res. 2013, 19, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Tsuda, H.; Shimizu, C.; Yamamoto, S.; Shibata, T.; Yamamoto, H.; Hirata, T.; Yonemori, K.; Ando, M.; Tamura, K.; et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res. Treat. 2012, 132, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer—The road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Jensen, K.C.; Vinayak, S.; Kurian, A.W.; Lipson, J.A.; Flaherty, P.J.; Timms, K.; Abkevich, V.; Schackmann, E.A.; Wapnir, I.L.; et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib as Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation–Associated Breast Cancer With Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. J. Clin. Oncol. 2015, 33, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, Y.; Sun, X.; Wang, B.; Wang, Z.; Luo, J.; Wang, L.; Zhang, S.; Cao, J.; Tao, Z.; et al. Biomarker assessment of the CBCSG006 trial: A randomized phase III trial of cisplatin plus gemcitabine compared with paclitaxel plus gemcitabine as first-line therapy for patients with metastatic triple-negative breast cancer. Ann. Oncol. 2018, 29, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Schafer, J.M.; Pendleton, C.S.; Tang, L.; Johnson, K.C.; Chen, X.; Balko, J.M.; Gómez, H.; Arteaga, C.L.; et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, M.; Xie, J.; Wang, Z.; Wang, B.; Zhang, S.; Wang, L.; Cao, J.; Tao, Z.; Li, T.; et al. Chemotherapy of metastatic triple negative breast cancer: Experience of using platinum-based chemotherapy. Oncotarget 2015, 6, 43135–43143. [Google Scholar] [CrossRef]
- Brok, W.D.D.; Speers, C.H.; Gondara, L.; Baxter, E.; Tyldesley, S.K.; Lohrisch, C.A. Survival with metastatic breast cancer based on initial presentation, de novo vs. relapsed. Breast Cancer Res. Treat. 2017, 161, 549–556. [Google Scholar] [CrossRef]
- Kassam, F.; Enright, K.; Dent, R.; Dranitsaris, G.; Myers, J.; Flynn, C.; Fralick, M.; Kumar, R.; Clemons, M. Survival Outcomes for Patients with Metastatic Triple-Negative Breast Cancer: Implications for Clinical Practice and Trial Design. Clin. Breast Cancer 2009, 9, 29–33. [Google Scholar] [CrossRef]
- Hu, X.-C.; Zhang, J.; Xu, B.-H.; Cai, L.; Ragaz, J.; Wang, Z.-H.; Wang, B.-Y.; Teng, Y.-E.; Tong, Z.-S.; Pan, Y.-Y.; et al. Cisplatin plus gemcitabine vs. paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; Park, Y.H.; Hall, P.S.; Perren, T.; et al. Capivasertib Plus Paclitaxel Vs. Placebo Plus Paclitaxel as First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J. Clin. Oncol. 2020, 38, 423–433. [Google Scholar] [CrossRef]
- Yardley, D.; Coleman, R.; Conte, P.; Cortes, J.; Brufsky, A.; Shtivelband, M.; Young, R.; Bengala, C.; Ali, H.; Eakel, J.; et al. nab-Paclitaxel plus carboplatin or gemcitabine vs. gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: Results from the tnAcity trial. Ann. Oncol. 2018, 29, 1763–1770. [Google Scholar] [CrossRef]
- Wang, B.; Sun, T.; Zhao, Y.; Wang, S.; Zhang, J.; Wang, Z.; Teng, Y.-E.; Cai, L.; Yan, M.; Wang, X.; et al. A randomized phase 3 trial of Gemcitabine or Nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer. Nat. Commun. 2022, 13, 4025. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Romieu, G.; Huober, J.; Delozier, T.; Tubiana-Hulin, M.; Schneeweiss, A.; Lluch, A.; Llombart, A.; du Bois, A.; Kreienberg, R.; et al. Phase III Study of Gemcitabine Plus Docetaxel Compared with Capecitabine Plus Docetaxel for Anthracycline-Pretreated Patients with Metastatic Breast Cancer. J. Clin. Oncol. 2009, 27, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, K.; Pang, D.; Wang, C.; Jiang, J.; Yang, S.; Liu, Y.; Fu, P.; Sheng, Y.; Zhang, G.; et al. Adjuvant Capecitabine With Docetaxel and Cyclophosphamide Plus Epirubicin for Triple-Negative Breast Cancer (CBCSG010): An Open-Label, Randomized, Multicenter, Phase III Trial. J. Clin. Oncol. 2020, 38, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy vs. placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Baraibar, I.; Espinós, J.; Legaspi, J.; López-Picazo, J.M.; Aramendía, J.M.; Fernández, O.A.; Santisteban, M. Combination of pegylated liposomal doxorubicin plus gemcitabine in heavily pretreated metastatic breast cancer patients: Long-term results from a single institution experience. Breast J. 2018, 24, 473–479. [Google Scholar] [CrossRef]
