Cognitive Alterations in Addictive Disorders: A Translational Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Alcohol Use Disorders
3.1.1. Clinical Studies
3.1.2. Preclinical Studies
Acute Alcohol Consumption
Chronic Alcohol Consumption
Binge-like Ethanol Exposure
Alcohol Withdrawal
3.2. Cannabis Use Disorders
3.2.1. Clinical Studies
Factors Associated with Regular and/or Chronic Cannabis Use
Ongoing Active Effects of Regular and/or Chronic Cannabis Consumption
Post-Detoxification Effects Related to Regular and/or Chronic Cannabis Consumption
3.2.2. Preclinical Studies
Acute Exposure to Δ9-THC
Chronic Exposure to Δ9-THC
| ACUTE Δ9-THC | |||||
|---|---|---|---|---|---|
| Pattern, Doses and Route of Administration | Strain and Stage of Administration | Test | Behavioral Alterations | Biological Alterations | References |
| Acute, (0.1–0.3 mg/kg, i.m.) 45 min before behavioral testing | Adult male rhesus macaques | Self-ordered spatial search Visuo-spatial paired associates learning PR schedule of reinforcement & bimanual motor skill | ↓ Working memory ↓ Visuo-Spatial associative memory↓ Incremental learning | - | [114] |
| Acute (30–240 μg/kg, i.v.) 30 min before behavioral testing | Spatial delayed response Delayed match-to-sample | =Object Working Memory ↓ Spatial Working Memory | [115] | ||
| Acute, (0.2, 0.5 mg/kg, i.m.) 30 min before behavioral testing | Bimanual motor skill Visuo-spatial paired associates learning Self-ordered spatial search PR schedule of reinforcement Rotating turntable task | ↓ Behavioral performance ↓ Visuospatial associative learning and memory ↓ Spatial working memory ↓ Number of reinforcements ↓ Threshold retrieval speed | [116] | ||
| Acute (6 mg/kg, i.p.) 60 min before behavioral testing | Adult male Wistar rats | Eight-arm radial maze | ↓ Spatial memory | - | [118] |
| Acute, (1.25–25 mg/kg, i.p.) 50 min before behavioral testing | Adult male CD1 mice | Y-maze | ↓ Spatial learning and memory performance | - | [119] |
| Acute, (0.1–3 mg/kg, i.p.) 15 min after training | Novel object recognition | ↓ Short- and long-term recognition memory (discrimination index) ↓ Total object exploration | = Synaptic excitatory transmission (hippocampal slices) = K+-evoked glutamate and GABA release (hippocampal slices) | [123] | |
| Acute, (0.1, 0.3, 1, 3 mg/kg, i.p.) 20 min after training | ↓ Recognition of short- and long-term memory (discrimination index) | ↑ Phosphorylated substrates of PKC in the HIPP ↑ Phosphorylated neurogranin in the HIPP | [124] | ||
| Acute, (0–10 mg/kg, i.p.) 15 min before behavioral testing | Adult male C57BL/6J mice | Conditional discrimination Barnes maze | =Discrimination ratio ↓ Spatial memory (THC-treated mice) | - | [120] |
| Acute (1 and 3 mg/kg, i.p.) immediately after training | Novel object recognition | ↓ Recognition memory (discrimination index) | Presence of A2Ar and CB1r heteromers at the presynaptic level in CA1 neurons in the HIPP | [125] | |
| Acute (3 and 10 mg/kg, i.p.) immediately after training | ↓ Recognition memory (discrimination index) in WT mice | CB1r-5HT2Ar heteromers are involved in the amnesic effect of Δ9-THC (cortex, striatum, NAcc, HIPP) | [122] | ||
| Acute, (0, 0.3, 1, 2, 3 mg/kg, i.p.) 30 min before behavioral testing | Adult male Long-Evans rats | Rat cognitive effort task | Impairment of decision-making involving cognitive effort costs | CB1r density in the mPFC correlated with Δ9-THC-induced choice impairments | [117] |
| Acute intra-PFC, (10, 50, 100 or 500 ng/0.5 µL) | Adult male Sprague-Dawley rats | Attentional set-shifting Y-maze | =Cognitive flexibility =Spatial working memory | - | [129] |
| Acute intra-vHipp, (10, 100 ng/0.5 µL) | Context-dependent and context-independent fear conditioning | ↑ Freezing time (to context and conditioned stimulus) | ↑ pERK1-2 protein expression in the vHipp ↑ VTA DA frequency and bursting rates ↓ VTA putative GABAergic neuronal activity | [130] | |
| Acute intra NAcc-Sh, (100 ng/0.5 μL) | Olfactory fear conditioning Prepulse inhibition Novel object recognition | ↑ Freezing time (posterior NAcc-Sh, sub-threshold fear memory 0.4 mA footshock) ↓ Freezing time (anterior NAcc-Sh, supra-threshold fear memory 0.8 mA footshock) ↓ PPI and ↑ PPF (posterior NAcc-Sh) ↓ Discrimination index (posterior NAcc-Sh) | ↓ pAktSer473 and ratio pAkt:tAktSer473 (anterior NAcc-Sh) ↓ pmTOR and ratio pmTOR:tmTOR (anterior NAcc-Sh) ↓ pGSK3a and pGSK3a:tGSK3a (posterior NAcc-Sh) ↑ β-catenin (posterior NAcc-Sh) | [131] | |
| Acute smoke exposure (0, 1, 3, 5 cigarettes with 5.6% of Δ9-THC, inh.) 15 min before behavioral testing Acute, (0, 0.3, 1, 3 mg/kg, i.p.) 10 min before behavioral testing | Adult male and female Long-Evans rats | Delayed response working memory task | ↑ Working memory accuracy (females) ↓ Working memory accuracy (males and females) | - | [121] |
| Acute, (3 mg/kg, i.p.) | Adolescent and adult female CD1 mice | - | - | Significant changes in the mouse brain lipidome (PND 35, PND 50 and adulthood) Significant changes in the brain levels of targeted lipids, including eCBs (PND 35, PND 50 and adulthood) Significant changes in the HIPP transcriptome (PND 35, PND 50 and adulthood) | [127] |
| Acute, (10 mg/kg, i.p.) | Adult male C57BL/6J mice | Impairment of the hippocampal synaptosomal proteome (metabolic pathways and proteasome system) | [128] | ||
| CHRONIC Δ9-THC | |||||
| Pattern, Doses and Route of Administration | Strain and Stage of Administration | Test | Behavioral Alterations | Biological Alterations | References |
| Acute (0.01–0.56 mg/kg, i.v.) and chronic (1.0–2.0 mg/kg, i.v., 12 weeks) | Adult male rhesus macaques | Delayed match-to-sample Stimulus discrimination Reversal learning Attentional set-shifting | ↓ Working memory (acute and chronic admin.) ↓ Compound discrimination (acute admin.) No significant effects ↓ Extradimensional set-shifting performance (acute admin.) | =D2/D3r striatal availability | [134] |
| Chronic (15–240 µg/kg, i.v., 5 days per week, 6 months) | Adolescent male rhesus macaques | Spatial memory task Object memory task | Impaired improvements in accuracy on the spatial working memory No significant effects | - | [138] |
| Chronic (15–240 µg/kg, 12 months, i.v.) | Spatial delayed response | Impairment of reinforcement-related learning processes required for improved performance on spatial working memory | [139] | ||
| Chronic, (week 1: 0.1 mg/kg; week 2: 0.3 mg/kg; week 3 and during 4 months: 1 mg/kg; i.m.) | Adolescent male Squirrel monkeys | Repeated acquisition Discrimination reversal | ↓ Discrimination learning =Cognitive flexibility | [140] | |
| Chronic (1 mg/kg, i.p., once daily for 21 days) | Adult male Sprague-Dawley rats | Prepulse inhibition | ↓ % of PPI | - | [141] |
| Chronic (10 mg/kg, i.p., once daily for 7 days) | Adult male C57BL/6J mice | Morris water maze Fear conditioning | ↓ Spatial memory ↓ Fear memory | ↑ COX-2 expression (CB1r-dependent) ↓Long-term potentiation at hippocampal CA3-CA1 synapses ↓ Dendritic spines and postsynaptic density ↓ GluA1, GluN2A, GluN2B protein expression in the hippocampal CA1 area | [142] |
| Chronic (5 mg/kg, 14 days, i.p.) | Adult male Long-Evans rats | - | - | Disruption of the balance of corticolimbic glutamatergic input to the NAcc (mostly prevented by CB1r antagonism) | [143] |
| Chronic (10 mg/kg, i.p., 30 days) | Adolescent male CD1 mice | Prepulse inhibition | ↑ PPI disruption induced by the activation of 5HT2Ar | =5HT2Ar protein density ↑ 5HT2Ar signaling through inhibitory G-proteins Involvement of Akt/mTOR intracellular signaling pathway | [144] |
| Chronic, (5 mg/kg, PND 30–45, i.p.) | Adolescent male C57BL/6J mice | Novel object recognition Social discrimination Task | ↓ Recognition memory (discrimination index) ↓ Social recognition memory (discrimination index) | Long-lasting activation of mTOR in the PFC that persisted in adulthood Impaired excitatory and inhibitory transmission in the PFC Impaired intrinsic properties of layer V pyramidal neurons Impaired LTD at PFC layer I/V synapses | [145] |
| Chronic (1.5 mg/kg, every 3rd day for a total of 8 Injections, i.p.) | Adolescent male Long-Evans rats | - | - | ↑ Plasma corticosterone Impaired dendritic arborization, spine density, and developmental trajectory of layer III PrL pyramidal neurons Impairment of normal developmental trajectory of the PrL pyramidal transcriptome | [147] |
| Chronic (5 mg/kg, 14 days PND 30–43, i.p.) | Adolescent male and female C57BL/6J mice Adolescent male and female Long-Evans rats | Serial “what” task and two-odor discrimination Novel object recognition (spatial version) | Impaired episodic memory (↓ discrimination) ↓ Discrimination index (female rats and mice) | Impaired mechanisms of enduring, memory-related synaptic plasticity (LTP) within HIPP (female rats and mice) | [146] |
| Self-admin. (3–100 µg/kg, i.v.) | Adolescent male and female Sprague-Dawley rats | Delayed match-to-sample | ↑ Working memory performance in male rats | ↓ CB1r protein expression in the PrL, IL and VTA ↓ GABAAR1α protein expression in the PrL and DH ↓ GABABr2 protein expression in the PrL ↓ GluR2/3 protein expression in the PrL | [148] |
| Chronic (60 mg/kg, 28 days, i.p.) | Adolescent and adult female Wistar rats | Morris water maze | ↑ Spatial and working memory | =BDNF protein levels | [149] |
| Chronic (7 mg/kg, i.p., every other day for 21 days) | Adolescent male St8sia2−/− and St8sia2+/+ mice (C57BL/6N background) | Hole board paradigm | ↓ Spatial reference memory in St8sia2−/− ↓ Learning index in St8sia2−/− | ↑ PolySia levels in the HIPP of St8sia2−/− ↓ PolySia-free NCAM-180 in the HIPP of St8sia2−/− ↑ PolySia in the molecular layer of the HIPP of St8sia2−/− | [150] |
| Chronic treatment (10 mg/kg, PND 42–51, i.p.) | Adolescent male and female DN-DISC1 and WT mice (C57BL/6J background) | Novel object recognition | ↓ Recognition memory (discrimination index) in DN-DISC1 mice | ↑ BDNF in the HIPP in WT mice | [151] |
| Chronic (8 mg/kg, 3 weeks from PND 30, s.c.) | Y maze Novel object recognition Novel place recognition test | ↓ Spatial recognition memory in DN-DISC1 male and female mice ↓ Recognition memory (discrimination index) in DN-DISC1 male mice↓ Preference for the novel place of one of two identical objects in DN-DISC1 male mice | ↑ Activation of the NF-kB-COX-2 pathway in astrocytes ↓ Immunoreactivity of parvalbumin-positive pre-synaptic inhibitory boutons around pyramidal neurons of the hippocampal CA3 area | [152] | |
| Chronic smoke exposure (5 cigarettes/day from PND 29 to 49, 5.6% of Δ9-THC, inh.) | Adolescent male Long-Evans rats | PR Delayed response task Set shifting and probabilistic reversal learning tasks Intertemporal choice task | =Motivation to work for food ↓ Working memory =Cognitive flexibility =Decision making | - | [153] |
| Chronic vapor exposure (10 mg/4 animals, PND 28–42 every other day, inh.) | Adolescent male Sprague-Dawley rats | Novel object recognition Conditioned avoidance response | =Discrimination index (↓ recognition memory when combined with alcohol adolescent exposure) No significant differences | - | [154] |
| Chronic vapor exposure (20 min of vapor exposure for 5 days) | Adolescent male Wistar rats | Fear conditioning | ↑ Freezing time in Δ9-THC and Δ9-THC+ Ethanol CIE animals | ↑ PrL signaling in response to the shock stimuli | [155] |
| Chronic (2.5 mg/kg PND 35–37, 5 mg/kg PND 38–41; 10 mg/kg PND 42–45, twice daily, i.p.) | Adolescent female Sprague-Dawley rats | Novel object recognition (classic and spatial versions) | ↓ Recognition and spatial memory (discrimination index) | ↑ Iba1, TNFα, COX-2, iNOS, and CB2 in the PFC ↓ CB1r and IL10 in the PFC | [156] |
| - | - | ↓ CB1r binding in the PFC Impaired eCB-LTD in the mPFC ↓ Dendritic length in DCX+ cells in the DG of the HIPP ↓ DCX+ cells in the DG of the HIPP Impaired newborn neurons-dependent LTP in the DG of the HIPP | [157] | ||
| Adolescent male Sprague-Dawley rats | NOR (classic and spatial versions) | ↓ Recognition and spatial memory (discrimination index) | ↑ SYP, PSD95, GluA1, GluA2, GluN2B in the HIPP ↑ GFAP, TNFα, iNOS ↓ IL10 in the HIPP ↑ COX2 in the PFC | [158] | |
| Social interaction test Novel object recognition Prepulse inhibition | ↓ Social recognition memory ↓ Object recognition memory ↓ % of prepulse inhibition | ↑ The activity of VTA DA and PFC pyramidal neurons ↓ GSK-3 (α and β isoforms) protein expression in the PFC ↓ Phosphorylated AKT protein expression in the PFC | [159] | ||
| Adolescent male Long-Evans rats | Prepulse inhibition Paired-associates learning | Impaired sensorimotor gating (4 months after Δ9-THC exposure) Delayed acquisition of learning criteria No differences in task performance | - | [160] | |
| Chronic, (0.3 mg/kg PND 35–37; 1 mg/kg PND 38–41; 3 mg/kg PND 42–45; i.p.) | Adolescent male Sprague-Dawley rats | Prepulse inhibition Attentional set-shifting test Novel object recognition (spatial version) Morris water maze | =% of prepulse inhibition No significant differences ↓ Discrimination index ↓ Spatial memory | ↑ CB1r protein expression in the PFC ↓ BDNF protein expression in the PFC and HIPP ↑ TrkB protein expression in the HIPP Impaired dopaminergic activity in the HIPP, PFC, dorsal striatum and NAcc ↓ SOX2+ and DCX+ cells in the HIPP (impaired hippocampal neurogenesis) | [164] |
| Chronic Adolescence (2.5 mg/kg PND 30–32; 5 mg/kg PND 33–36; 10 mg/kg PND 37–41; i.p.) Late adolescence (2.5 mg/kg PND 45–47; 5 mg/kg PND 48–51; 10 mg/kg PND 52–56; i.p.) | Adolescent male Sprague-Dawley rats | Novel object recognition (spatial version) Novel object recognition (classical version) Water T-maze | ↑ Discrimination index in late adolescent-treated rats ↓ Discrimination index in adolescent-treated rats ↑ Number of trials to reach learning criteria | Impairment of vSub-NAc LTP amplitude | [165] |
| Δ9-THC pure (2.5 mg/kg PND 35–37; 5 mg/kg PND 38–41; 10 mg/kg PND 42–45; i.p.) Δ9-THC-rich/CBD-poor (THC 2.5 mg/kg + CBD 0.83 mg/kg PND 35–37; THC 5 mg/kg + CBD 1.66 mg/kg PND 38–41; THC 10 mg/kg + CBD 3.32 mg/kg PND 42–45; i.p.) CBD-rich/THC-poor (THC 0.15 mg/kg + CBD 5 mg/kg PND 35–45, i.p.). | Adolescent female Sprague-Dawley rats | Novel object recognition | ↓ Object recognition memory (discrimination index) | ↓ CB1r protein expression (Δ9-THC pure) in the PFC ↓ GAD67 (Δ9-THC pure) and ↑ GAD67 (Δ9-THC-rich/CBD-poor, CBD-rich/THC-poor) protein expression in the PFC ↑ CD11b (Δ9-THC pure, Δ9-THC-rich/CBD-poor) in the PFC | [161] |
| Adolescent chronic treatment (2.5 mg/kg, PND 35–37; 5 mg/kg, PND 38–41; 10 mg/kg, PND 42–45; i.p.) Adult chronic treatment (2.5 mg/kg, PND 75–77; 5 mg/kg, PND 78–81; 10 mg/kg, PND 82–85; i.p.) | Adolescent and adult female Sprague-Dawley rats | Novel object recognition | ↓ Recognition memory (discrimination index) | Impairment of histone modifications (mainly H3K9me3) and expression of plasticity genes (more widespread and intense after adolescence treatment) in the PFC | [162] |
| Chronic (2.5 mg/kg from pnd 28 to 34; 5 mg/kg from pnd 35 to 40; 10 mg/kg from pnd 41 to 45; i.p.) | Adolescent male and female Wistar rats | Novel object recognition Prepulse inhibition | ↓ Recognition memory (discrimination index) in THC-treated female rats No significant effects | ↓ Leptin plasma levels in THC-treated female rats ↓ Arc in the PFC of THC-treated male and female rats ↓ Prepro-orexin in the Hyp of THC-treated male rats | [166] |
| Chronic smoke exposure (5 cigarettes/day from PND 29 to 49, 5.6% of Δ9-THC, inh.) Chronic (2.5 mg/kg, PND 35–37; 5 mg/kg, PND 38–41; 10 mg/kg, PND 42–45; i.p.) | Adolescent male and female Long-Evans rats | Novel object recognition | No significant differences in the discrimination index | - | [163] |
3.3. Psychostimulant Use Disorders
3.3.1. Clinical Studies
Cocaine Use Disorders
3.3.2. Preclinical Studies
| COCAINE | |||||
|---|---|---|---|---|---|
| Drug and Pattern of Administration | Strain and Stage of Administration | Test | Behavioral Alterations | Biological Alterations | References |
| Cocaine self-administration 1 h/day (short access; ShA) 6 h/day (long access; LgA) | Wistar rats Adulthood | Sustained attention test | Impaired on the sustained attention task of the LgA animals. ↓ Vigilance index | ↓ D2 gene expression in the medial and orbital prefrontal cortex and D2 protein expression in the medial prefrontal cortex in LgA animals | [200] |
| Cocaine self-administration 14 days | Male Long-Evans rats Adulthood | Reversal learning | Alterations in reversal learning even one month after withdrawal | - | [201] |
| Cocaine self-administration | Glu-CB1r vs. GABA-CB1r C mice | Associative process | T-Maze Learning and Reversal | CB1r expression in cortical glutamatergic neurons controlled the associative learning processes | [202] |
| Cocaine self-administration | Long-Evans rats | fMRI | - | ↓ Neurobiological interaction between prelimbic cortex and entopeduncular nucleus and NAcc and DMPFC | [203] |
| Acute cocaine administration in the abstinence period after cocaine self-administration | Female rhesus monkeys | fMRI | - | ↓ Global functional connectivity in the prefrontal circuitry | [204] |
| Cocaine self-administration 14 days | Long Evans rats | fMRI | - | After 1 day of abstinence high clustering coefficient in the AMY, hypothalamus, striatum, HIPP, and thalamus After 14 days of abstinence ↓ clustering coefficient | [205] |
| 30 mg/kg/day 14 days After 2 weeks during the withdrawal period | C57BL/6 | Reversal learning task Three-choice serial reaction time taskDelayed matching-to-position task | Impaired learning and working memory | - | [206] |
| 20 mg/kg/day, i.p. 12 days | C57BL/6 J | Y Maze Open field exploration Spontaneous behavior Forced swimming test | Cognitive deficits in spontaneous alternation behavior and place recognition memory | - | [207] |
| Acute and chronic 5 mg/kg, i.p 7 days | Marmoset monkeys | Spontaneous object location | Acute administration improved the marmoset’s recognition memory Detrimental effects after the repeated exposure | - | [208] |
| 5 sessions of crack inhalation once a day | Wistar rats | Eight-arm radial maze | Impaired long-term spatial working memory | ↑ AOPP and SOD activity | [209] |
| Acute i.c.v AEME | Wistar rats | Eight-arm radial maze | 32 µg and 100 µg impaired working memory | ↑ 100 μg i.c.v glutathione peroxidase | [210] |
3.4. Opioid Use Disorders
3.4.1. Clinical Studies
3.4.2. Preclinical Studies
| MORPHINE | |||||
|---|---|---|---|---|---|
| Pattern, Doses and Route of Administration | Strain and Stage of Administration | Test | Behavioral Alterations | Biological Alterations | References |
| 1, 3 and 10 mg/kg; i.p.; pre and post-training, acute | Adult male Swiss albino mice | Step-through passive avoidance test | No alteration in memory retention Impairment of memory retrieval | - | [223] |
| 1 and 2.5 mg/kg; i.p.; post-training, acute | Adult male DBA/2 mice | Impairment of memory retrieval | - | [224] | |
| 3 and 5 mg/kg; i.p.; post-training, acute | Adult male Wistar rats | Impairment of memory retrieval | - | [226] | |
| 3 and 6 mg/kg; i.p.; post-training, acute | Adult male Wistar rats | Impairment of memory retrieval | - | [227] | |
| 2.5, 5 and 7.5 mg/kg; i.p., acute | Adult male Wistar rats | Morris water maze | ↓ Spatial memory acquisition | - | [225] |
| 0.01, 0.1 and 0.2 mg/kg; i.p., acute | Adult male Rhesus monkey | Impairment of spatial working memory | [245] | ||
| 2.5, 5 and 7.5 mg/kg i.p.; 24 h/3 days; pre-training (morphine sensitization), subchronic | Adult male Wistar rats | Morris water maze | Reverses the impairment of morphine | - | [225] |
| Increasing doses (from 30 to 90 kg/kg s.c.; 12 h/3 days, subchronic | Male NMRI mice | Novel object recognition | Short-term memory alterations | ↑ Brain corticosterone concentrations in the whole brain | [252] |
| Increasing doses from 5–50 mg/kg s.c.; 12 h/6 days, subchronic | Adult male Wistar rats | A cost-benefit decision-making T-maze | Impaired effort and delay-based form of cost-benefit decision making | BDNF, p-CREB/CREB and p-GSK3β/GSK3β in the AMY | [247] |
| 10 mg/kg; s.c.;12 h/10 days, chronic | Adult male Sprague-Dawley rats | - | - | ↓ LTP in CA1 | [228,229] |
| 3 mg/kg; i.p.; 12 h/14 days | Adult male C57BL/6 and BALB/cJ mice | Novel object recognition Social recognition test | ↓ Discrimination ratio for both strains in novel object recognition ↓ Discrimination ratio of C57BL/6 in the social recognition test | - | [230] |
| 10 mg/kg; i.p.; 24 h/14–17 days | Adult male C57BL/6J mice | 5-choice serial reaction time task | ↓attentional responses ↓processing speed ↑ impulsivity | ↓ PSD95 and ↓ CREB in PFC and HIPP | [231,233] |
| Increasing doses from 2 to 30 mg/kg i.p.; 21 days | Adult male Wistar rats | Step-through passive avoidance test | Impairment of memory | ↑ PGC-1α and ↓ CART in the HIPP | [239] |
| 10 mg/kg; i.p.; 12 h/10 days | Female Wistar rats | T-maze Step-through passive avoidance test NOR | Impairment in all tests | ↓ BDNF in HIPP | [232,253] |
| 0.75 mg/rat; i.p.; 14 days | Adult male Sprague-Dawley rats | Morris water maze | Impair spatial memory | ↓ neurogenesis ↓ BDNF in HIPP | [246] |
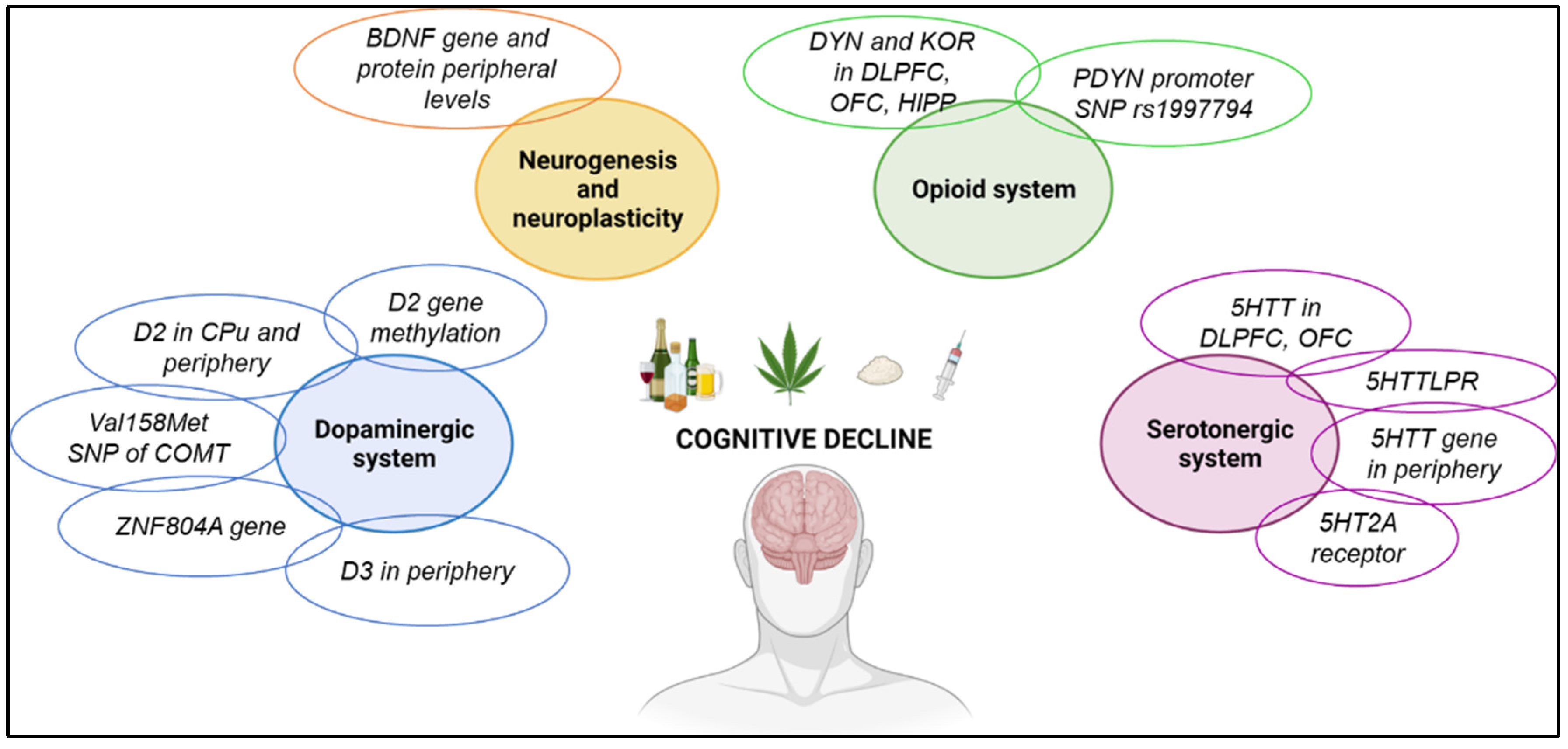
3.5. Molecular Alterations Associated with Cognitive Decline in Addicted Patients
3.5.1. Dopaminergic System
3.5.2. Brain-Derived Neurotrophic Factor
3.5.3. Opioid System
3.5.4. Serotonergic System
3.5.5. Immune System
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- APA. Diagnostic and Statistical Manual of Mental Disorders 5th edn. DSM-V; American Psychiatric Association (APA): Washington, DC, USA, 2013. [Google Scholar]
- Robinson, T.E.; Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004, 47 (Suppl. 1), 33–46. [Google Scholar] [CrossRef] [PubMed]
- Morie, K.P.; DeVito, E.E.; Potenza, M.N.; Worhunsky, P.D. Longitudinal changes in network engagement during cognitive control in cocaine use disorder. Drug. Alcohol. Depend. 2021, 229, 109151. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.D.; Pittman, B.; Petrakis, I.; Yoon, G. Donepezil and cognitive remediation therapy to augment treatment of alcohol use disorder related mild cognitive impairment (AUD-MCI): An open label pilot study with historical controls. Subst. Abus. 2021, 42, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Garavan, H. Cognitive predictors of problem drinking and AUDIT scores among college students. Drug. Alcohol. Depend. 2011, 115, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.R.; Dodd, A.B.; Wilcox, C.E.; Klimaj, S.D.; Claus, E.D.; Bryan, A.D. Effects of attentional bias modification therapy on the cue reactivity and cognitive control networks in participants with cocaine use disorders. Am. J. Drug Alcohol Abus. 2020, 46, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Seemiller, L.R.; Gould, T.J. The effects of adolescent alcohol exposure on learning and related neurobiology in humans and rodents. Neurobiol. Learn. Mem. 2020, 172, 107234. [Google Scholar] [CrossRef]
- Copersino, M.L.; Fals-Stewart, W.; Fitzmaurice, G.; Schretlen, D.J.; Sokoloff, J.; Weiss, R.D. Rapid cognitive screening of patients with substance use disorders. Exp. Clin. Psychopharmacol. 2009, 17, 337–344. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Li, Y.; Deng, Y.; Huang, Y.; Yuan, J.; Lv, R.; Wang, Y.; Zhang, G.; Guo, Z.; et al. Longitudinal changes of dopamine transporters in heroin users during abstinence. Psychopharmacology 2015, 232, 3391–3401. [Google Scholar] [CrossRef]
- Giessing, C.; Thiel, C.M.; Alexander-Bloch, A.F.; Patel, A.X.; Bullmore, E.T. Human brain functional network changes associated with enhanced and impaired attentional task performance. J. Neurosci. 2013, 33, 5903–5914. [Google Scholar] [CrossRef]
- Stavro, K.; Pelletier, J.; Potvin, S. Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addict. Biol. 2013, 18, 203–213. [Google Scholar] [CrossRef]
- Reed, L.J.; Lasserson, D.; Marsden, P.; Stanhope, N.; Stevens, T.; Bello, F.; Kingsley, D.; Colchester, A.; Kopelman, M.D. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex 2003, 39, 1027–1045. [Google Scholar] [CrossRef] [PubMed]
- Kessels, R.P.C.; Murk, S.; Walvoort, S.J.W.; Hampstead, B.M. The effects of strategy training on spatial memory in diencephalic amnesia: A randomized controlled study. Cogn. Process. 2020, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Arts, N.J.; Walvoort, S.J.; Kessels, R.P. Korsakoff’s syndrome: A critical review. Neuropsychiatr. Dis. Treat. 2017, 13, 2875–2890. [Google Scholar] [CrossRef] [PubMed]
- Angarita, G.A.; Canavan, S.V.; Forselius, E.; Bessette, A.; Pittman, B.; Morgan, P.T. Abstinence-related changes in sleep during treatment for cocaine dependence. Drug. Alcohol. Depend. 2014, 134, 343–347. [Google Scholar] [CrossRef]