- Khallaf, S.M.; Roshdy, J.; Ibrahim, A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J. Egypt. Natl. Cancer Inst. 2020, 32, 20. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Karachaliou, N.; Ziras, N.; Syrigos, K.; Tryfonidis, K.; Papadimitraki, E.; Kontopodis, E.; Bozionelou, V.; Kalykaki, A.; Georgoulias, V.; Mavroudis, D. A multicenter phase II trial of docetaxel and capecitabine as salvage treatment in anthracycline- and taxane-pretreated patients with metastatic breast cancer. Cancer Chemother. Pharmacol. 2012, 70, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Im, S.-A.; Jung, K.H.; Sohn, J.H.; Park, Y.H.; Lee, K.S.; Sim, S.H.; Park, K.-H.; Kim, J.H.; Nam, B.H.; et al. Randomized Open Label Phase III Trial of Irinotecan Plus Capecitabine vs. Capecitabine Monotherapy in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane: PROCEED Trial (KCSG BR 11-01). Cancer Res. Treat. 2019, 51, 43–52. [Google Scholar] [CrossRef]
- Albain, K.S.; Nag, S.M.; Calderillo-Ruiz, G.; Jordaan, J.P.; Llombart, A.C.; Pluzanska, A.; Rolski, J.; Melemed, A.S.; Reyes-Vidal, J.M.; Sekhon, J.S.; et al. Gemcitabine Plus Paclitaxel Vs. Paclitaxel Monotherapy in Patients with Metastatic Breast Cancer and Prior Anthracycline Treatment. J. Clin. Oncol. 2008, 26, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Maisano, R.; Zavettieri, M.; Azzarello, D.; Raffaele, M.; Bottari, M.; Nardi, M.; Maisano, M. Carboplatin and Gemcitabine Combination in Metastatic Triple-Negative Anthracycline- and Taxane-Pretreated Breast Cancer Patients: A Phase II Study. J. Chemother. 2011, 23, 40–43. [Google Scholar] [CrossRef]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Diéras, V.; Delozier, T.; et al. Eribulin monotherapy vs. treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Mougalian, S.S.; Copher, R.; Kish, J.K.; McAllister, L.; Wang, Z.; Broscious, M.; Garofalo, D.; Radtchenko, J.; Feinberg, B.A. Clinical benefit of treatment with eribulin mesylate for metastatic triple-negative breast cancer: Long-term outcomes of patients treated in the US community oncology setting. Cancer Med. 2018, 7, 4371–4378. [Google Scholar] [CrossRef]
- Liu, C.-T.; Hsieh, M.-C.; Su, Y.-L.; Hung, C.-M.; Pei, S.-N.; Liao, C.-K.; Tsai, Y.-F.; Liao, H.-Y.; Liu, W.-C.; Chiu, C.-C.; et al. Metronomic vinorelbine is an excellent and safe treatment for advanced breast cancer: A retrospective, observational study. J. Cancer 2021, 12, 5355–5364. [Google Scholar] [CrossRef]
- Valerio, M.R.; Spadaro, P.; Arcanà, C.; Borsellino, N.; Cipolla, C.; Vigneri, P.; Piazza, D.; Gebbia, V. Oral vinorelbine and capecitabine as first-line therapy in metastatic breast cancer: A retrospective analysis. Futur. Sci. OA 2021, 7, FSO750. [Google Scholar] [CrossRef]
- Li, M.; Fan, Y.; Li, Q.; Zhang, P.; Yuan, P.; Ma, F.; Wang, J.; Luo, Y.; Cai, R.; Chen, S.; et al. Vinorelbine Plus Platinum in Patients with Metastatic Triple Negative Breast Cancer and Prior Anthracycline and Taxane Treatment. Medicine 2015, 94, e1928. [Google Scholar] [CrossRef]
- Li, D.-D.; Tao, Z.-H.; Wang, B.-Y.; Wang, L.-P.; Cao, J.; Hu, X.-C.; Zhang, J. Apatinib plus vinorelbine vs. vinorelbine for metastatic triple-negative breast cancer who failed first/second-line treatment: The NAN trial. NPJ Breast Cancer 2022, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Wang, Z.; Zhonghua, W.; Wang, B.; Cao, J.; Lv, F.; Zhen, C.; Zhang, S.; Shao, Z. A phase II trial of biweekly vinorelbine and oxaliplatin in second- or third-line metastatic triple-negative breast cancer. Cancer Biol. Ther. 2015, 16, 225–232. [Google Scholar] [CrossRef]
- Ibrahim, N.K. Ixabepilone: Overview of Effectiveness, Safety, and Tolerability in Metastatic Breast Cancer. Front. Oncol. 2021, 11, 617874. [Google Scholar] [CrossRef]
- Rugo, H.S.; Roche, H.; Thomas, E.; Chung, H.C.; Lerzo, G.L.; Vasyutin, I.; Patel, A.; Vahdat, L. Efficacy and Safety of Ixabepilone and Capecitabine in Patients with Advanced Triple-negative Breast Cancer: A Pooled Analysis from Two Large Phase III, Randomized Clinical Trials. Clin. Breast Cancer 2018, 18, 489–497. [Google Scholar] [CrossRef]
- Korkmaz, A.; Topal, T.; Oter, S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol. Toxicol. 2007, 23, 303–312. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Anders, C.K.; Woodcock, M.G.; Swearingen, A.E.D.V.; Moore, D.T.; Sambade, M.J.; Laurie, S.; Robeson, A.; Kolupaev, O.; A Cuaboy, L.; Garrett, A.L.; et al. Evaluating the efficacy of a priming dose of cyclophosphamide prior to pembrolizumab to treat metastatic triple negative breast cancer. J. Immunother. Cancer 2022, 10, e003427. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.; Vick, S.C.; Iglesia, M.D.; Brickey, W.J.; Midkiff, B.R.; McKinnon, K.P.; Reisdorf, S.; Anders, C.K.; Carey, L.A.; Parker, J.S.; et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J. Clin. Investig. 2017, 127, 3472–3483. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, R.H.; Van Alphen, R.J.; Verweij, J.; Loos, W.J.; Nooter, K.; Stoter, G.; Sparreboom, A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 2001, 7, 2182–2194. [Google Scholar]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- O’shaughnessy, J.; Brufsky, A.; Rugo, H.S.; Tolaney, S.M.; Punie, K.; Sardesai, S.; Hamilton, E.; Loirat, D.; Traina, T.; Leon-Ferre, R.; et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res. Treat. 2022, 195, 127–139. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody–Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Diéras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.-Y.; Viret, F.; Levy, C.; Salabert, L.; Le Du, F.; et al. Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res. 2022, 82, PD8-02. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- John, P.; Wei, Y.; Liu, W.; Du, M.; Guan, F.; Zang, X. The B7x Immune Checkpoint Pathway: From Discovery to Clinical Trial. Trends Pharmacol. Sci. 2019, 40, 883–896. [Google Scholar] [CrossRef]
- Stagg, J.; Allard, B. Immunotherapeutic approaches in triple-negative breast cancer: Latest research and clinical prospects. Ther. Adv. Med. Oncol. 2013, 5, 169–181. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.; Pohlmann, P.R.; Tolaney, S.M.; Chang, C.-W.; Zhang, W.; Iizuka, K.; Foster, P.G.; Molinero, L.; et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up. JAMA Oncol. 2019, 5, 334. [Google Scholar] [CrossRef]
- Røssevold, A.H.; Andresen, N.K.; Bjerre, C.A.; Gilje, B.; Jakobsen, E.H.; Raj, S.X.; Falk, R.S.; Russnes, H.G.; Jahr, T.; Mathiesen, R.R.; et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: The randomized, double-blind phase 2b ALICE trial. Nat. Med. 2022, 28, 2573–2583. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab vs. investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Singh, V.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors 2022, 12, 617. [Google Scholar] [CrossRef]
- Henry, N.L.; Somerfield, M.R.; Dayao, Z.; Elias, A.; Kalinsky, K.; McShane, L.M.; Moy, B.; Park, B.H.; Shanahan, K.M.; Sharma, P.; et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 3205–3221. [Google Scholar] [CrossRef]
- Jasin, M.; Rothstein, R. Repair of Strand Breaks by Homologous Recombination. Cold Spring Harb. Perspect. Biol. 2013, 5, a012740. [Google Scholar] [CrossRef]
- Chapman, J.R.; Taylor, M.R.; Boulton, S.J. Playing the End Game: DNA Double-Strand Break Repair Pathway Choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H.J. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef]
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef]
- Featherstone, C.; Jackson, S.P. DNA double-strand break repair. Curr. Biol. 1999, 9, R759–R761. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib vs. chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef]
- de Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Telli, M.L.; Rugo, H.S.; Mailliez, A.; Ettl, J.; Grischke, E.-M.; Mina, L.A.; Balmaña, J.; Fasching, P.A.; Hurvitz, S.A.; et al. A Phase II Study of Talazoparib after Platinum or Cytotoxic Nonplatinum Regimens in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO). Clin. Cancer Res. 2019, 25, 2717–2724. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef]