- DeVito, E.E.; Herman, A.I.; Konkus, N.S.; Zhang, H.; Sofuoglu, M. Atomoxetine in abstinent cocaine users: Cognitive, subjective and cardiovascular effects. Pharmacol. Biochem. Behav. 2017, 159, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Aharonovich, E.; Nunes, E.; Hasin, D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug. Alcohol. Depend. 2003, 71, 207–211. [Google Scholar] [CrossRef]
- Lyvers, M.; Yakimoff, M. Neuropsychological correlates of opioid dependence and withdrawal. Addict. Behav. 2003, 28, 605–611. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef]
- Spronk, D.B.; van Wel, J.H.; Ramaekers, J.G.; Verkes, R.J. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci. Biobehav. Rev. 2013, 37, 1838–1859. [Google Scholar] [CrossRef]
- van Holst, R.J.; Schilt, T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr. Drug. Abuse Rev. 2011, 4, 42–56. [Google Scholar] [CrossRef]
- Sorkhou, M.; Rabin, R.A.; Rabin, J.S.; Kloiber, S.; McIntyre, R.S.; George, T.P. Effects of 28 days of cannabis abstinence on cognition in major depressive disorder: A pilot study. Am. J. Addict. 2022, 31, 454–462. [Google Scholar] [CrossRef]
- Wallace, A.L.; Wade, N.E.; Lisdahl, K.M. Impact of 2 Weeks of Monitored Abstinence on Cognition in Adolescent and Young Adult Cannabis Users. J. Int. Neuropsychol. Soc. JINS 2020, 26, 776–784. [Google Scholar] [CrossRef]
- Kober, H.; DeVito, E.E.; DeLeone, C.M.; Carroll, K.M.; Potenza, M.N. Cannabis abstinence during treatment and one-year follow-up: Relationship to neural activity in men. Neuropsychopharmacology 2014, 39, 2288–2298. [Google Scholar] [CrossRef]
- Bosker, W.M.; Karschner, E.L.; Lee, D.; Goodwin, R.S.; Hirvonen, J.; Innis, R.B.; Theunissen, E.L.; Kuypers, K.P.; Huestis, M.A.; Ramaekers, J.G. Psychomotor function in chronic daily Cannabis smokers during sustained abstinence. PLoS ONE 2013, 8, e53127. [Google Scholar] [CrossRef] [PubMed]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Su, H.; Jiang, H.; Li, X.; Zhong, N.; Du, J.; Meng, Y.; Duan, C.; Zhang, C.; Xiao, K.; et al. Cognitive and emotional predictors of real versus sham repetitive transcranial magnetic stimulation treatment response in methamphetamine use disorder. J. Psychiatr. Res. 2020, 126, 73–80. [Google Scholar] [CrossRef]
- Bates, M.E.; Buckman, J.F.; Nguyen, T.T. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol. Rev. 2013, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Blevins, D.; Wang, X.Q.; Sharma, S.; Ait-Daoud, N. Impulsiveness as a predictor of topiramate response for cocaine use disorder. Am. J. Addict. 2019, 28, 71–76. [Google Scholar] [CrossRef]
- Bruijnen, C.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Markus, W.; VanDerNagel, J.E.L.; Kessels, R.P.C.; CAJ, D.E.J. Prevalence of cognitive impairment in patients with substance use disorder. Drug. Alcohol. Rev. 2019, 38, 435–442. [Google Scholar] [CrossRef]
- Kwako, L.E.; Momenan, R.; Litten, R.Z.; Koob, G.F.; Goldman, D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol. Psychiatry 2016, 80, 179–189. [Google Scholar] [CrossRef]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Irshaid, S.; Ludtke, T.; Schafer, I.; Hauschildt, M.; Lipp, M. Neurocognitive Functioning in Alcohol Use Disorder: Cognitive Test Results Do not Tell the Whole Story. Eur. Addict. Res. 2018, 24, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Fama, R.; Le Berre, A.P.; Hardcastle, C.; Sassoon, S.A.; Pfefferbaum, A.; Sullivan, E.V.; Zahr, N.M. Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict. Biol. 2019, 24, 290–302. [Google Scholar] [CrossRef]
- Topiwala, A.; Allan, C.L.; Valkanova, V.; Zsoldos, E.; Filippini, N.; Sexton, C.; Mahmood, A.; Fooks, P.; Singh-Manoux, A.; Mackay, C.E.; et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ 2017, 357, j2353. [Google Scholar] [CrossRef] [PubMed]
- Sion, A.; Bruna Fernandez, R.; Martinez Maldonado, A.; Dominguez Centeno, I.; Torrado-Carvajal, A.; Rubio, G.; Pereda, E.; Jurado-Barba, R. Resting-state connectivity and network parameter analysis in alcohol-dependent males. A simultaneous EEG-MEG study. J. Neurosci. Res. 2020, 98, 1857–1876. [Google Scholar] [CrossRef]
- Moggi, F.; Ossola, N.; Graser, Y.; Soravia, L.M. Trail Making Test: Normative Data for Patients with Severe Alcohol Use Disorder. Subst. Use Misuse 2020, 55, 1790–1799. [Google Scholar] [CrossRef]
- Molina, P.E.; Nelson, S. Binge Drinking’s Effects on the Body. Alcohol. Res. 2018, 39, 99–109. [Google Scholar]
- Crowe, S.F.; Cammisuli, D.M.; Stranks, E.K. Widespread Cognitive Deficits in Alcoholism Persistent Following Prolonged Abstinence: An Updated Meta-analysis of Studies That Used Standardised Neuropsychological Assessment Tools. Arch. Clin. Neuropsychol. 2019, 35, 31–45. [Google Scholar] [CrossRef]
- Pocas, I.M.; Grilo, A.; Lino, P.; Cabrita, A.; Carvalho, A.; Ruivo, C.; Rocha, R.; Cairrao, S. Visual function and psychological variables in alcohol dependency syndrome. Strabismus 2021, 29, 130–137. [Google Scholar] [CrossRef]
- Le Berre, A.-P.; Fama, R.; Sullivan, E.V. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol. Clin. Exp. Res. 2017, 41, 1432–1443. [Google Scholar] [CrossRef]
- Fama, R.; Le Berre, A.P.; Sassoon, S.A.; Zahr, N.M.; Pohl, K.M.; Pfefferbaum, A.; Sullivan, E.V. Relations between cognitive and motor deficits and regional brain volumes in individuals with alcoholism. Brain Struct. Funct. 2019, 224, 2087–2101. [Google Scholar] [CrossRef]
- Khemiri, L.; Brynte, C.; Stunkel, A.; Klingberg, T.; Jayaram-Lindstrom, N. Working Memory Training in Alcohol Use Disorder: A Randomized Controlled Trial. Alcohol. Clin. Exp. Res. 2019, 43, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Bertoux, M.; Turner, J.J.D.; Moss, A.; Locker, K.; Riggs, K. Aspects of alcohol use disorder affecting social cognition as assessed using the Mini Social and Emotional Assessment (mini-SEA). Drug. Alcohol. Depend. 2018, 187, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Meredith, L.R.; Lim, A.C.; Ray, L.A. Neurocognitive performance in alcohol use disorder using the NIH toolbox: Role of severity and sex differences. Drug. Alcohol. Depend. 2020, 216, 108269. [Google Scholar] [CrossRef]
- Kaur, P.; Sidana, A.; Malhotra, N.; Gupta, A. Effects of abstinence of alcohol on neurocognitive functioning in patients with alcohol dependence syndrome. Asian J. Psychiatr. 2020, 50, 101997. [Google Scholar] [CrossRef]
- Cox, T. The effects of caffeine, alcohol, and previous exposure to the test situation on spontaneous alternation. Psychopharmacologia 1970, 17, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Givens, B.; McMahon, K. Effects of ethanol on nonspatial working memory and attention in rats. Behav. Neurosci. 1997, 111, 275–282. [Google Scholar] [CrossRef]
- Devenport, L.D.; Cater, N. Ethanol blockade of context change effects. Behav. Neural Biol. 1986, 45, 135–142. [Google Scholar] [CrossRef]
- Devenport, L.D.; Merriman, V.J. Ethanol and behavioral variability in the radial-arm maze. Psychopharmacology 1983, 79, 21–24. [Google Scholar] [CrossRef]
- Vilpoux, C.; Fouquet, G.; Deschamps, C.; Lefebvre, E.; Gosset, P.; Antol, J.; Zabijak, L.; Marcq, I.; Naassila, M.; Pierrefiche, O. Astrogliosis and compensatory neurogenesis after the first ethanol binge drinking-like exposure in the adolescent rat. Alcohol. Clin. Exp. Res. 2022, 46, 207–220. [Google Scholar] [CrossRef]
- Alvandi, M.S.; Bourmpoula, M.; Homberg, J.R.; Fathollahi, Y. Association of contextual cues with morphine reward increases neural and synaptic plasticity in the ventral hippocampus of rats. Addict. Biol. 2017, 22, 1883–1894. [Google Scholar] [CrossRef]
- Hefner, K.; Holmes, A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology 2007, 191, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Alijanpour, S.; Rezayof, A.; Sepehri, H.; Delphi, L. Alterations in the hippocampal phosphorylated CREB expression in drug state-dependent learning. Behav. Brain Res. 2015, 292, 109–115. [Google Scholar] [CrossRef]
- Broadwater, M.; Spear, L.P. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav. Brain Res. 2013, 256, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ryabinin, A.E.; Miller, M.N.; Durrant, S. Effects of acute alcohol administration on object recognition learning in C57BL/6J mice. Pharmacol. Biochem. Behav. 2002, 71, 307–312. [Google Scholar] [CrossRef]
- Garcia-Moreno, L.M.; Cimadevilla, J.M. Acute and chronic ethanol intake: Effects on spatial and non-spatial memory in rats. Alcohol 2012, 46, 757–762. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 1189–1201, quiz 1285. [Google Scholar] [CrossRef]
- Zeiss, C.J. Comparative Milestones in Rodent and Human Postnatal Central Nervous System Development. Toxicol. Pathol. 2021, 49, 1368–1373. [Google Scholar] [CrossRef]
- Fernandez, G.M.; Lew, B.J.; Vedder, L.C.; Savage, L.M. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience 2017, 348, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Hosova, D.; Towner, T.; Werner, D.F.; Spear, L.P. Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behav. Brain Res. 2020, 378, 112292. [Google Scholar] [CrossRef]
- Kim, A.; Zamora-Martinez, E.R.; Edwards, S.; Mandyam, C.D. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct. Funct. 2015, 220, 1705–1720. [Google Scholar] [CrossRef]
- Kroener, S.; Mulholland, P.J.; New, N.N.; Gass, J.T.; Becker, H.C.; Chandler, L.J. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS ONE 2012, 7, e37541. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.T.; Glen, W.B., Jr.; McGonigal, J.T.; Trantham-Davidson, H.; Lopez, M.F.; Randall, P.K.; Yaxley, R.; Floresco, S.B.; Chandler, L.J. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 2014, 39, 2570–2583. [Google Scholar] [CrossRef] [PubMed]
- Trantham-Davidson, H.; Burnett, E.J.; Gass, J.T.; Lopez, M.F.; Mulholland, P.J.; Centanni, S.W.; Floresco, S.B.; Chandler, L.J. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J. Neurosci. 2014, 34, 3706–3718. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, Z.; Xie, X.; Cheng, Y.; Zhuang, X.; Childs, M.J.; Gangal, H.; Wang, X.; Smith, L.N.; Smith, R.J.; et al. Chronic alcohol drinking persistently suppresses thalamostriatal excitation of cholinergic neurons to impair cognitive flexibility. J. Clin. Investig. 2022, 132, e154969. [Google Scholar] [CrossRef] [PubMed]
- Sircar, R.; Sircar, D. Repeated ethanol treatment in adolescent rats alters cortical NMDA receptor. Alcohol 2006, 39, 51–58. [Google Scholar] [CrossRef]
- Pascual, M.; Blanco, A.M.; Cauli, O.; Minarro, J.; Guerri, C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007, 25, 541–550. [Google Scholar] [CrossRef]
- Golub, H.M.; Zhou, Q.G.; Zucker, H.; McMullen, M.R.; Kokiko-Cochran, O.N.; Ro, E.J.; Nagy, L.E.; Suh, H. Chronic Alcohol Exposure is Associated with Decreased Neurogenesis, Aberrant Integration of Newborn Neurons, and Cognitive Dysfunction in Female Mice. Alcohol. Clin. Exp. Res. 2015, 39, 1967–1977. [Google Scholar] [CrossRef]
- Meunier, J.; Demeilliers, B.; Celerier, A.; Maurice, T. Compensatory effect by sigma1 (sigma1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav. Brain Res. 2006, 166, 166–176. [Google Scholar] [CrossRef]
- Risher, M.L.; Fleming, R.L.; Risher, W.C.; Miller, K.M.; Klein, R.C.; Wills, T.; Acheson, S.K.; Moore, S.D.; Wilson, W.A.; Eroglu, C.; et al. Adolescent intermittent alcohol exposure: Persistence of structural and functional hippocampal abnormalities into adulthood. Alcohol. Clin. Exp. Res. 2015, 39, 989–997. [Google Scholar] [CrossRef]
- Li, Q.; Fleming, R.L.; Acheson, S.K.; Madison, R.D.; Moore, S.D.; Risher, M.L.; Wilson, W.A.; Swartzwelder, H.S. Long-term modulation of A-type K(+) conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcohol. Clin. Exp. Res. 2013, 37, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Nixon, K.; Shetty, A.K.; Crews, F.T. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur. J. Neurosci. 2005, 21, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Stragier, E.; Martin, V.; Davenas, E.; Poilbout, C.; Mongeau, R.; Corradetti, R.; Lanfumey, L. Brain plasticity and cognitive functions after ethanol consumption in C57BL/6J mice. Transl. Psychiatry 2015, 5, e696. [Google Scholar] [CrossRef] [PubMed]