- Hart, L.L.; Ferrarotto, R.; Andric, Z.G.; Beck, J.T.; Subramanian, J.; Radosavljevic, D.Z.; Zaric, B.; Hanna, W.T.; Aljumaily, R.; Owonikoko, T.K.; et al. Myelopreservation with Trilaciclib in Patients Receiving Topotecan for Small Cell Lung Cancer: Results from a Randomized, Double-Blind, Placebo-Controlled Phase II Study. Adv. Ther. 2021, 38, 350–365. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Roberts, P.J.; Sorrentino, J.A.; Bisi, J.E.; Storrie-White, H.; Tiessen, R.G.; Makhuli, K.M.; Wargin, W.A.; Tadema, H.; van Hoogdalem, E.-J.; et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci. Transl. Med. 2017, 9, eaal3986. [Google Scholar] [CrossRef]
- Tan, A.R.; Wright, G.S.; Thummala, A.R.; Danso, M.A.; Popovic, L.; Pluard, T.J.; Han, H.S.; Vojnović, Ž.; Vasev, N.; Ma, L.; et al. Trilaciclib plus chemotherapy vs. chemotherapy alone in patients with metastatic triple-negative breast cancer: A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019, 20, 1587–1601. [Google Scholar] [CrossRef]
- Davidson, D.; O’Carroll, R.; Bancroft, J. Increasing circulating androgens with oral testosterone undecanoate in eugonadal men. J. Steroid Biochem. 1987, 26, 713–715. [Google Scholar] [CrossRef]
- Janku, F.; Wheler, J.J.; Westin, S.N.; Moulder, S.L.; Naing, A.; Tsimberidou, A.M.; Fu, S.; Falchook, G.S.; Hong, D.S.; Garrido-Laguna, I.; et al. PI3K/AKT/mTOR Inhibitors in Patients with Breast and Gynecologic Malignancies Harboring PIK3CA Mutations. J. Clin. Oncol. 2012, 30, 777–782. [Google Scholar] [CrossRef]
- Massihnia, D.; Galvano, A.; Fanale, D.; Perez, A.; Castiglia, M.; Incorvaia, L.; Listì, A.; Rizzo, S.; Cicero, G.; Bazan, V.; et al. Triple negative breast cancer: Shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget 2016, 7, 60712–60722. [Google Scholar] [CrossRef]
- Li, J.; Davies, B.R.; Han, S.; Zhou, M.; Bai, Y.; Zhang, J.; Xu, Y.; Tang, L.; Wang, H.; Liu, Y.J.; et al. The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J. Transl. Med. 2013, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Abramson, V.G.; Sanders, M.E.; Mayer, E.L.; Haddad, T.C.; Nanda, R.; Van Poznak, C.; Storniolo, A.M.; Nangia, J.R.; Gonzalez-Ericsson, P.I.; et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.C.; Brokx, R.D.; Denny, T.A.; Hembrough, T.A.; Plum, S.M.; Fogler, W.E.; Sidor, C.F.; Bray, M.R. ENMD-2076 Is an Orally Active Kinase Inhibitor with Antiangiogenic and Antiproliferative Mechanisms of Action. Mol. Cancer Ther. 2011, 10, 126–137. [Google Scholar] [CrossRef]
- Diamond, J.R.; Eckhardt, S.G.; Pitts, T.M.; Van Bokhoven, A.; Aisner, D.; Gustafson, D.L.; Capasso, A.; Sams, S.; Kabos, P.; Zolman, K.; et al. A phase II clinical trial of the Aurora and angiogenic kinase inhibitor ENMD-2076 for previously treated, advanced, or metastatic triple-negative breast cancer. Breast Cancer Res. 2018, 20, 82. [Google Scholar] [CrossRef]
- Franklin, D.A.; Sharick, J.T.; Ericsson-Gonzalez, P.I.; Sanchez, V.; Dean, P.T.; Opalenik, S.R.; Cairo, S.; Judde, J.-G.; Lewis, M.T.; Chang, J.C.; et al. MEK activation modulates glycolysis and supports suppressive myeloid cells in TNBC. JCI Insight 2020, 5, e134290. [Google Scholar] [CrossRef]
- MacKeigan, J.; Collins, T.S.; Ting, J.P.-Y. MEK Inhibition Enhances Paclitaxel-induced Tumor Apoptosis. J. Biol. Chem. 2000, 275, 38953–38956. [Google Scholar] [CrossRef]
- Brufsky, A.; Kim, S.; Zvirbule, Ž.; Eniu, A.; Mebis, J.; Sohn, J.; Wongchenko, M.; Chohan, S.; Amin, R.; Yan, Y.; et al. A phase II randomized trial of cobimetinib plus chemotherapy, with or without atezolizumab, as first-line treatment for patients with locally advanced or metastatic triple-negative breast cancer (COLET): Primary analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 652–660. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Medford, A.J.; Oshry, L.; Boyraz, B.; Kiedrowski, L.; Menshikova, S.; Butusova, A.; Dai, C.S.; Gogakos, T.; Keenan, J.C.; Occhiogrosso, R.H.; et al. TRK inhibitor in a patient with metastatic triple-negative breast cancer and NTRK fusions identified via cell-free DNA analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359231152844. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P.; Bonnefoi, H.; Becette, V.; Tubiana-Hulin, M.; Fumoleau, P.; Larsimont, D.; MacGrogan, G.; Bergh, J.; Cameron, D.; Goldstein, D.; et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 2005, 24, 4660–4671. [Google Scholar] [CrossRef] [PubMed]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple Molecular Subtypes of Triple-Negative Breast Cancer Critically Rely on Androgen Receptor and Respond to Enzalutamide In Vivo. Mol. Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Androgen biosynthesis from cholesterol to DHEA. Mol. Cell. Endocrinol. 2002, 198, 7–14. [Google Scholar] [CrossRef]