- Vaghef, L.; Farajdokht, F.; Erfani, M.; Majdi, A.; Sadigh-Eteghad, S.; Karimi, P.; Sandoghchian Shotorbani, S.; Seyedi Vafaee, M.; Mahmoudi, J. Cerebrolysin attenuates ethanol-induced spatial memory impairments through inhibition of hippocampal oxidative stress and apoptotic cell death in rats. Alcohol 2019, 79, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Staples, M.C.; Kim, A.; Mandyam, C.D. Dendritic remodeling of hippocampal neurons is associated with altered NMDA receptor expression in alcohol dependent rats. Mol. Cell. Neurosci. 2015, 65, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Lopez-Hidalgo, R.; Montagud-Romero, S.; Urena-Peralta, J.R.; Rodriguez-Arias, M.; Guerri, C. Role of mTOR-regulated autophagy in spine pruning defects and memory impairments induced by binge-like ethanol treatment in adolescent mice. Brain Pathol. 2021, 31, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Yaxley, R.; Paniagua, B.; Crews, F.T. Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addict. Biol. 2016, 21, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Reitz, N.L.; Nunes, P.T.; Savage, L.M. Adolescent Binge-Type Ethanol Exposure in Rats Mirrors Age-Related Cognitive Decline by Suppressing Cholinergic Tone and Hippocampal Neurogenesis. Front. Behav. Neurosci. 2021, 15, 772857. [Google Scholar] [CrossRef]
- Buck, S.A.; Torregrossa, M.M.; Logan, R.W.; Freyberg, Z. Roles of dopamine and glutamate co-release in the nucleus accumbens in mediating the actions of drugs of abuse. FEBS J. 2021, 288, 1462–1474. [Google Scholar] [CrossRef]
- Galaj, E.; Kipp, B.T.; Floresco, S.B.; Savage, L.M. Persistent Alterations of Accumbal Cholinergic Interneurons and Cognitive Dysfunction after Adolescent Intermittent Ethanol Exposure. Neuroscience 2019, 404, 153–164. [Google Scholar] [CrossRef]
- Cannizzaro, C.; Talani, G.; Brancato, A.; Mulas, G.; Spiga, S.; De Luca, M.A.; Sanna, A.; Marino, R.A.M.; Biggio, G.; Sanna, E.; et al. Dopamine Restores Limbic Memory Loss, Dendritic Spine Structure, and NMDAR-Dependent LTD in the Nucleus Accumbens of Alcohol-Withdrawn Rats. J. Neurosci. 2019, 39, 929–943. [Google Scholar] [CrossRef]
- Swartzwelder, H.S.; Hogan, A.; Risher, M.L.; Swartzwelder, R.A.; Wilson, W.A.; Acheson, S.K. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol 2014, 48, 353–360. [Google Scholar] [CrossRef]
- Chefer, V.; Meis, J.; Wang, G.; Kuzmin, A.; Bakalkin, G.; Shippenberg, T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict. Biol. 2011, 16, 229–237. [Google Scholar] [CrossRef]
- Irimia, C.; Wiskerke, J.; Natividad, L.A.; Polis, I.Y.; de Vries, T.J.; Pattij, T.; Parsons, L.H. Increased impulsivity in rats as a result of repeated cycles of alcohol intoxication and abstinence. Addict. Biol. 2015, 20, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Mitrirattanakul, S.; Lopez-Valdes, H.E.; Liang, J.; Matsuka, Y.; Mackie, K.; Faull, K.F.; Spigelman, I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol. Clin. Exp. Res. 2007, 31, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Balino, P.; Alfonso-Loeches, S.; Aragon, C.M.; Guerri, C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav. Immun. 2011, 25 (Suppl. 1), S80–S91. [Google Scholar] [CrossRef] [PubMed]
- Aharonovich, E.; Campbell, A.N.C.; Shulman, M.; Hu, M.-C.; Kyle, T.; Winhusen, T.; Nunes, E.V. Neurocognitive Profiling of Adult Treatment Seekers Enrolled in a Clinical Trial of a Web-delivered Intervention for Substance Use Disorders. J. Addict. Med. 2018, 12, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Blithikioti, C.; Miquel, L.; Batalla, A.; Rubio, B.; Maffei, G.; Herreros, I.; Gual, A.; Verschure, P.; Balcells-Oliveró, M. Cerebellar alterations in cannabis users: A systematic review. Addict. Biol. 2019, 24, 1121–1137. [Google Scholar] [CrossRef]
- Burggren, A.C.; Shirazi, A.; Ginder, N.; London, E.D. Cannabis effects on brain structure, function, and cognition: Considerations for medical uses of cannabis and its derivatives. Am. J. Drug Alcohol Abus. 2019, 45, 563–579. [Google Scholar] [CrossRef]
- Debenham, J.; Birrell, L.; Champion, K.; Lees, B.; Yücel, M.; Newton, N. Neuropsychological and neurophysiological predictors and consequences of cannabis and illicit substance use during neurodevelopment: A systematic review of longitudinal studies. Lancet Child Adolesc. Health 2021, 5, 589–604. [Google Scholar] [CrossRef]
- Figueiredo, P.R.; Tolomeo, S.; Steele, J.D.; Baldacchino, A. Neurocognitive consequences of chronic cannabis use: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 108, 358–369. [Google Scholar] [CrossRef]
- Kroon, E.; Kuhns, L.; Hoch, E.; Cousijn, J. Heavy cannabis use, dependence and the brain: A clinical perspective. Addiction 2020, 115, 559–572. [Google Scholar] [CrossRef]
- Polles, A.G.; Williams, M.K.; Phalin, B.R.; Teitelbaum, S.; Merlo, L.J. Neuropsychological impairment associated with substance use by physicians. J. Neurol. Sci. 2020, 411, 116714. [Google Scholar] [CrossRef]
- Stringfield, S.J.; Torregrossa, M.M. Disentangling the lasting effects of adolescent cannabinoid exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110067. [Google Scholar] [CrossRef] [PubMed]
- Cengel, H.Y.; Bozkurt, M.; Evren, C.; Umut, G.; Keskinkilic, C.; Agachanli, R. Evaluation of cognitive functions in individuals with synthetic cannabinoid use disorder and comparison to individuals with cannabis use disorder. Psychiatry Res. 2018, 262, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jacobus, J.; Squeglia, L.M.; Infante, M.A.; Castro, N.; Brumback, T.; Meruelo, A.D.; Tapert, S.F. Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology 2015, 29, 829–843. [Google Scholar] [CrossRef]
- Aharonovich, E.; Hasin, D.S.; Nunes, E.V.; Stohl, M.; Cannizzaro, D.; Sarvet, A.; Bolla, K.; Carroll, K.M.; Genece, K.G. Modified cognitive behavioral therapy (M-CBT) for cocaine dependence: Development of treatment for cognitively impaired users and results from a Stage 1 trial. Psychol. Addict. Behav. 2018, 32, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.E.; Wallace, A.L.; Swartz, A.M.; Lisdahl, K.M. Aerobic Fitness Level Moderates the Association Between Cannabis Use and Executive Functioning and Psychomotor Speed Following Abstinence in Adolescents and Young Adults. J. Int. Neuropsychol. Soc. 2019, 25, 134–145. [Google Scholar] [CrossRef]
- Schnakenberg Martin, A.M.; D’Souza, D.C.; Newman, S.D.; Hetrick, W.P.; O’Donnell, B.F. Differential Cognitive Performance in Females and Males with Regular Cannabis Use. J. Int. Neuropsychol. Soc. JINS 2021, 27, 570–580. [Google Scholar] [CrossRef]
- King, G.R.; Ernst, T.; Deng, W.; Stenger, A.; Gonzales, R.M.; Nakama, H.; Chang, L. Altered brain activation during visuomotor integration in chronic active cannabis users: Relationship to cortisol levels. J. Neurosci. 2011, 31, 17923–17931. [Google Scholar] [CrossRef]
- Wadsworth, E.J.; Moss, S.C.; Simpson, S.A.; Smith, A.P. Cannabis use, cognitive performance and mood in a sample of workers. J. Psychopharmacol. 2006, 20, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Nader, D.A.; Sanchez, Z.M. Effects of regular cannabis use on neurocognition, brain structure, and function: A systematic review of findings in adults. Am. J. Drug Alcohol Abus. 2018, 44, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.P.; Collins, P.F.; Schultz, A.; Urošević, S.; Schmaling, B.; Luciana, M. Longitudinal changes in cognition in young adult cannabis users. J. Clin. Exp. Neuropsychol. 2018, 40, 529–543. [Google Scholar] [CrossRef]
- Petker, T.; Owens, M.M.; Amlung, M.T.; Oshri, A.; Sweet, L.H.; MacKillop, J. Cannabis involvement and neuropsychological performance: Findings from the Human Connectome Project. J. Psychiatry Neurosci. JPN 2019, 44, 414–422. [Google Scholar] [CrossRef]
- Schuster, R.M.; Hareli, M.; Moser, A.D.; Lowman, K.; Gilman, J.; Ulysse, C.; Schoenfeld, D.; Evins, A.E. Cross-domain correlates of cannabis use disorder severity among young adults. Addict. Behav. 2019, 93, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.E.; Bruno, R.; Johnston, J.; Matthews, A.; McGregor, I.; Allsop, D.J.; Lintzeris, N. Cognitive, physical, and mental health outcomes between long-term cannabis and tobacco users. Addict. Behav. 2018, 79, 178–188. [Google Scholar] [CrossRef]
- Rolland, B.; D’Hondt, F.; Montègue, S.; Brion, M.; Peyron, E.; D’Aviau de Ternay, J.; de Timary, P.; Nourredine, M.; Maurage, P. A Patient-Tailored Evidence-Based Approach for Developing Early Neuropsychological Training Programs in Addiction Settings. Neuropsychol. Rev. 2019, 29, 103–115. [Google Scholar] [CrossRef]
- Block, R.I.; O’Leary, D.S.; Hichwa, R.D.; Augustinack, J.C.; Boles Ponto, L.L.; Ghoneim, M.M.; Arndt, S.; Hurtig, R.R.; Watkins, G.L.; Hall, J.A.; et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol. Biochem. Behav. 2002, 72, 237–250. [Google Scholar] [CrossRef]
- Blest-Hopley, G.; O’Neill, A.; Wilson, R.; Giampietro, V.; Bhattacharyya, S. Disrupted parahippocampal and midbrain function underlie slower verbal learning in adolescent-onset regular cannabis use. Psychopharmacology 2021, 238, 1315–1331. [Google Scholar] [CrossRef]
- Ross, J.M.; Ellingson, J.M.; Rhee, S.H.; Hewitt, J.K.; Corley, R.P.; Lessem, J.M.; Friedman, N.P. Investigating the causal effect of cannabis use on cognitive function with a quasi-experimental co-twin design. Drug Alcohol Depend. 2020, 206, 107712. [Google Scholar] [CrossRef]
- Hirst, R.B.; Young, K.R.; Sodos, L.M.; Wickham, R.E.; Earleywine, M. Trying to remember: Effort mediates the relationship between frequency of cannabis use and memory performance. J. Clin. Exp. Neuropsychol. 2017, 39, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.; Lauzon, S.; Nietert, P.J.; McRae-Clark, A.; Sherman, B.J. Self-reported cognition and marijuana use in older adults: Results from the national epidemiologic survey on alcohol and related conditions-III. Addict. Behav. 2020, 108, 106437. [Google Scholar] [CrossRef]
- Taffe, M.A. Delta(9)Tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J. Psychopharmacol. 2012, 26, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Verrico, C.D.; Liu, S.; Bitler, E.J.; Gu, H.; Sampson, A.R.; Bradberry, C.W.; Lewis, D.A. Delay- and dose-dependent effects of Delta(9)-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology 2012, 37, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.J., Jr.; Vandewater, S.A.; Taffe, M.A. Cannabidiol attenuates deficits of visuospatial associative memory induced by Delta(9) tetrahydrocannabinol. Br. J. Pharmacol. 2013, 170, 1365–1373. [Google Scholar] [CrossRef]
- Silveira, M.M.; Adams, W.K.; Morena, M.; Hill, M.N.; Winstanley, C.A. Delta(9)-Tetrahydrocannabinol decreases willingness to exert cognitive effort in male rats. J. Psychiatry Neurosci. 2017, 42, 131–138. [Google Scholar] [CrossRef]
- Egashira, N.; Manome, N.; Mishima, K.; Iwasaki, K.; Oishi, R.; Fujiwara, M. Involvement of opioid system in cognitive deficits induced by (9)-tetrahydrocannabinol in rats. Psychopharmacology 2012, 219, 1111–1118. [Google Scholar] [CrossRef]
- Schreiber, S.; Bader, M.; Lenchinski, T.; Meningher, I.; Rubovitch, V.; Katz, Y.; Cohen, E.; Gabet, Y.; Rotenberg, M.; Wolf, E.U.; et al. Functional effects of synthetic cannabinoids versus Delta(9) -THC in mice on body temperature, nociceptive threshold, anxiety, cognition, locomotor/exploratory parameters and depression. Addict. Biol. 2019, 24, 414–425. [Google Scholar] [CrossRef]
- Myers, A.M.; Siegele, P.B.; Foss, J.D.; Tuma, R.F.; Ward, S.J. Single and combined effects of plant-derived and synthetic cannabinoids on cognition and cannabinoid-associated withdrawal signs in mice. Br. J. Pharmacol. 2019, 176, 1552–1567. [Google Scholar] [CrossRef]
- Blaes, S.L.; Orsini, C.A.; Holik, H.M.; Stubbs, T.D.; Ferguson, S.N.; Heshmati, S.C.; Bruner, M.M.; Wall, S.C.; Febo, M.; Bruijnzeel, A.W.; et al. Enhancing effects of acute exposure to cannabis smoke on working memory performance. Neurobiol. Learn. Mem. 2019, 157, 151–162. [Google Scholar] [CrossRef]