- Speers, C.; Zhao, S.G.; Chandler, B.; Liu, M.; Wilder-Romans, K.; Olsen, E.; Nyati, S.; Ritter, C.; Alluri, P.G.; Kothari, V.; et al. Androgen receptor as a mediator and biomarker of radioresistance in triple-negative breast cancer. NPJ Breast Cancer 2017, 3, 29. [Google Scholar] [CrossRef]
- Michmerhuizen, A.R.; Chandler, B.; Olsen, E.; Wilder-Romans, K.; Moubadder, L.; Liu, M.; Pesch, A.; Zhang, A.; Ritter, C.; Ward, S.T.; et al. Seviteronel, a Novel CYP17 Lyase Inhibitor and Androgen Receptor Antagonist, Radiosensitizes AR-Positive Triple Negative Breast Cancer Cells. Front. Endocrinol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Li, H.; Xue, M.; Ji, A.; Li, Y. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2015, 2015, 186908. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Liu, S.-J.; Liu, Y.-J.; Wang, S.; Ni, X. Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages. Cell. Mol. Life Sci. 2010, 67, 1119–1132. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- World Health Organization; Sharma, K.; Kathait, A.; Jain, A.; Kujur, K.; Raghuwanshi, S.; Bharti, A.C.; Saklani, A.C.; Das, B.C.; Ault, K.A.; et al. Co-Infections of HPV16/18 with Other High-Risk HPV Types and the Risk of Cervical Carcinogenesis: A Large Population-Based Study. Gynecol. Oncol. 2019, 155, 436–443. [Google Scholar] [CrossRef]
- Kashfi, K.; Xu, S.; Yang, C.-T.; Meng, F.-H.; Pacheco, A.; Chen, L.; Xian, M.; Liu, M.; Wu, L.; Montaut, S.; et al. Anti-Cancer Activity of New Designer Hydrogen Sulfide-Donating Hybrids. Antioxid. Redox Signal. 2014, 20, 831–846. [Google Scholar] [CrossRef]
- Reis, A.K.C.A.; Stern, A.; Monteiro, H.P. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in cancer. Redox Biol. 2019, 27, 101190. [Google Scholar] [CrossRef]
- Li, H.; Xu, F.; Gao, G.; Gao, X.; Wu, B.; Zheng, C.; Wang, P.; Li, Z.; Hua, H.; Li, D. Hydrogen sulfide and its donors: Novel antitumor and antimetastatic therapies for triple-negative breast cancer. Redox Biol. 2020, 34, 101564. [Google Scholar] [CrossRef] [PubMed]
- Berke, T.P.; Slight, S.H.; Hyder, S.M. Role of Reactivating Mutant p53 Protein in Suppressing Growth and Metastasis of Triple-Negative Breast Cancer. OncoTargets Ther. 2022, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res. Treat. 2018, 170, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Menichini, P.; Monti, P.; Speciale, A.; Cutrona, G.; Matis, S.; Fais, F.; Taiana, E.; Neri, A.; Bomben, R.; Gentile, M.; et al. Antitumor Effects of PRIMA-1 and PRIMA-1Met (APR246) in Hematological Malignancies: Still A Mutant P53-Dependent Affair? Cells 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Salim, K.Y.; Vareki, S.M.; Danter, W.R.; San-Marina, S.; Koropatnick, J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget 2016, 7, 41363–41379. [Google Scholar] [CrossRef]
- Synnott, N.C.; O’connell, D.; Crown, J.; Duffy, M.J. COTI-2 reactivates mutant p53 and inhibits growth of triple-negative breast cancer cells. Breast Cancer Res. Treat. 2020, 179, 47–56. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Vs. miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-C.; Chuang, C.-H.; Huang, W.-C.; Weng, S.-L.; Chen, C.-H.; Chang, K.-H.; Liao, K.-W.; Huang, H.-D. A panel of eight microRNAs is a good predictive parameter for triple-negative breast cancer relapse. Theranostics 2020, 10, 8771–8789. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.K.; Saeed, M.; Malik, M. Clinical and Therapeutic Implications of Histone Acetylation in Breast Cancer. West Indian Med. J. 2015, 65, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, H.; Xu, J.; Sun, T.; Feng, X. Autophagic Vacuole Secretion Triggered by Chidamide Participates in TRAIL Apoptosis Effect in Breast Cancer Cells. Curr. Pharm. Des. 2021, 27, 2366–2380. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Jiang, H.; Han, G.; He, Q. Chidamide suppresses the glycolysis of triple negative breast cancer cells partially by targeting the miR-33a-5p-LDHA axis. Mol. Med. Rep. 2019, 20, 1857–1865. [Google Scholar] [CrossRef]