- Vinals, X.; Moreno, E.; Lanfumey, L.; Cordomi, A.; Pastor, A.; de La Torre, R.; Gasperini, P.; Navarro, G.; Howell, L.A.; Pardo, L.; et al. Cognitive Impairment Induced by Delta9-tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB1 and Serotonin 5-HT2A Receptors. PLoS Biol. 2015, 13, e1002194. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Ossato, A.; Canazza, I.; Trapella, C.; Borelli, A.C.; Beggiato, S.; Rimondo, C.; Serpelloni, G.; Ferraro, L.; Marti, M. Synthetic cannabinoid JWH-018 and its halogenated derivatives JWH-018-Cl and JWH-018-Br impair Novel Object Recognition in mice: Behavioral, electrophysiological and neurochemical evidence. Neuropharmacology 2016, 109, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Garcia, A.; Gomis-Gonzalez, M.; Salgado-Mendialdua, V.; Galera-Lopez, L.; Puighermanal, E.; Martin-Garcia, E.; Maldonado, R.; Ozaita, A. Hippocampal Protein Kinase C Signaling Mediates the Short-Term Memory Impairment Induced by Delta9-Tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Aso, E.; Fernandez-Duenas, V.; Lopez-Cano, M.; Taura, J.; Watanabe, M.; Ferrer, I.; Lujan, R.; Ciruela, F. Adenosine A2A-Cannabinoid CB1 Receptor Heteromers in the Hippocampus: Cannabidiol Blunts Delta(9)-Tetrahydrocannabinol-Induced Cognitive Impairment. Mol. Neurobiol. 2019, 56, 5382–5391. [Google Scholar] [CrossRef]
- Rubino, T.; Parolaro, D. Cannabis abuse in adolescence and the risk of psychosis: A brief review of the preclinical evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 52, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Leishman, E.; Murphy, M.; Mackie, K.; Bradshaw, H.B. Delta(9)-Tetrahydrocannabinol changes the brain lipidome and transcriptome differentially in the adolescent and the adult. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1863, 479–492. [Google Scholar] [CrossRef]
- Salgado-Mendialdua, V.; Aguirre-Plans, J.; Guney, E.; Reig-Viader, R.; Maldonado, R.; Bayes, A.; Oliva, B.; Ozaita, A. Delta9-tetrahydrocannabinol modulates the proteasome system in the brain. Biochem. Pharmacol. 2018, 157, 159–168. [Google Scholar] [CrossRef]
- Szkudlarek, H.J.; Desai, S.J.; Renard, J.; Pereira, B.; Norris, C.; Jobson, C.E.L.; Rajakumar, N.; Allman, B.L.; Laviolette, S.R. Delta-9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology 2019, 44, 817–825. [Google Scholar] [CrossRef]
- Hudson, R.; Renard, J.; Norris, C.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol Counteracts the Psychotropic Side-Effects of Delta-9-Tetrahydrocannabinol in the Ventral Hippocampus through Bidirectional Control of ERK1-2 Phosphorylation. J. Neurosci. 2019, 39, 8762–8777. [Google Scholar] [CrossRef]
- Hudson, R.; Norris, C.; Szkudlarek, H.J.; Khan, D.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Anxiety and cognitive-related effects of Delta (9)-tetrahydrocannabinol (THC) are differentially mediated through distinct GSK-3 vs. Akt-mTOR pathways in the nucleus accumbens of male rats. Psychopharmacology 2022, 239, 509–524. [Google Scholar] [CrossRef]
- Paylor, R.; Crawley, J.N. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 1997, 132, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Perez-Ortiz, J.M.; Manzanares, J. Cannabinoid CB(2) receptor-mediated regulation of impulsive-like behaviour in DBA/2 mice. Br. J. Pharmacol. 2012, 165, 260–273. [Google Scholar] [CrossRef]
- John, W.S.; Martin, T.J.; Solingapuram Sai, K.K.; Nader, S.H.; Gage, H.D.; Mintz, A.; Nader, M.A. Chronic Delta(9)-THC in Rhesus Monkeys: Effects on Cognitive Performance and Dopamine D2/D3 Receptor Availability. J. Pharmacol. Exp. Ther. 2018, 364, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Stokes, P.R.; Egerton, A.; Watson, B.; Reid, A.; Lappin, J.; Howes, O.D.; Nutt, D.J.; Lingford-Hughes, A.R. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J. Psychopharmacol. 2012, 26, 144–149. [Google Scholar] [CrossRef]
- Urban, N.B.; Slifstein, M.; Thompson, J.L.; Xu, X.; Girgis, R.R.; Raheja, S.; Haney, M.; Abi-Dargham, A. Dopamine release in chronic cannabis users: A [11c]raclopride positron emission tomography study. Biol. Psychiatry 2012, 71, 677–683. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Alexoff, D.; Logan, J.; Jayne, M.; Wong, C.; Tomasi, D. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc. Natl. Acad. Sci. USA 2014, 111, E3149–E3156. [Google Scholar] [CrossRef] [PubMed]
- Verrico, C.D.; Gu, H.; Peterson, M.L.; Sampson, A.R.; Lewis, D.A. Repeated Delta9-tetrahydrocannabinol exposure in adolescent monkeys: Persistent effects selective for spatial working memory. Am. J. Psychiatry 2014, 171, 416–425. [Google Scholar] [CrossRef]
- Verrico, C.D.; Mathai, D.S.; Gu, H.; Sampson, A.R.; Lewis, D.A. Recovery from impaired working memory performance during chronic Delta-9-tetrahydrocannabinol administration to adolescent rhesus monkeys. J. Psychopharmacol. 2020, 34, 211–220. [Google Scholar] [CrossRef]
- Withey, S.L.; Kangas, B.D.; Charles, S.; Gumbert, A.B.; Eisold, J.E.; George, S.R.; Bergman, J.; Madras, B.K. Effects of daily Delta(9)-Tetrahydrocannabinol (THC) alone or combined with cannabidiol (CBD) on cognition-based behavior and activity in adolescent nonhuman primates. Drug. Alcohol. Depend. 2021, 221, 108629. [Google Scholar] [CrossRef]
- Tournier, B.B.; Ginovart, N. Repeated but not acute treatment with (9)-tetrahydrocannabinol disrupts prepulse inhibition of the acoustic startle: Reversal by the dopamine D(2)/(3) receptor antagonist haloperidol. Eur. Neuropsychopharmacol. 2014, 24, 1415–1423. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Fan, N.; Teng, Z.Q.; Wu, Y.; Yang, H.; Tang, Y.P.; Sun, H.; Song, Y.; Chen, C. Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 2013, 155, 1154–1165. [Google Scholar] [CrossRef]
- Hwang, E.K.; Lupica, C.R. Altered Corticolimbic Control of the Nucleus Accumbens by Long-term Delta(9)-Tetrahydrocannabinol Exposure. Biol. Psychiatry 2020, 87, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lecue, I.; Mollinedo-Gajate, I.; Meana, J.J.; Callado, L.F.; Diez-Alarcia, R.; Uriguen, L. Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology 2018, 43, 2028–2035. [Google Scholar] [CrossRef]
- Berthoux, C.; Hamieh, A.M.; Rogliardo, A.; Doucet, E.L.; Coudert, C.; Ango, F.; Grychowska, K.; Chaumont-Dubel, S.; Zajdel, P.; Maldonado, R.; et al. Early 5-HT6 receptor blockade prevents symptom onset in a model of adolescent cannabis abuse. EMBO Mol. Med. 2020, 12, e10605. [Google Scholar] [CrossRef]
- Le, A.A.; Quintanilla, J.; Amani, M.; Piomelli, D.; Lynch, G.; Gall, C.M. Persistent sexually dimorphic effects of adolescent THC exposure on hippocampal synaptic plasticity and episodic memory in rodents. Neurobiol. Dis. 2022, 162, 105565. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.L.; Chadwick, B.; Dickstein, D.L.; Purushothaman, I.; Egervari, G.; Rahman, T.; Tessereau, C.; Hof, P.R.; Roussos, P.; Shen, L.; et al. Adolescent exposure to Delta(9)-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol. Psychiatry 2019, 24, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Stringfield, S.J.; Torregrossa, M.M. Intravenous self-administration of delta-9-THC in adolescent rats produces long-lasting alterations in behavior and receptor protein expression. Psychopharmacology 2021, 238, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Chahkandi, M.; Sepehri, G.; Komeili, G.; Hadad, M.K.; Haghparast, E.; Chahkandi, M. The different role of G-protein-coupled receptor 30 (GPR30) in the interaction effects of marijuana and estradiol on spatial learning and memory at different ages. Brain Res. Bull. 2022, 178, 155–163. [Google Scholar] [CrossRef]
- Tantra, M.; Krocher, T.; Papiol, S.; Winkler, D.; Rockle, I.; Jatho, J.; Burkhardt, H.; Ronnenberg, A.; Gerardy-Schahn, R.; Ehrenreich, H.; et al. St8sia2 deficiency plus juvenile cannabis exposure in mice synergistically affect higher cognition in adulthood. Behav. Brain Res. 2014, 275, 166–175. [Google Scholar] [CrossRef]
- Segal-Gavish, H.; Gazit, N.; Barhum, Y.; Ben-Zur, T.; Taler, M.; Hornfeld, S.H.; Gil-Ad, I.; Weizman, A.; Slutsky, I.; Niwa, M.; et al. BDNF overexpression prevents cognitive deficit elicited by adolescent cannabis exposure and host susceptibility interaction. Hum. Mol. Genet. 2017, 26, 2462–2471. [Google Scholar] [CrossRef]
- Jouroukhin, Y.; Zhu, X.; Shevelkin, A.V.; Hasegawa, Y.; Abazyan, B.; Saito, A.; Pevsner, J.; Kamiya, A.; Pletnikov, M.V. Adolescent Delta(9)-Tetrahydrocannabinol Exposure and Astrocyte-Specific Genetic Vulnerability Converge on Nuclear Factor-kappaB-Cyclooxygenase-2 Signaling to Impair Memory in Adulthood. Biol. Psychiatry 2019, 85, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.M.; Orsini, C.A.; Blaes, S.L.; Bizon, J.L.; Febo, M.; Bruijnzeel, A.W.; Setlow, B. Effects of repeated adolescent exposure to cannabis smoke on cognitive outcomes in adulthood. J. Psychopharmacol. 2021, 35, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Hamidullah, S.; Lutelmowski, C.D.; Creighton, S.D.; Luciani, K.R.; Frie, J.A.; Winters, B.D.; Khokhar, J.Y. Effects of vapourized THC and voluntary alcohol drinking during adolescence on cognition, reward, and anxiety-like behaviours in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110141. [Google Scholar] [CrossRef] [PubMed]
- Smiley, C.E.; Saleh, H.K.; Nimchuk, K.E.; Garcia-Keller, C.; Gass, J.T. Adolescent exposure to delta-9-tetrahydrocannabinol and ethanol heightens sensitivity to fear stimuli. Behav. Brain Res. 2021, 415, 113517. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Gabaglio, M.; Prini, P.; Rubino, T.; Parolaro, D. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. Eur. Neuropsychopharmacol. 2015, 25, 2404–2415. [Google Scholar] [CrossRef]
- Cuccurazzu, B.; Zamberletti, E.; Nazzaro, C.; Prini, P.; Trusel, M.; Grilli, M.; Parolaro, D.; Tonini, R.; Rubino, T. Adult Cellular Neuroadaptations Induced by Adolescent THC Exposure in Female Rats Are Rescued by Enhancing Anandamide Signaling. Int. J. Neuropsychopharmacol. 2018, 21, 1014–1024. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Grilli, M.; Prini, P.; Catanese, A.; Pittaluga, A.; Marchi, M.; Rubino, T.; Parolaro, D. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol. Res. 2016, 111, 459–470. [Google Scholar] [CrossRef]
- De Felice, M.; Renard, J.; Hudson, R.; Szkudlarek, H.J.; Pereira, B.J.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. l-Theanine Prevents Long-Term Affective and Cognitive Side Effects of Adolescent Delta-9-Tetrahydrocannabinol Exposure and Blocks Associated Molecular and Neuronal Abnormalities in the Mesocorticolimbic Circuitry. J. Neurosci. 2021, 41, 739–750. [Google Scholar] [CrossRef]
- Abela, A.R.; Rahbarnia, A.; Wood, S.; Le, A.D.; Fletcher, P.J. Adolescent exposure to Delta9-tetrahydrocannabinol delays acquisition of paired-associates learning in adulthood. Psychopharmacology 2019, 236, 1875–1886. [Google Scholar] [CrossRef]
- Gabaglio, M.; Zamberletti, E.; Manenti, C.; Parolaro, D.; Rubino, T. Long-Term Consequences of Adolescent Exposure to THC-Rich/CBD-Poor and CBD-Rich/THC-Poor Combinations: A Comparison with Pure THC Treatment in Female Rats. Int. J. Mol. Sci. 2021, 22, 8899. [Google Scholar] [CrossRef]
- Prini, P.; Rusconi, F.; Zamberletti, E.; Gabaglio, M.; Penna, F.; Fasano, M.; Battaglioli, E.; Parolaro, D.; Rubino, T. Adolescent THC exposure in female rats leads to cognitive deficits through a mechanism involving chromatin modifications in the prefrontal cortex. J. Psychiatry Neurosci. 2018, 43, 87–101. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W.; Knight, P.; Panunzio, S.; Xue, S.; Bruner, M.M.; Wall, S.C.; Pompilus, M.; Febo, M.; Setlow, B. Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology 2019, 236, 2773–2784. [Google Scholar] [CrossRef] [PubMed]
- Poulia, N.; Delis, F.; Brakatselos, C.; Polissidis, A.; Koutmani, Y.; Kokras, N.; Dalla, C.; Politis, P.K.; Antoniou, K. Detrimental effects of adolescent escalating low-dose Delta(9) -tetrahydrocannabinol leads to a specific bio-behavioural profile in adult male rats. Br. J. Pharmacol. 2021, 178, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Sabran-Cohen, T.; Bright, U.; Mizrachi Zer-Aviv, T.; Akirav, I. Rapamycin prevents the long-term impairing effects of adolescence Delta-9-tetrahydrocannabinol on memory and plasticity in male rats. Eur. J. Neurosci. 2021, 54, 6104–6122. [Google Scholar] [CrossRef]
- Llorente-Berzal, A.; Puighermanal, E.; Burokas, A.; Ozaita, A.; Maldonado, R.; Marco, E.M.; Viveros, M.P. Sex-dependent psychoneuroendocrine effects of THC and MDMA in an animal model of adolescent drug consumption. PLoS ONE 2013, 8, e78386. [Google Scholar] [CrossRef]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Lubman, D.I.; Cheetham, A.; Yucel, M. Cannabis and adolescent brain development. Pharmacol. Ther. 2015, 148, 1–16. [Google Scholar] [CrossRef]
- Prini, P.; Zamberletti, E.; Manenti, C.; Gabaglio, M.; Parolaro, D.; Rubino, T. Neurobiological mechanisms underlying cannabis-induced memory impairment. Eur. Neuropsychopharmacol. 2020, 36, 181–190. [Google Scholar] [CrossRef]
- Augustin, S.M.; Lovinger, D.M. Synaptic changes induced by cannabinoid drugs and cannabis use disorder. Neurobiol. Dis. 2022, 167, 105670. [Google Scholar] [CrossRef]
- van ROSSUM, J.; van der SCHOOT, J.; Hurkmans, J.A. Mechanism of action of cocaine and amphetamine in the brain. Experientia 1962, 18, 229–231. [Google Scholar] [CrossRef]
- Frazer, K.M.; Richards, Q.; Keith, D.R. The long-term effects of cocaine use on cognitive functioning: A systematic critical review. Behav. Brain Res. 2018, 348, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Rasgado-Toledo, J.; Shah, A.; Ingalhalikar, M.; Garza-Villarreal, E.A. Neurite orientation dispersion and density imaging in cocaine use disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 113, 110474. [Google Scholar] [CrossRef] [PubMed]
- Parvaz, M.A.; Rabin, R.A.; Adams, F.; Goldstein, R.Z. Structural and functional brain recovery in individuals with substance use disorders during abstinence: A review of longitudinal neuroimaging studies. Drug Alcohol Depend. 2022, 232, 109319. [Google Scholar] [CrossRef]
- Costumero, V.; Rosell-Negre, P.; Bustamante, J.C.; Fuentes-Claramonte, P.; Llopis, J.J.; Ávila, C.; Barrós-Loscertales, A. Left frontoparietal network activity is modulated by drug stimuli in cocaine addiction. Brain Imaging Behav. 2018, 12, 1259–1270. [Google Scholar] [CrossRef]
- Wang, W.; Worhunsky, P.D.; Zhang, S.; Le, T.M.; Potenza, M.N.; Li, C.-S.R. Response inhibition and fronto-striatal-thalamic circuit dysfunction in cocaine addiction. Drug Alcohol Depend. 2018, 192, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.P.; Beatty, G.K.; Anderson, R.E.; Kodituwakku, P.; Phillips, J.P.; Lane, T.D.R.; Kiehl, K.A.; Calhoun, V.D. Reduced fMRI activity predicts relapse in patients recovering from stimulant dependence. Hum. Brain Mapp. 2014, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, O.M.; Goldenberg, D.; Thayer, R.; Migliorini, R.; Simmons, A.N.; Tapert, S.F. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addict. Behav. 2013, 38, 1435–1441. [Google Scholar] [CrossRef]
- Worhunsky, P.D.; Stevens, M.C.; Carroll, K.M.; Rounsaville, B.J.; Calhoun, V.D.; Pearlson, G.D.; Potenza, M.N. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol. Addict. Behav. 2013, 27, 477–488. [Google Scholar] [CrossRef]
- Mahoney, J.J. Cognitive dysfunction in individuals with cocaine use disorder: Potential moderating factors and pharmacological treatments. Exp. Clin. Psychopharmacol. 2019, 27, 203–214. [Google Scholar] [CrossRef]
- Fernández-Serrano, M.J.; Pérez-García, M.; Verdejo-García, A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci. Biobehav. Rev. 2011, 35, 377–406. [Google Scholar] [CrossRef]
- Jiménez, S.; Angeles-Valdez, D.; Villicaña, V.; Reyes-Zamorano, E.; Alcala-Lozano, R.; Gonzalez-Olvera, J.J.; Garza-Villarreal, E.A. Identifying cognitive deficits in cocaine dependence using standard tests and machine learning. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109709. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, M.T.; Rush, C.R.; Hays, L. Cocaine improves inhibitory control in a human model of response conflict. Exp. Clin. Psychopharmacol. 2005, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Garavan, H.; Kaufman, J.N.; Hester, R. Acute effects of cocaine on the neurobiology of cognitive control. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3267–3276. [Google Scholar] [CrossRef]
- Garavan, H.; Stout, J.C. Neurocognitive insights into substance abuse. Trends Cogn. Sci. 2005, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.M.; Gonçalves, P.D.; Ometto, M.; dos Santos, B.; Cavallet, M.; Chaim-Avancini, T.M.; Serpa, M.H.; Nicastri, S.; Malbergier, A.; Busatto, G.F.; et al. Distinct cognitive performance and patterns of drug use among early and late onset cocaine users. Addict. Behav. 2017, 73, 41–47. [Google Scholar] [CrossRef]
- Almeida, P.P.; de Araujo Filho, G.M.; Malta, S.M.; Laranjeira, R.R.; Marques, A.C.R.P.; Bressan, R.A.; Lacerda, A.L.T. Attention and memory deficits in crack-cocaine users persist over four weeks of abstinence. J. Subst. Abus. Treat. 2017, 81, 73–78. [Google Scholar] [CrossRef]
- Chao, T.; Haney, M.; Cooper, Z.D.; Vadhan, N.P.; Van Dam, N.T.; Van Snellenberg, J.; Bedi, G. Cognitive function in aging cocaine smokers. J. Psychopharmacol. 2019, 33, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vonmoos, M.; Hulka, L.M.; Preller, K.H.; Minder, F.; Baumgartner, M.R.; Quednow, B.B. Cognitive impairment in cocaine users is drug-induced but partially reversible: Evidence from a longitudinal study. Neuropsychopharmacology 2014, 39, 2200–2210. [Google Scholar] [CrossRef]
- Blanco-Presas, L.; Moreno-Alcázar, A.; Alonso-Lana, S.; Salvador, R.; Pomarol-Clotet, E.; McKenna, P. Cognitive impairment associated with cocaine use: The role of co-existent alcohol abuse/dependence. Drug Alcohol Depend. 2018, 189, 70–75. [Google Scholar] [CrossRef]
- Czermainski, F.R.; Willhelm, A.R.; Santos, Á.Z.; Pachado, M.P.; de Almeida, R.M.M. Assessment of inhibitory control in crack and/or cocaine users: A systematic review. Trends Psychiatry Psychother. 2017, 39, 216–225. [Google Scholar] [CrossRef]
- Fillmore, M.T.; Rush, C.R.; Hays, L. Acute effects of cocaine in two models of inhibitory control: Implications of non-linear dose effects. Addiction 2006, 101, 1323–1332. [Google Scholar] [CrossRef]
- Vergara-Moragues, E.; Verdejo-García, A.; Lozano, O.M.; Santiago-Ramajo, S.; González-Saiz, F.; Betanzos Espinosa, P.; Pérez García, M. Association between executive function and outcome measure of treatment in therapeutic community among cocaine dependent individuals. J. Subst. Abus. Treat. 2017, 78, 48–55. [Google Scholar] [CrossRef]
- Gowin, J.L.; Sloan, M.E.; Ramchandani, V.A.; Paulus, M.P.; Lane, S.D. Differences in decision-making as a function of drug of choice. Pharmacol. Biochem. Behav. 2018, 164, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Garcia, A.; Chong, T.T.J.; Stout, J.C.; Yücel, M.; London, E.D. Stages of dysfunctional decision-making in addiction. Pharmacol. Biochem. Behav. 2018, 164, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Garcia, A.; Benbrook, A.; Funderburk, F.; David, P.; Cadet, J.-L.; Bolla, K.I. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007, 90, 2–11. [Google Scholar] [CrossRef]
- Hulka, L.M.; Vonmoos, M.; Preller, K.H.; Baumgartner, M.R.; Seifritz, E.; Gamma, A.; Quednow, B.B. Changes in cocaine consumption are associated with fluctuations in self-reported impulsivity and gambling decision-making. Psychol. Med. 2015, 45, 3097–3110. [Google Scholar] [CrossRef] [PubMed]
- Ersche, K.D.; Jones, P.S.; Williams, G.B.; Robbins, T.W.; Bullmore, E.T. Cocaine dependence: A fast-track for brain ageing? Mol. Psychiatry 2013, 18, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Bachi, K.; Mani, V.; Jeyachandran, D.; Fayad, Z.A.; Goldstein, R.Z.; Alia-Klein, N. Vascular disease in cocaine addiction. Atherosclerosis 2017, 262, 154–162. [Google Scholar] [CrossRef]
- Briand, L.A.; Flagel, S.B.; Garcia-Fuster, M.J.; Watson, S.J.; Akil, H.; Sarter, M.; Robinson, T.E. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 2008, 33, 2969–2980. [Google Scholar] [CrossRef]
- Calu, D.J.; Stalnaker, T.A.; Franz, T.M.; Singh, T.; Shaham, Y.; Schoenbaum, G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn. Mem. 2007, 14, 325–328. [Google Scholar] [CrossRef]
- Martin-Garcia, E.; Bourgoin, L.; Cathala, A.; Kasanetz, F.; Mondesir, M.; Gutierrez-Rodriguez, A.; Reguero, L.; Fiancette, J.F.; Grandes, P.; Spampinato, U.; et al. Differential Control of Cocaine Self-Administration by GABAergic and Glutamatergic CB1 Cannabinoid Receptors. Neuropsychopharmacology 2016, 41, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zou, Q.; Chefer, S.; Ross, T.J.; Vaupel, D.B.; Guillem, K.; Rea, W.P.; Yang, Y.; Peoples, L.L.; Stein, E.A. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connect. 2014, 4, 499–510. [Google Scholar] [CrossRef]
- Murnane, K.S.; Gopinath, K.S.; Maltbie, E.; Daunais, J.B.; Telesford, Q.K.; Howell, L.L. Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology 2015, 232, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Orsini, C.A.; Colon-Perez, L.M.; Heshmati, S.C.; Setlow, B.; Febo, M. Functional Connectivity of Chronic Cocaine Use Reveals Progressive Neuroadaptations in Neocortical, Striatal, and Limbic Networks. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Krueger, D.D.; Howell, J.L.; Oo, H.; Olausson, P.; Taylor, J.R.; Nairn, A.C. Prior chronic cocaine exposure in mice induces persistent alterations in cognitive function. Behav. Pharmacol. 2009, 20, 695–704. [Google Scholar] [CrossRef]
- Manas-Padilla, M.C.; Avila-Gamiz, F.; Gil-Rodriguez, S.; Ladron de Guevara-Miranda, D.; Rodriguez de Fonseca, F.; Santin, L.J.; Castilla-Ortega, E. Persistent changes in exploration and hyperactivity coexist with cognitive impairment in mice withdrawn from chronic cocaine. Physiol. Behav. 2021, 240, 113542. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.L.; de Jesus, F.M.; Aquino, J.; Vannuchi, C.R.S.; Duarte, R.B.M.; Maior, R.S.; Tomaz, C.; Barros, M. Differential modulatory effects of cocaine on marmoset monkey recognition memory. Prog. Brain Res. 2017, 235, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Lipaus, I.F.S.; Gomes, E.F.; Martins, C.W.; CM, E.S.; Pires, R.G.W.; Malgarin, F.; Schuck, P.F.; Palacios, E.M.N.; de Melo Rodrigues, L.C. Impairment of spatial working memory and oxidative stress induced by repeated crack cocaine inhalation in rats. Behav. Brain Res. 2019, 359, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.F.; Lipaus, I.F.S.; Martins, C.W.; Araujo, A.M.; Mendonca, J.B.; Pelicao, F.S.; Lebarch, E.C.; de Melo Rodrigues, L.C.; Nakamura-Palacios, E.M. Anhydroecgonine Methyl Ester (AEME), a Product of Cocaine Pyrolysis, Impairs Spatial Working Memory and Induces Striatal Oxidative Stress in Rats. Neurotox. Res. 2018, 34, 834–847. [Google Scholar] [CrossRef]
- Tolomeo, S.; Steele, J.D.; Ekhtiari, H.; Baldacchino, A. Chronic heroin use disorder and the brain: Current evidence and future implications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110148. [Google Scholar] [CrossRef]
- Ma, B.; Mei, D.; Wang, F.; Liu, Y.; Zhou, W. Cognitive enhancers as a treatment for heroin relapse and addiction. Pharmacol. Res. 2019, 141, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.; Frischknecht, U.; Reinhard, I.; Bekier, N.; Demirakca, T.; Ende, G.; Vollstädt-Klein, S.; Kiefer, F.; Hermann, D. Impaired working memory performance in opioid-dependent patients is related to reduced insula gray matter volume: A voxel-based morphometric study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cook, J.; Rassbach, B.; Lemus, A.; DeArmond, S.J.; Mastrianni, J.A. A new transgenic mouse model of Gerstmann-Sträussler-Scheinker syndrome caused by the A117V mutation of PRNP. J. Neurosci. 2009, 29, 10072–10080. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver, M.M.; Sanborn, V.; Shrestha, R.; Mistler, C.B.; Sullivan, M.C.; Gunstad, J. Developing a cognitive dysfunction risk score for use with opioid-dependent persons in drug treatment. Drug Alcohol Depend. 2021, 224, 108726. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.K.; Stathopoulou, G.; Otto, M.W. Emotion regulation and motives for illicit drug use in opioid-dependent patients. Cogn. Behav. Ther. 2020, 49, 74–80. [Google Scholar] [CrossRef]
- Mahlberg, J.; Haber, P.; Morley, K.; Weidemann, G.; Hogarth, L.; Beck, K.D.; Myers, C.E.; Moustafa, A.A. Reward and punishment-based compound cue learning and generalization in opiate dependency. Exp. Brain Res. 2017, 235, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.A.; Raaen, L.; Chen, C.; Azhar, G.; Shahidinia, N.; Shen, M.; Maksabedian, E.; Shanman, R.M.; Newberry, S.; Hempel, S. Effects of medication assisted treatment (MAT) for opioid use disorder on functional outcomes: A systematic review. J. Subst. Abus. Treat. 2018, 89, 28–51. [Google Scholar] [CrossRef]
- Wollman, S.C.; Hauson, A.O.; Hall, M.G.; Connors, E.J.; Allen, K.E.; Stern, M.J.; Stephan, R.A.; Kimmel, C.L.; Sarkissians, S.; Barlet, B.D.; et al. Neuropsychological functioning in opioid use disorder: A research synthesis and meta-analysis. Am. J. Drug Alcohol Abus. 2019, 45, 11–25. [Google Scholar] [CrossRef]