- Meng, Y.; Jin, J.; Gong, C.; Miao, H.; Tao, Z.; Li, T.; Cao, J.; Wang, L.; Wang, B.; Zhang, J.; et al. Phase II study of chidamide in combination with cisplatin in patients with metastatic triple-negative breast cancer. Ann. Palliat. Med. 2021, 10, 11255–11264. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Wicinski, J.; Cervera, N.; Finetti, P.; Hur, M.-H.; Diebel, M.E.; Monville, F.; Dutcher, J.; et al. Breast Cancer Cell Lines Contain Functional Cancer Stem Cells with Metastatic Capacity and a Distinct Molecular Signature. Cancer Res. 2009, 69, 1302–1313. [Google Scholar] [CrossRef]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D.; et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef]
- Schott, A.F.; Goldstein, L.J.; Cristofanilli, M.; Ruffini, P.A.; McCanna, S.; Reuben, J.M.; Perez, R.P.; Kato, G.; Wicha, M. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2–Negative Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5358–5365. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausovà, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Rashid, S.; Al-Bozom, I.A. PD−L1 immunostaining: What pathologists need to know. Diagn. Pathol. 2021, 16, 94. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, A.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Landry, I.; Sumbly, V.; Vest, M. Advancements in the Treatment of Triple-Negative Breast Cancer: A Narrative Review of the Literature. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Hosseini, M.; Seyedpour, S.; Khodaei, B.; Loghman, A.-H.; Seyedpour, N.; Yazdi, M.-H.; Rezaei, N. Cancer Vaccines for Triple-Negative Breast Cancer: A Systematic Review. Vaccines 2023, 11, 146. [Google Scholar] [CrossRef]
- Gholami, S.; Chen, C.-H.; Gao, S.; Lou, E.; Fujisawa, S.; Carson, J.; Nnoli, J.E.; Chou, T.-C.; Bromberg, J.; Fong, Y. Role of MAPK in oncolytic herpes viral therapy in triple-negative breast cancer. Cancer Gene Ther. 2014, 21, 283–289. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Nguyen, H.-M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef]
- Tao, J.J.; Visvanathan, K.; Wolff, A.C. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast 2015, 24, S149–S153. [Google Scholar] [CrossRef]
- Sandigursky, S.; Mor, A. Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Rheumatol. Rep. 2018, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.M.; Danos, D.M.; Fu, Q.; Wang, X.; Scribner, R.A.; Chu, S.T.; Horswell, R.L.; Price-Haywood, E.G.; Collins-Burow, B.M.; Wu, X.-C.; et al. Association of Obesity and Diabetes With the Incidence of Breast Cancer in Louisiana. Am. J. Prev. Med. 2022, 63, S83–S92. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, A.; Mykała-Cieśla, J.; Pogoda, K.; Jagiełło-Gruszfeld, A.; Kunkiel, M.; Winder, M.; Chudek, J. Limitations of Systemic Oncological Therapy in Breast Cancer Patients with Chronic Kidney Disease. J. Oncol. 2020, 2020, 7267083. [Google Scholar] [CrossRef] [PubMed]

| Authors | Trial Registration Code | Sample Size, n | Study Design | Study Type | Study Duration | Treatment | PFS and/or OS | Other Clinical Results |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. (CBCSG006 trial) [14] | NCT01287624 | 240 | Phase III | RCT | 11 January–13 November | Cisplatin + gemcitabine (GP) vs. paclitaxel + gemcitabine (GT) | PFS: 7.73 (95% CI 6.46–9.00) for GP vs. 6.07 (95% CI 5.32–6.83) for GT arm OS: NS | - |
| Yardley et al. (tnAcity trial) [23] | NCT01881230 | 191 | Phase II | RCT | 13 September–15 April | Nab-paclitaxel + carboplatin (nab-P/C) vs. nab-paclitaxel + gemcitabine (nab-P/G) vs. gemcitabine + carboplatin (G/C) | PFS: nab-P/C vs. nab-P/G 8.3 vs. 5.5 (HR 0.59, 95% CI 0.38–0.92); nab-P/C vs. G/C 8.3 vs. 6.0 (HR 0.58, 95% CI 0.37–0.90) OS: NS | ORR: 73% for nab-P/C, 39% for nag-P/G, 44% for G/C |