- Rezapour, T.; Hatami, J.; Farhoudian, A.; Noroozi, A.; Daneshmand, R.; Sofuoglu, M.; Baldacchino, A.; Ekhtiari, H. Baseline executive functions and receiving cognitive rehabilitation can predict treatment response in people with opioid use disorder. J. Subst. Abus. Treat. 2021, 131, 108558. [Google Scholar] [CrossRef]
- Liang, C.S.; Ho, P.S.; Yen, C.H.; Chen, C.Y.; Kuo, S.C.; Huang, C.C.; Yeh, Y.W.; Ma, K.H.; Huang, S.Y. The relationship between the striatal dopamine transporter and novelty seeking and cognitive flexibility in opioid dependence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 74, 36–42. [Google Scholar] [CrossRef]
- Murray, D.E.; Durazzo, T.C.; Schmidt, T.P.; Murray, T.A.; Abé, C.; Guydish, J.; Meyerhoff, D.J. Regional cerebral blood flow in opiate dependence relates to substance use and neuropsychological performance. Addict. Biol. 2018, 23, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Datta, H.; Sharma, P.L. Effects of morphine on memory: Interactions with naloxone, propranolol and haloperidol. Pharmacology 1991, 42, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Castellano, C.; Pavone, F.; Puglisi Allegra, S. Morphine and memory in DBA/2 mice: Effects of stress and of prior experience. Behav. Brain Res. 1984, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, M.; Karimian, S.M.; Naghdi, N.; Zarrindast, M.R.; Kadivar, M. Morphine-induced impairment of spatial memory acquisition reversed by morphine sensitization in rats. Behav. Brain Res. 2010, 211, 156–163. [Google Scholar] [CrossRef]
- Sharifi, K.A.; Rezayof, A.; Alijanpour, S.; Zarrindast, M.R. GABA-cannabinoid interplays in the dorsal hippocampus and basolateral amygdala mediate morphine-induced amnesia. Brain Res. Bull. 2020, 157, 61–68. [Google Scholar] [CrossRef]
- Sharifi, K.A.; Rezayof, A.; Torkaman-Boutorabi, A.; Zarrindast, M.R. The major neurotransmitter systems in the basolateral amygdala and the ventral tegmental area mediate morphine-induced memory consolidation impairment. Neuroscience 2017, 353, 7–16. [Google Scholar] [CrossRef]
- Bao, G.; Kang, L.; Li, H.; Li, Y.; Pu, L.; Xia, P.; Ma, L.; Pei, G. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology 2007, 32, 1738–1749. [Google Scholar] [CrossRef]
- Salmanzadeh, F.; Fathollahi, Y.; Semnanian, S.; Shafizadeh, M. Dependence on morphine impairs the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Brain Res. 2003, 965, 108–113. [Google Scholar] [CrossRef]
- Gong, D.; Zhao, H.; Liang, Y.; Chao, R.; Chen, L.; Yang, S.; Yu, P. Differences in cocaine- and morphine-induced cognitive impairments and serum corticosterone between C57BL/6J and BALB/cJ mice. Pharmacol. Biochem. Behav. 2019, 182, 1–6. [Google Scholar] [CrossRef]
- Guo, H.; Xie, Q.; Cui, J.; Xu, D.; Deji, C.; Chen, Y.; Wang, Y.; Lai, J. Naloxone reversed cognitive impairments induced by repeated morphine under heavy perceptual load in the 5-choice serial reaction time task. J. Neurosci. Res. 2019, 97, 1051–1065. [Google Scholar] [CrossRef]
- Ghodrati-Jaldbakhan, S.; Ahmadalipour, A.; Rashidy-Pour, A.; Vafaei, A.A.; Miladi-Gorji, H.; Alizadeh, M. Low- and high-intensity treadmill exercise attenuates chronic morphine-induced anxiogenesis and memory impairment but not reductions in hippocampal BDNF in female rats. Brain Res. 2017, 1663, 20–28. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, J.; Lu, Y.; Zhang, Y.; Liu, J.; Deji, C.; Qiao, X.; Gao, K.; Xu, M.; Lai, J.; et al. Modafinil rescues repeated morphine-induced synaptic and behavioural impairments via activation of D1R-ERK-CREB pathway in medial prefrontal cortex. Addict. Biol. 2022, 27, e13103. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, L.A.; Moussawi, K.; Lalumiere, R.; Schwendt, M.; Klugmann, M.; Kalivas, P.W. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J. Neurosci. 2010, 30, 7984–7992. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Chandler, L.J. The thorny side of addiction: Adaptive plasticity and dendritic spines. Sci. World J. 2007, 7, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Kogan, J.H.; Frankland, P.W.; Kida, S. CREB and memory. Annu. Rev. Neurosci. 1998, 21, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, P.; Hui, T.; Zhang, J. Epigenetic upregulation of PSD-95 contributes to the rewarding behavior by morphine conditioning. Eur. J. Pharmacol. 2014, 732, 123–129. [Google Scholar] [CrossRef]
- Khavandgar, S.; Homayoun, H.; Torkaman-Boutorabi, A.; Zarrindast, M.R. The effects of adenosine receptor agonists and antagonists on morphine state-dependent memory of passive avoidance. Neurobiol. Learn. Mem. 2002, 78, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Malboosi, N.; Nasehi, M.; Hashemi, M.; Vaseghi, S.; Zarrindast, M.R. The neuroprotective effect of NeuroAid on morphine-induced amnesia with respect to the expression of TFAM, PGC-1alpha, DeltafosB and CART genes in the hippocampus of male Wistar rats. Gene 2020, 742, 144601. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef]
- Bakhtazad, A.; Vousooghi, N.; Garmabi, B.; Zarrindast, M.R. CART peptide and opioid addiction: Expression changes in male rat brain. Neuroscience 2016, 325, 63–73. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Ardjmand, A.; Rezayof, A.; Ahmadi, S. The time profile of morphine effect on different phases of inhibitory avoidance memory in rat. Arch. Iran. Med. 2013, 16, 34–37. [Google Scholar] [PubMed]
- Wang, J.H.; Rizak, J.D.; Chen, Y.M.; Li, L.; Hu, X.T.; Ma, Y.Y. Interactive effects of morphine and dopaminergic compounds on spatial working memory in rhesus monkeys. Neurosci. Bull. 2013, 29, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Famitafreshi, H.; Karimian, M.; Fatima, S. Synergistic Effects of Social Isolation and Morphine Addiction on Reduced Neurogenesis and BDNF Levels and the Resultant Deficits in Cognition and Emotional State in Male Rats. Curr. Mol. Pharmacol. 2016, 9, 337–347. [Google Scholar] [CrossRef]
- Fatahi, Z.; Zeinaddini-Meymand, A.; Karimi-Haghighi, S.; Moradi, M.; Khodagholi, F.; Haghparast, A. Naloxone-precipitated withdrawal ameliorates impairment of cost-benefit decision making in morphine-treated rats: Involvement of BDNF, p-GSK3-beta, and p-CREB in the amygdala. Neurobiol. Learn. Mem. 2020, 167, 107138. [Google Scholar] [CrossRef]
- Le Greves, P.; Huang, W.; Zhou, Q.; Thornwall, M.; Nyberg, F. Acute effects of morphine on the expression of mRNAs for NMDA receptor subunits in the rat hippocampus, hypothalamus and spinal cord. Eur. J. Pharmacol. 1998, 341, 161–164. [Google Scholar] [CrossRef]
- Johansson, T.; Elfverson, M.; Zhou, Q.; Nyberg, F. Allosteric modulation of the NMDA receptor by neurosteroids in rat brain and the impact of long term morphine administration. Biochem. Biophys. Res. Commun. 2010, 401, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sheng, W.S.; Lokensgard, J.R.; Peterson, P.K. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology 2002, 42, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Ardjmand, A.; Rezayof, A.; Zarrindast, M.R. Involvement of central amygdala NMDA receptor mechanism in morphine state-dependent memory retrieval. Neurosci. Res. 2011, 69, 25–31. [Google Scholar] [CrossRef]
- Rabbani, M.; Hajhashemi, V.; Mesripour, A. Increase in brain corticosterone concentration and recognition memory impairment following morphine withdrawal in mice. Stress 2009, 12, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Shahroodi, A.; Mohammadi, F.; Vafaei, A.A.; Miladi-Gorji, H.; Bandegi, A.R.; Rashidy-Pour, A. Impact of different intensities of forced exercise on deficits of spatial and aversive memory, anxiety-like behavior, and hippocampal BDNF during morphine abstinence period in male rats. Metab. Brain Dis. 2020, 35, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.E.; Dean, A.C.; Mandelkern, M.A.; London, E.D. Striatal Dopamine D2/D3 Receptor Availability Is Associated with Executive Function in Healthy Controls but Not Methamphetamine Users. PLoS ONE 2015, 10, e0143510. [Google Scholar] [CrossRef]
- Moeller, S.J.; Tomasi, D.; Honorio, J.; Volkow, N.D.; Goldstein, R.Z. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl. Psychiatry 2012, 2, e176. [Google Scholar] [CrossRef]
- Cherner, M.; Bousman, C.; Everall, I.; Barron, D.; Letendre, S.; Vaida, F.; Atkinson, J.H.; Heaton, R.; Grant, I. Cytochrome P450-2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: Preliminary findings. J. Int. Neuropsychol. Soc. JINS 2010, 16, 890–901. [Google Scholar] [CrossRef]
- Spronk, D.B.; Van der Schaaf, M.E.; Cools, R.; De Bruijn, E.R.; Franke, B.; van Wel, J.H.; Ramaekers, J.G.; Verkes, R.J. Acute effects of cocaine and cannabis on reversal learning as a function of COMT and DRD2 genotype. Psychopharmacology 2016, 233, 199–211. [Google Scholar] [CrossRef]
- Tunbridge, E.M.; Dunn, G.; Murray, R.M.; Evans, N.; Lister, R.; Stumpenhorst, K.; Harrison, P.J.; Morrison, P.D.; Freeman, D. Genetic moderation of the effects of cannabis: Catechol-O-methyltransferase (COMT) affects the impact of Δ9-tetrahydrocannabinol (THC) on working memory performance but not on the occurrence of psychotic experiences. J. Psychopharmacol. 2015, 29, 1146–1151. [Google Scholar] [CrossRef]
- Hagerty, S.L.; YorkWilliams, S.L.; Bidwell, L.C.; Weiland, B.J.; Sabbineni, A.; Blaine, S.K.; Bryan, A.D.; Hutchison, K.E. DRD2 methylation is associated with executive control network connectivity and severity of alcohol problems among a sample of polysubstance users. Addict. Biol. 2020, 25, e12684. [Google Scholar] [CrossRef]
- Leamy, T.E.; Connor, J.P.; Voisey, J.; Young, R.M.; Gullo, M.J. Alcohol misuse in emerging adulthood: Association of dopamine and serotonin receptor genes with impulsivity-related cognition. Addict. Behav. 2016, 63, 29–36. [Google Scholar] [CrossRef]
- Schellekens, A.F.; van Oosterwijck, A.W.; Ellenbroek, B.; de Jong, C.A.; Buitelaar, J.K.; Cools, L.; Verkes, R.J. The dopamine agonist apomorphine differentially affects cognitive performance in alcohol dependent patients and healthy controls. Eur. Neuropsychopharmacol. 2009, 19, 68–73. [Google Scholar] [CrossRef]
- Liu, F.; Xuan, A.; Chen, Y.; Zhang, J.; Xu, L.; Yan, Q.; Long, D. Combined effect of nerve growth factor and brain-derived neurotrophic factor on neuronal differentiation of neural stem cells and the potential molecular mechanisms. Mol. Med. Rep. 2014, 10, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Cuello, A.C.; Pentz, R.; Hall, H. The Brain NGF Metabolic Pathway in Health and in Alzheimer’s Pathology. Front. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Requena-Ocaña, N.; Araos, P.; Flores, M.; García-Marchena, N.; Silva-Peña, D.; Aranda, J.; Rivera, P.; Ruiz, J.J.; Serrano, A.; Pavón, F.J.; et al. Evaluation of neurotrophic factors and education level as predictors of cognitive decline in alcohol use disorder. Sci. Rep. 2021, 11, 15583. [Google Scholar] [CrossRef] [PubMed]
- Silva-Peña, D.; García-Marchena, N.; Alén, F.; Araos, P.; Rivera, P.; Vargas, A.; García-Fernández, M.I.; Martín-Velasco, A.I.; Villanúa, M.; Castilla-Ortega, E.; et al. Alcohol-induced cognitive deficits are associated with decreased circulating levels of the neurotrophin BDNF in humans and rats. Addict. Biol. 2019, 24, 1019–1033. [Google Scholar] [CrossRef]
- García-Marchena, N.; Pizarro, N.; Pavón, F.J.; Martínez-Huélamo, M.; Flores-López, M.; Requena-Ocaña, N.; Araos, P.; Silva-Peña, D.; Suárez, J.; Santín, L.J.; et al. Potential association of plasma lysophosphatidic acid (LPA) species with cognitive impairment in abstinent alcohol use disorders outpatients. Sci. Rep. 2020, 10, 17163. [Google Scholar] [CrossRef]
- Su, H.; Tao, J.; Zhang, J.; Xie, Y.; Wang, Y.; Zhang, Y.; Han, B.; Lu, Y.; Sun, H.; Wei, Y.; et al. The Effects of BDNF Val66Met Gene Polymorphism on Serum BDNF and Cognitive Function in Methamphetamine-Dependent Patients and Normal Controls: A Case-Control Study. J. Clin. Psychopharmacol. 2015, 35, 517–524. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Q.; Wang, Y.; Hao, Y.; Jing, P.; Jiao, S.; Ma, L.; Pan, C.; Wu, Y. MMP-9-BDNF pathway is implicated in cognitive impairment of male individuals with methamphetamine addiction during early withdrawal. Behav. Brain Res. 2019, 366, 29–35. [Google Scholar] [CrossRef]
- Viola, T.W.; Tractenberg, S.G.; Kluwe-Schiavon, B.; Levandowski, M.L.; Sanvicente-Vieira, B.; Wearick-Silva, L.E.; de Azeredo, L.A.; Teixeira, A.L., Jr.; Grassi-Oliveira, R. Brain-Derived Neurotrophic Factor and Delayed Verbal Recall in Crack/Cocaine Dependents. Eur. Addict. Res. 2015, 21, 273–278. [Google Scholar] [CrossRef]
- Fatjó-Vilas, M.; Soler, J.; Ibáñez, M.I.; Moya-Higueras, J.; Ortet, G.; Guardiola-Ripoll, M.; Fañanás, L.; Arias, B. The effect of the AKT1 gene and cannabis use on cognitive performance in healthy subjects. J. Psychopharmacol. 2020, 34, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Tao, J.; Zhang, J.; Xie, Y.; Zhang, X.; Su, H.; He, J. Increased BDNF may not be associated with cognitive impairment in heroin-dependent patients. Medicine 2017, 96, e6582. [Google Scholar] [CrossRef]
- Bazov, I.; Kononenko, O.; Watanabe, H.; Kuntić, V.; Sarkisyan, D.; Taqi, M.M.; Hussain, M.Z.; Nyberg, F.; Yakovleva, T.; Bakalkin, G. The endogenous opioid system in human alcoholics: Molecular adaptations in brain areas involved in cognitive control of addiction. Addict. Biol. 2013, 18, 161–169. [Google Scholar] [CrossRef] [PubMed]
- McCann, U.D.; Szabo, Z.; Vranesic, M.; Palermo, M.; Mathews, W.B.; Ravert, H.T.; Dannals, R.F.; Ricaurte, G.A. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/-)3,4-methylenedioxymethamphetamine (“ecstasy”) users: Relationship to cognitive performance. Psychopharmacology 2008, 200, 439–450. [Google Scholar] [CrossRef]
- Thomasius, R.; Petersen, K.; Buchert, R.; Andresen, B.; Zapletalova, P.; Wartberg, L.; Nebeling, B.; Schmoldt, A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology 2003, 167, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Rogers, R.D.; Cook, L.J.; Sahakian, B.J. The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology 2006, 188, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Havranek, M.M.; Vonmoos, M.; Müller, C.P.; Büetiger, J.R.; Tasiudi, E.; Hulka, L.M.; Preller, K.H.; Mössner, R.; Grünblatt, E.; Seifritz, E.; et al. Serotonin Transporter and Tryptophan Hydroxylase Gene Variations Mediate Working Memory Deficits of Cocaine Users. Neuropsychopharmacology 2015, 40, 2929–2937. [Google Scholar] [CrossRef]
- Galindo, L.; Moreno, E.; López-Armenta, F.; Guinart, D.; Cuenca-Royo, A.; Izquierdo-Serra, M.; Xicota, L.; Fernandez, C.; Menoyo, E.; Fernández-Fernández, J.M.; et al. Cannabis Users Show Enhanced Expression of CB(1)-5HT(2A) Receptor Heteromers in Olfactory Neuroepithelium Cells. Mol. Neurobiol. 2018, 55, 6347–6361. [Google Scholar] [CrossRef]
- Hanak, C.; Benoit, J.; Fabry, L.; Hein, M.; Verbanck, P.; de Witte, P.; Walter, H.; Dexter, D.T.; Ward, R.J. Changes in Pro-Inflammatory Markers in Detoxifying Chronic Alcohol Abusers, Divided by Lesch Typology, Reflect Cognitive Dysfunction. Alcohol Alcohol. 2017, 52, 529–534. [Google Scholar] [CrossRef]
- Wang, T.Y.; Lee, S.Y.; Chang, Y.H.; Chen, S.L.; Chen, P.S.; Chu, C.H.; Huang, S.Y.; Tzeng, N.S.; Lee, I.H.; Chen, K.C.; et al. Correlation of cytokines, BDNF levels, and memory function in patients with opioid use disorder undergoing methadone maintenance treatment. Drug. Alcohol. Depend. 2018, 191, 6–13. [Google Scholar] [CrossRef]
- Dominguez-Salas, S.; Diaz-Batanero, C.; Lozano-Rojas, O.M.; Verdejo-Garcia, A. Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neurosci. Biobehav. Rev. 2016, 71, 772–801. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Garcia, A.; Garcia-Fernandez, G.; Dom, G. Cognition and addiction. Dialogues Clin. Neurosci. 2019, 21, 281–290. [Google Scholar] [CrossRef] [PubMed]
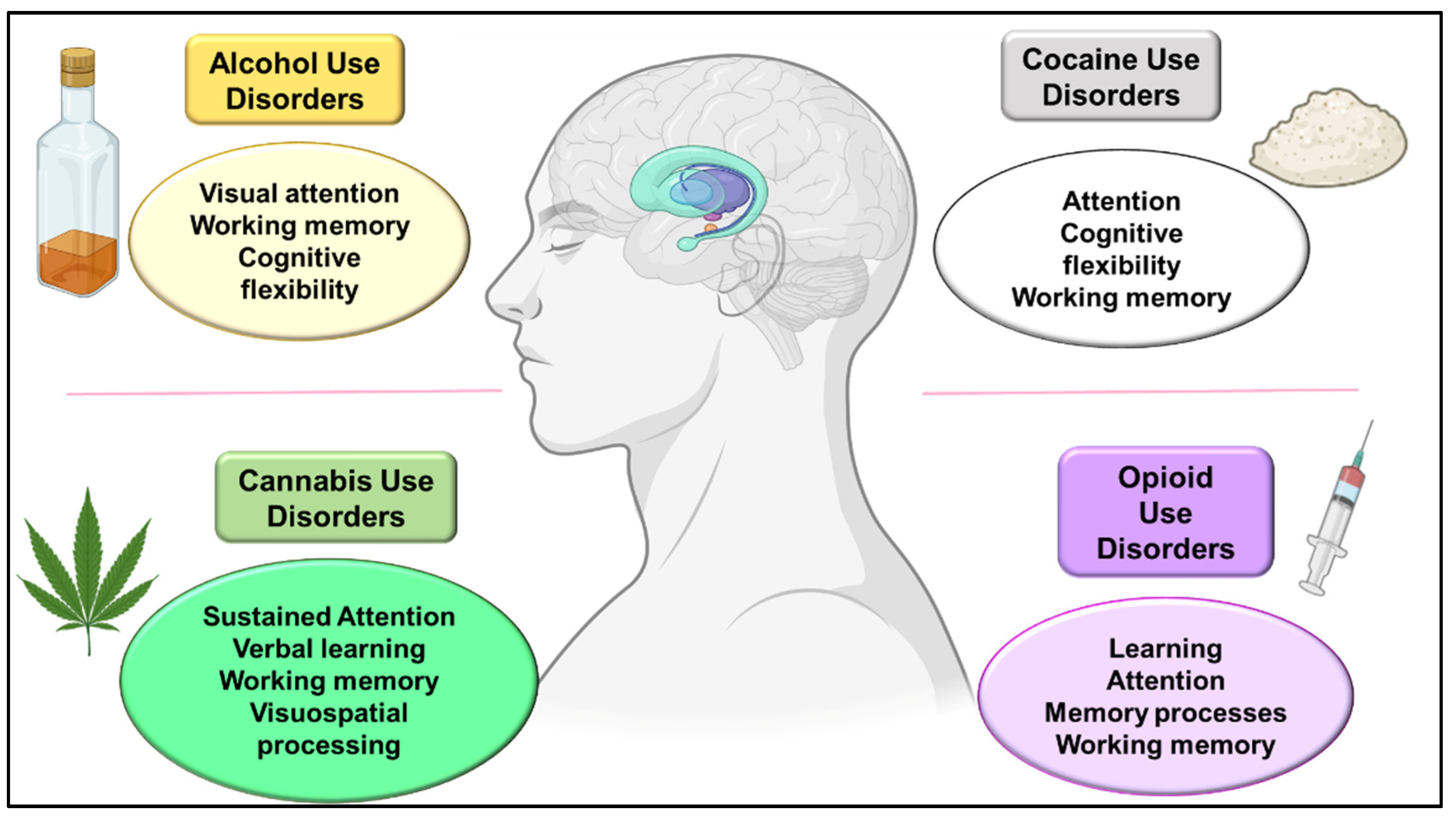
| ALCOHOL | |||||
|---|---|---|---|---|---|
| Drug and Pattern of Administration | Strain and Stage of Administration | Test | Behavioral Alterations | Biological Alterations | References |
| EtOH 2 g/kg, i.p., acute | Female Wistar albino rats, Adulthood | Spontaneous alternation in the Y maze | Reduced spontaneous alternation | - | [47] |
| EtOH 1.5 g/kg, i.p., acute | Male Sprague-Dawley rats, Adulthood | Eight-arm radial maze | Impaired contextual memory | - | [49] |
| EtOH 0.75, 1.5 and 2 g/kg, i.p., acute | Male Sprague-Dawley rats, Adulthood | Eight-arm radial maze | Impaired behavioral variability | - | [50] |
| EtOH 0.75 g/kg, i.p., acute | Male Long-Evans rats, Adulthood | Operant chambers | Working memory impairment | - | [48] |
| EtOH 3 g/kg, i.p., two administrations | Male Sprague-Dawley rats, Adulthood | - | - | ↑ Doublecortin, astrogliosis and ↓ mature neurons in the SGZ of the DG ↓ Neuronal TLR4 in CA1 and DG | [51] |
| EtOH 2 g/kg, i.p., acute | Male C57BL/6J mice at 3, 5 and 7 weeks of age | Fear conditioning | No changes were observed | - | [53] |
| EtOH 1 g/kg, i.p., Acute, pre-training | Male NMRI mice, Adulthood | Step-down inhibitory avoidance | Reduction in aversive memory retention 24 h after the training session | Reduced pCREB/CREB protein ratio in the HIPP | [54] |
| EtOH 1.6 and 2.4 g/kg, i.p., Acute | Male C57BL/6J mice | Novel object recognition | Reduced object recognition | - | [56] |
| Chronic EtOH → EtOH 2% v/v to 20% v/v solution | Male Wistar rats, Adulthood | Novel object recognition Spatial recognition test | Impaired recognition in both tests after acute and chronic EtOH, with worse results in chronically treated rats | - | [57] |
| EtOH → 2 g/kg, i.p., once a week | |||||
| EtOH 5 g/kg, p.o., 2-day on/off cycle, 13 administrations | Male Sprague-Dawley rats Early Adolescence PND28 Male Sprague-Dawley rats Mid-adolescence PND35 Male Sprague-Dawley rats Adult PND65 | Spontaneous alternation in a plus maze Non-spatial discrimination learning and reversal task | Impaired behavioral flexibility in the plus maze test Impaired acquisition and learning in the non-spatial discrimination test | Reduced BDNF protein levels in the PFC immediately after EtOH administration in all ages. In abstinence, BDNF reduction was maintained in early adolescents, whereas it increased in adults | [60] |
| EtOH 4 g/kg, p.o. daily for 11 days | Male and female Sprague-Dawley rats Early adolescence PND25 Male and female Sprague-Dawley rats Late adolescence PND45 | Behavioral flexibility by operant set-shifting task | Males showed deficits in behavioral flexibility only after early ethanol exposure | - | [61] |
| EtOH vapor 14 h/day, 7–10 weeks | Male Wistar rats, Adulthood PND56 | - | - | ↓ Proliferation and differentiation of oligodendrocyte progenitor cells and ↓ Myelin expression in the mPFC | [62] |
| EtOH vapor (15–17 mg/L air), 16 h for 4 consecutive days | Male C57BL/6J mice, Adulthood | Reversal learning task Attentional set-shifting task | No changes in the reversal learning task ↑ Number of errors in the attentional set-shifting | ↑ NMDA/AMPA ratio in the mPFC, with an increase of NMDA component | [63] |
| EtOH vapor 14 h/day, 15 days | Male Long-Evans rats, Adulthood | Operant set-shifting task | ↑ Number of errors in the attentional set-shifting | ↓ D2/D4 modulatory actions in the mPFC | [65] |
| EtOH vapor, 14 h/day, 4 cycles of 2-daily exposure | Male Long-Evans rats, Adolescence | Operant set-shifting task | ↓ Behavioral flexibility | Positive allosteric modulation of mGluR5 reverses behavioral deficits | [64] |
| EtOH 3 g/kg, i.p., 8 intermittent administrations | Wistar rats, Adolescence | Conditional discrimination learning NOR | Cognitive deficits in adolescence and adulthood | ↑ COX2 and iNOS levels ↑ cell death in the neocortex, HIPP and cerebellum | [68] |
| EtOH liquid diet over 4 weeks | Female C57BL/6J mice, Adulthood | NOR Y-maze | ↓ Recognition of the novel object | ↓ Neural stem cell proliferation and survival in the HIPP | [69] |
| EtOH 2–10%, 4 months, p.o. | Male Swiss mice, Late adolescence | NOR | ↓ Recognition of the novel object | ↑ γ protein levels | [70] |
| EtOH 5 g/kg (p.o.), intermittent administration for 16 days | Male Sprague-Dawley rats, Adolescence | - | - | ↓ PSD-95 and SAP102 | [71] |
| EtOH 5 g/kg (p.o.), intermittent administration for 20 days | Male Sprague-Dawley rats, Adolescence And Adults | - | - | ↓ IA mean peak amplitude | [72] |
| EtOH liquid diet with 7% alcohol, 1, 2 or 4 weeks (p.o.) | Male Sprague-Dawley rats, | - | - | ↓ PCNA, DCX in the HIPP | [73] |
| EtOH 3–10% (p.o.), voluntary consumption | Male C57BL/6J mice, Adults | Fear conditioning test NOR Barnes maze test | ↓ Behavioral flexibility, context-induced freezing and novel object recognition | ↓ BDNF gene methylation and ↑ BDNF signaling pathway in CA1 and CA3 of the HIPP | [74] |
| EtOH 4 g/kg (p.o.) for 30 consecutive days | Male Wistar rats, Adults | Morris water maze | ↓ spatial memory | ↑ MDA, ↓ SOD and GPx in the HIPP | [75] |
| EtOH vapor 14 h/day, 7 weeks | Male Wistar rats, Adults | - | - | Spine density in the HIPP ↑ after EtOH exposure and ↓ in abstinence | [76] |
| EtOH 2 g/kg (p.o.) for 5 consecutive days | Male Sprague-Dawley rats, Adolescence | - | - | ↑ NMDA receptor in the frontal cortex in the abstinence phase | [67] |
| Intermittent access to 20% of voluntary ethanol consumption followed by operant conditioning | Chat-eGFP, Char-Cre, VGlut2-Cre, Drd1a-tdTomato and Ai32 mice with C57BL/6J background | - | - | ↓ Thalamic excitation of DMS CINs | [66] |
| EtOH 3 g/kg on 2 consecutive days with 48 h intervals over 2 weeks | Male and female C57BL6J mice, Adolescence | Hebb-Williams maze NOR Passive avoidance test | ↓ Learning and memory in all paradigms | ↓ P-CREB and P-ERK protein expression | [77] |
| EtOH intermittent exposure 5 g/kg, 2 days on and 2 days off | Male Wistar rats, Adolescence | NOR | ↓ Recognition memory | ↓ Axial and radial diffusivity in several brain regions | [78] |
| EtOH intermittent exposure 5 g/kg, 2 days on and 2 days off | Male and female rats | Spontaneous alternation NOR | No changes in adolescence, but a slight reduction in adult rats | Blunted behavioral-evoked HIPP acetylcholine efflux | [79] |
| EtOH intermittent exposure 5 g/kg (p.o.), 2 days on and 2 days off | Male and female Sprague-Dawley rats | Social recognition | ↓ Social memory and learning | ↓ Number of cholinergic interneurons in the NAc | [81] |
| Liquid EtOH diet 2.4–7.2%, p.o. for 20 days | Male Sprague-Dawley rats | Emotional novel object recognition | ↓ Emotional learning | Loss of dendritic “long thin” spines in the NAc during abstinence | [82] |
| EtOH 4 g/kg, i.p. at 24 h of intervals for 5 days | Male Long-Evans rats, Adolescence and Adulthood | Morris water maze | ↓ Spatial learning in adolescents but not in adults during the abstinence phase | - | [83] |
| EtOH 3.4 g/kg for 6 consecutive days | Male Wistar rats | - | - | ↑ GLU release in the HIPP during the abstinence phase | [84] |
| 54 cycles of liquid 10% EtOH diet followed by an abstinence period | Male Wistar rats | 5-Choice serial reaction time task | ↓ Behavioral inhibition and attentional capacity at 7 and 34 days of abstinence | - | [85] |
| EtOH 5 g/kg for 5 days + 6 g/kg for 55 additional days, followed by abstinence period | Male Sprague-Dawley rats | - | - | ↓ Short and ↑ long-term HIPP CB1r protein levels | [86] |
| 2 to 10% of EtOH for 5 months, followed by an abstinence period | Male C57BL/6WT and TLR4 knock-out mice | NOR | ↓ Discrimination index | ↑ Neuroinflammation damage | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparyan, A.; Maldonado Sanchez, D.; Navarrete, F.; Sion, A.; Navarro, D.; García-Gutiérrez, M.S.; Rubio Valladolid, G.; Jurado Barba, R.; Manzanares, J. Cognitive Alterations in Addictive Disorders: A Translational Approach. Biomedicines 2023, 11, 1796. https://doi.org/10.3390/biomedicines11071796
Gasparyan A, Maldonado Sanchez D, Navarrete F, Sion A, Navarro D, García-Gutiérrez MS, Rubio Valladolid G, Jurado Barba R, Manzanares J. Cognitive Alterations in Addictive Disorders: A Translational Approach. Biomedicines. 2023; 11(7):1796. https://doi.org/10.3390/biomedicines11071796
Chicago/Turabian StyleGasparyan, Ani, Daniel Maldonado Sanchez, Francisco Navarrete, Ana Sion, Daniela Navarro, María Salud García-Gutiérrez, Gabriel Rubio Valladolid, Rosa Jurado Barba, and Jorge Manzanares. 2023. "Cognitive Alterations in Addictive Disorders: A Translational Approach" Biomedicines 11, no. 7: 1796. https://doi.org/10.3390/biomedicines11071796
APA StyleGasparyan, A., Maldonado Sanchez, D., Navarrete, F., Sion, A., Navarro, D., García-Gutiérrez, M. S., Rubio Valladolid, G., Jurado Barba, R., & Manzanares, J. (2023). Cognitive Alterations in Addictive Disorders: A Translational Approach. Biomedicines, 11(7), 1796. https://doi.org/10.3390/biomedicines11071796