| Wang et al. (GAP trial) [24] | NCT02546934 | 254 | Phase III | RCT | 16 March–19 October | Nab-paclitaxel + cisplatin (AP) vs. gemcitabine + cisplatin (GP) | PFS: 9.8 for AP vs. 7.4 for GP OS: HR 0.62 (95% CI 0.44–0.90) in favor of AP | ORR: 81.1% for AP vs. 56.3% for GP |
| Cortes et al. (EMBRACE trial) [36] | NCT00388726 | 762 | Phase III | RCT | 6–8 November | Eribulin mesilate (EM) vs. treatment of physician’s choice (TPC) | OS: 13.1 (95% CI 11.8–14.3) vs. 10.6 (95% CI 9.3–12.5); HR 0.81 (95% CI 0.66–0.99) in favor of EM arm | - |
| Anders et al. (LCCC 1525 trial) [47] | NCT02768701 | 40 | Phase II, single arm | SAT | 16 November–18 February | Cyclophosphamide prior to pembrolizumab | PFS: 1.8 months | ORR: 21% |
| Bardia et al. (ASCENT trial) [50] | NCT02574455 | 468 | Phase III | RCT | 17 November–19 September | Sacituzumab govitecan (SG) vs. chemotherapy (CT) | PFS: 5.6 (95% CI 4.3–6.3) for SG arm vs. 1.7 (95% CI 1.5–2.6) for CT arm OS: 12.1 (95% 10.7–14.0) for SG vs. 6.7 (95% CI 5.8–7.7) for CT arm | ORR: 35% for SG vs. 5% for CT arm |
| Schmid et al. (IMpassion130 trial) [60] | NCT02425891 | 902 | Phase III | RCT | 15 June–17 May | Atezolizumab + nab-paclitaxel (AT-P) vs. placebo + nab-paclitaxel (PP) | PFS: 7.5 vs. 5.0; HR 0.62 (95% CI 0.49–0.78) in favor of AT-P arm OS: NS | - |
| Røssevold et al. (ALICE trial) [62] | NCT03164993 | 70 | Phase IIb | RCT | 17 August–21 December | Atezolizumab + pegylated liposomal doxorubin + cyclophosphamide (AT-CT) vs. placebo + doxorubicin + cyclophosphamide (P-CT) | PFS: 4.3 vs. 3.5; HR 0.57 (95% CI 0.33–0.99) in favor of AT-CT arm | - |
| Miles et al. (IMpassion131 trial) [63] | NCT03125902 | 651 | Phase III | RCT | 17 August–19 September | Atezolizumab + paclitaxel (AT-P) vs. placebo + paclitaxel (PP) | PFS: 6.0 for AT-P arm vs. 5.7 for PP arm; HR 0.82 (95% CI 0.60–1.12) OS: NS | - |
| Adams et al. (KEYNOTE-086 trial) [64] | NCT02447003 | 170 | Phase II, single arm | SAT | 15 July–16 January | Pembrolizumab | PFS: 2.0 (95% CI 1.9–2.0) OS: 9.0 (95% CI 7.6–11.2) | ORR: 5.3% in the total and 5.7% in the PD-L1+ population |
| Ho et al. [65] | NCT02730130 | 17 | Phase II, single arm | SAT | 16 June–17 May | Pembrolizumab + radiotherapy | PFS: 2.6 months OS: 8.25 months | ORR: 17.6% (95% CI 4.7–44.2) |
| Winer et al. (KEYNOTE-119 trial) [67] | NCT02555657 | 622 | Phase III | RCT | 15 November–17 April | Pembrolizumab (Pem) vs. chemotherapy of physician’s choice (TPC) | OS: 9.9 (95% CI 8.3–11.4) for Pem arm and 10.2 (95% CI 7.9–12.6) for TPC arm | - |
| Cortes et al. (KEYNOTE-355 trial) [68] | NCT02819518 | 847 | Phase III | RCT | 17 January–18 June | Pembrolizumab (Pem) vs. chemotherapy treatment of physician’s choice (TPC) | OS: 23.0 for Pem arm (with PD-L1 CPS ≥10) vs. 16.1 for TPC arm; HR 0.86 (95% CI 0.72–1.04) | - |
| Tan et al. [87] | NCT02978716 | 34 | Phase II | RCT | 17 February–18 May | Trilaciclib (Tr) prior to gemcitabine + carboplatin (G/C) vs. G/C alone | OS: 12.6 for G/C arm, not reached for Tr prior to G/C arm, and 19.8 for Tr + Tr prior to G/C arm | - |
| Schmid et al. (PAKT trial) [22] | NCT02423603 | 140 | Phase II | RCT | 14 May–17 June | Capivasertib + paclitaxel (CP) vs. placebo + paclitaxel (PP) | PFS: 5.9 for CP arm vs. 4.2 for PP arm, HR 0.74 (95% CI 0.50–1.08) OS: 19.1 for CP arm vs. 12.6 for PP arm, HR 0.61 (95% CI 0.37–0.99) | - |
| Garrido-Castro et al. [92] | NCT01790932 | 50 | Phase II, single arm | SAT | 12 June–14 September | Buparlisib | PFS: 1.8 (95% CI 1.6–2.3) OS: 11.2 (95% CI 6.2–25) | - |
| Lehmann et al. (TBCRC 032 trial) [93] | NCT02457910 | 17 | Phase IB/II | RCT | 15 May–18 August | Enzalutamide alone (Ez) vs. enzalutamide + taselisib (Ez/T) | PFS: 3.4 months | CBR: 35.7% |
| Diamond et al. [95] | NCT01639248 | 41 | Phase II, single arm | SAT | 12 July–16 October | ENMD-2076 | PFS: 1.84 (95% CI 1.73–3.73) | CBR: 16.7% (95% CI 6–32.8%) |
| Brufsky et al. (COLET trial) [98] | NCT02322814 | 106 | Phase II | RCT | 15 March–16 October | Cobimetinib + paclitaxel (CoP) vs. placebo + paclitaxel (PP) | PFS: 5.5 for CoP arm vs. 3.8 for PP arm, HR 0.73 (95% CI 0.43–1.24) | ORR: 38.3% (95% CI 24.4–52.2) for CoP arm vs. 20.9% (95% CI 8.8–33.1) for PP arm |
| Goldstein et al. (fRIDA trial) [131] | NCT01861054 | 123 | Phase II | RCT | 15 July–18 May | Reparixin + paclitaxel (RP) vs. placebo + paclitaxel (PP) | PFS: 5.5 for RP arm vs. 5.6 vs. PP arm | - |
| Setting | Subtype/Biomarkers | Regimen |
|---|---|---|
| First-line | PD-L1 CPS ≥ 10 regardless of germline BRCA mutation | Pembrolizumab + chemotherapy (nab-paclitaxel, or gemcitabine and carboplatin) (Category 1) |
| PD-L1 CPS < 10 and no germline BRCA1/2 mutation | Systemic chemotherapy (see Table 3) | |
| PD-L1 CPS < 10 and germline BRCA1/2 mutation | PARPi (olaparib, talazoparib) or platinum (carboplatin or cisplatin) (both Category 1) | |
| Second-line | Germline BRCA1/2 mutation | PARPi (olaparib, talazoparib) (Category 1) |
| Any | Sacituzumab govitecan (Category 1) | |
| No germline BRCA1/2 mutation and HER2 IHC 1+ or 2 + /ISH negative | Fam-trastuzumab deruxtecan-nxki (Category 1) | |
| Third-line and beyond | Biomarker positive | Targeted agents (see Table 4) |
| Any | Systemic chemotherapy (see Table 3) |
| Preferred Regimens | Other Recommended Regimens | Useful in Certain Circumstances |
|---|---|---|
| Anthracyclines (doxorubicin or liposomal doxorubicin) | Cyclophosphamide | AC (doxorubicin and cyclophosphamide) |
| Taxanes (paclitaxel) | Docetaxel | EC (epirubicin and cyclophosphamide) |
| Anti-metabolites (capecitabine or gemcitabine) | Nab-paclitaxel | CMF (cyclophosphamide and methotrexate and fluorouracil) |
| Microtubule inhibitors (vinorelbine or eribulin) | Epirubicin | Docetaxel and capecitabine |
| Ixabepilone | GT (gemcitabine and paclitaxel) | |
| Carboplatin and paclitaxel or nab-paclitaxel | ||
| Gemcitabine and carboplatin |
| Biomarker | Detection Method | FDA-Approved Agents |
|---|---|---|
| NTRK fusion | FISH, NGS, PCR (tissue block) | Larotrectinib or entrectinib (Category 2A) |
| MSI-H/dMMR | IHC, NGS, PCR (tissue block) | Pembrolizumab or dostarlimab-gxly (Category 2A) |
| TMB-H (≥ 10 mut/Mb) | NGS | Pembrolizumab (Category 2A) |
| RET-fusion | NGS | Selpercatinib (Category 2A) |
| Antineoplastic Agents | Main Mechanism of Action |
|---|---|
| Chemotherapeutic agents | |
| Platinum-based chemotherapy | Covalent binding to DNA, leading to the formation of DNA cross-links |
| Taxanes | Binding to microtubules, preventing their depolymerization |
| Anthracyclines | Disruption of DNA by poisoning topoisomerase |
| Microtubule inhibitors | Inhibition of the AKT/mTOR signaling pathway |
| Alkylating agents | Direct action on DNA, resulting in crosslinking and strand breaks |
| Antibody–drug conjugates | Delivery of deactivated cytotoxins to specific cancer cells |
| Immune checkpoint inhibitors | Targeted inhibition of the bindings between cancer cell checkpoint ligands and their complementary receptors on the CD8+ cell |
| PARP inhibitors | Inhibition of DNA repair pathways, leading to apoptosis of cancer cells, especially in homologous recombination deficient cells |
| Tyrosine kinase inhibitors | Phosphorylation of specific amino acids on substrate enzymes, causing altered signal transduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, S.; Fabbri, N.; Spaggiari, R.; De Giorgi, A.; Fabbian, F.; Giovine, A. Update on Classic and Novel Approaches in Metastatic Triple-Negative Breast Cancer Treatment: A Comprehensive Review. Biomedicines 2023, 11, 1772. https://doi.org/10.3390/biomedicines11061772
Greco S, Fabbri N, Spaggiari R, De Giorgi A, Fabbian F, Giovine A. Update on Classic and Novel Approaches in Metastatic Triple-Negative Breast Cancer Treatment: A Comprehensive Review. Biomedicines. 2023; 11(6):1772. https://doi.org/10.3390/biomedicines11061772
Chicago/Turabian StyleGreco, Salvatore, Nicolò Fabbri, Riccardo Spaggiari, Alfredo De Giorgi, Fabio Fabbian, and Antonio Giovine. 2023. "Update on Classic and Novel Approaches in Metastatic Triple-Negative Breast Cancer Treatment: A Comprehensive Review" Biomedicines 11, no. 6: 1772. https://doi.org/10.3390/biomedicines11061772
APA StyleGreco, S., Fabbri, N., Spaggiari, R., De Giorgi, A., Fabbian, F., & Giovine, A. (2023). Update on Classic and Novel Approaches in Metastatic Triple-Negative Breast Cancer Treatment: A Comprehensive Review. Biomedicines, 11(6), 1772. https://doi.org/10.3390/biomedicines11061772






