Cocaine Regulates NLRP3 Inflammasome Activity and CRF Signaling in a Region- and Sex-Dependent Manner in Rat Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rat Brain Tissues
2.2. RNA Extraction, Reverse Transcription, and Quantitative Polymerase Chain Reaction (qPCR)
2.3. Western Blots
2.4. Statistical Analysis
3. Results
3.1. Cocaine Upregulates Neuroinflammation in the Striatum and HP of Male and Female Rats
3.2. Cocaine Increases Microglial Markers in the Male Striatum
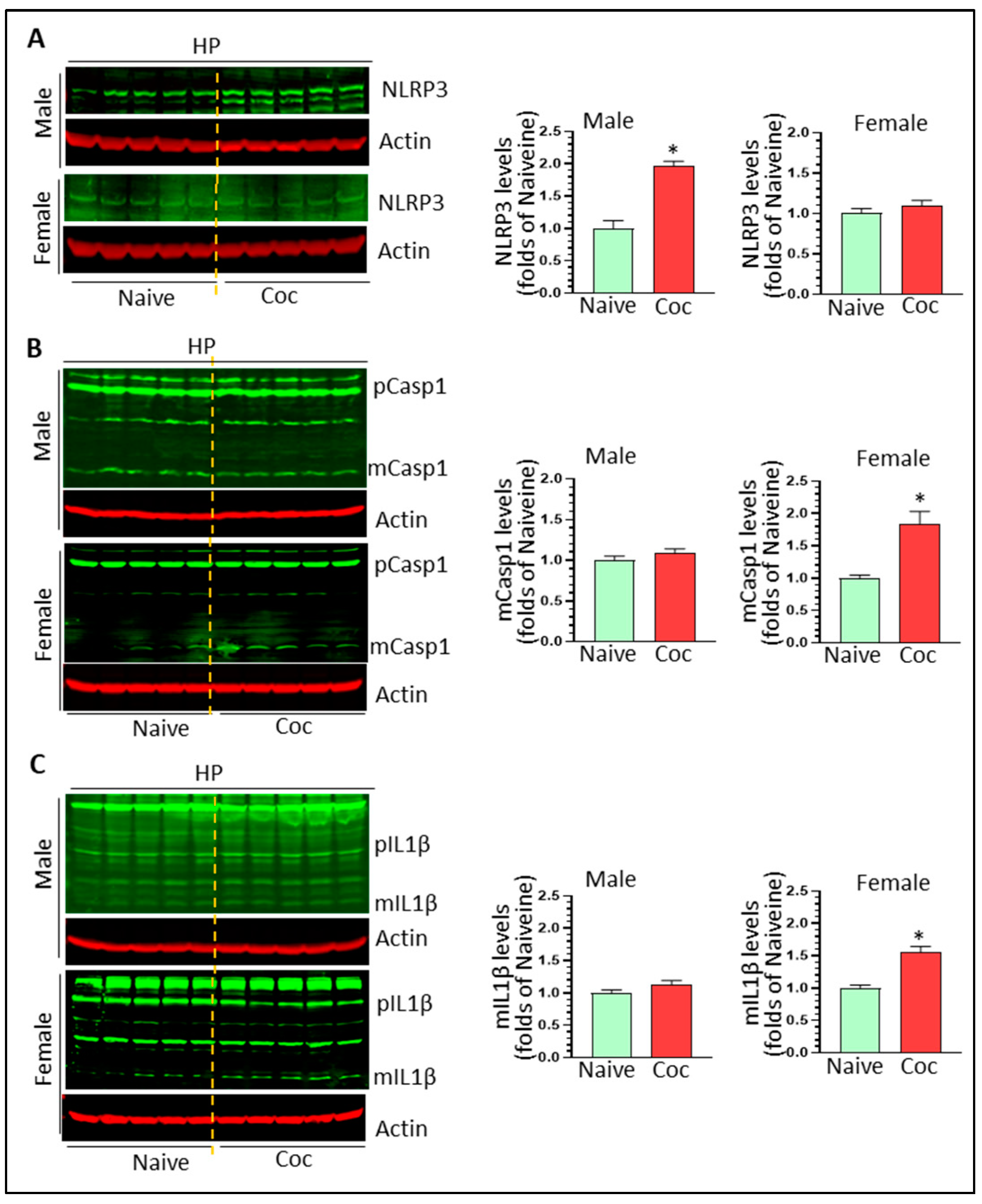
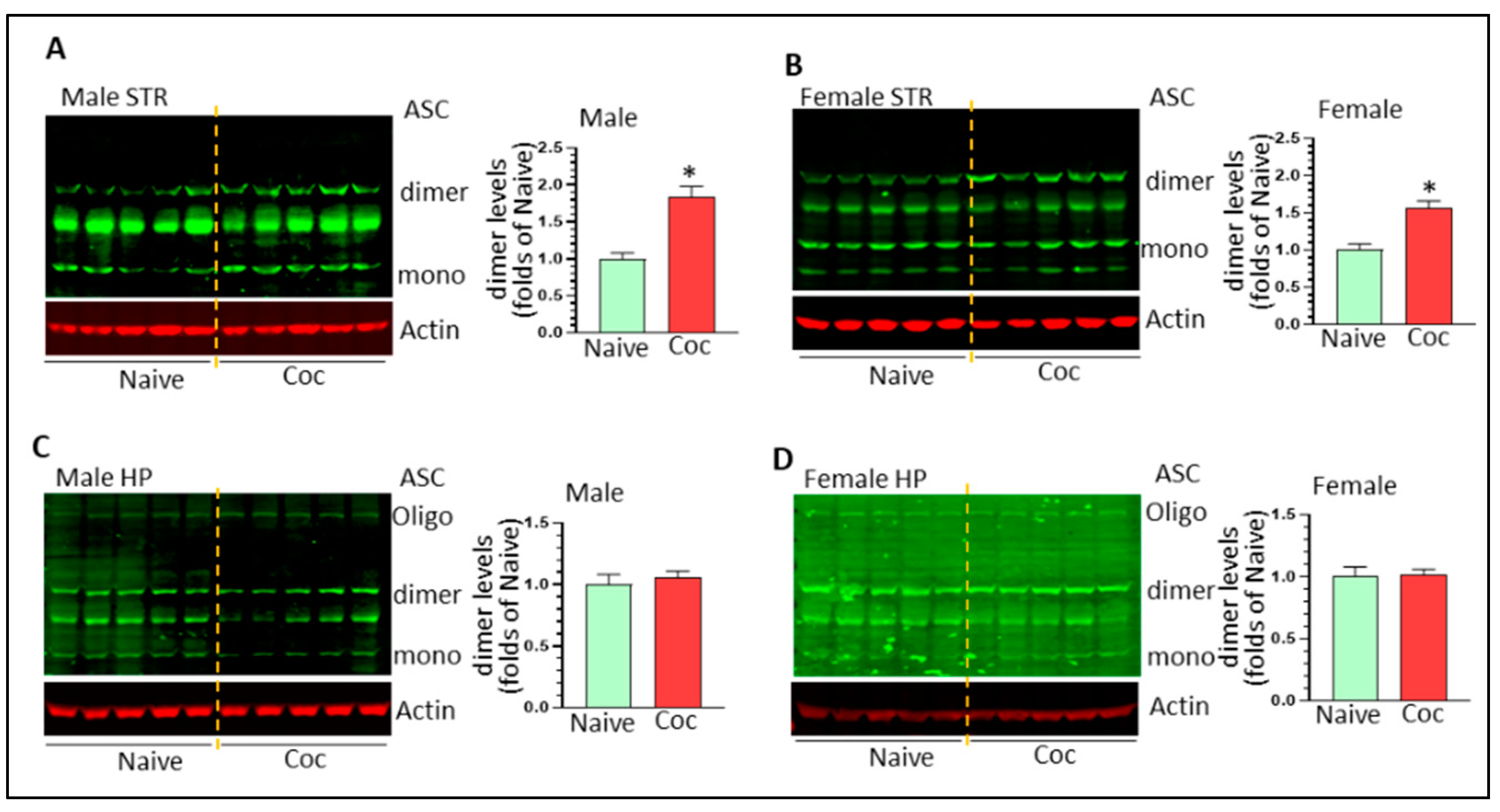
3.3. Cocaine Differentially Upregulates NLPR3 Inflammasome Activity in Rat Brain
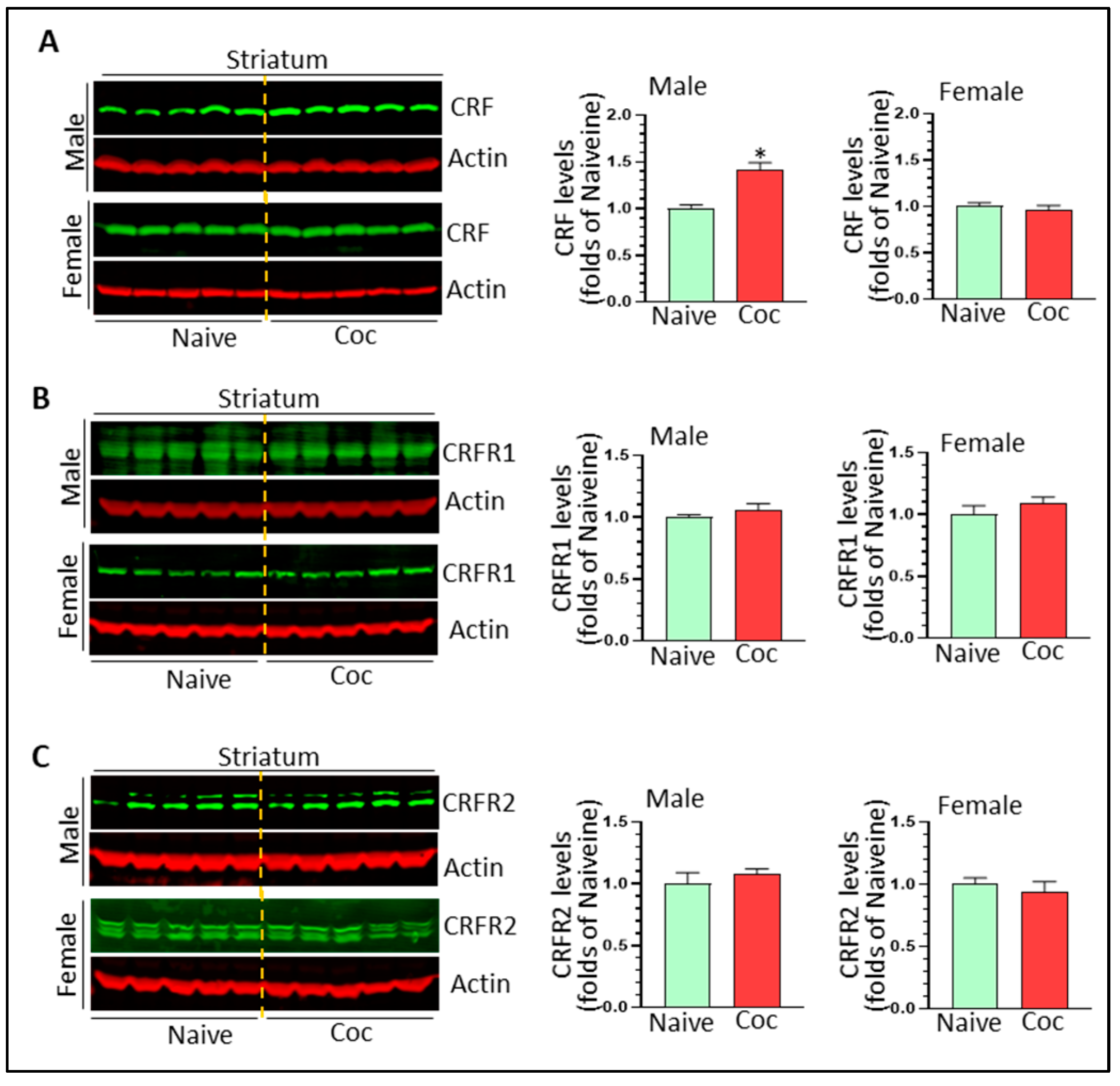
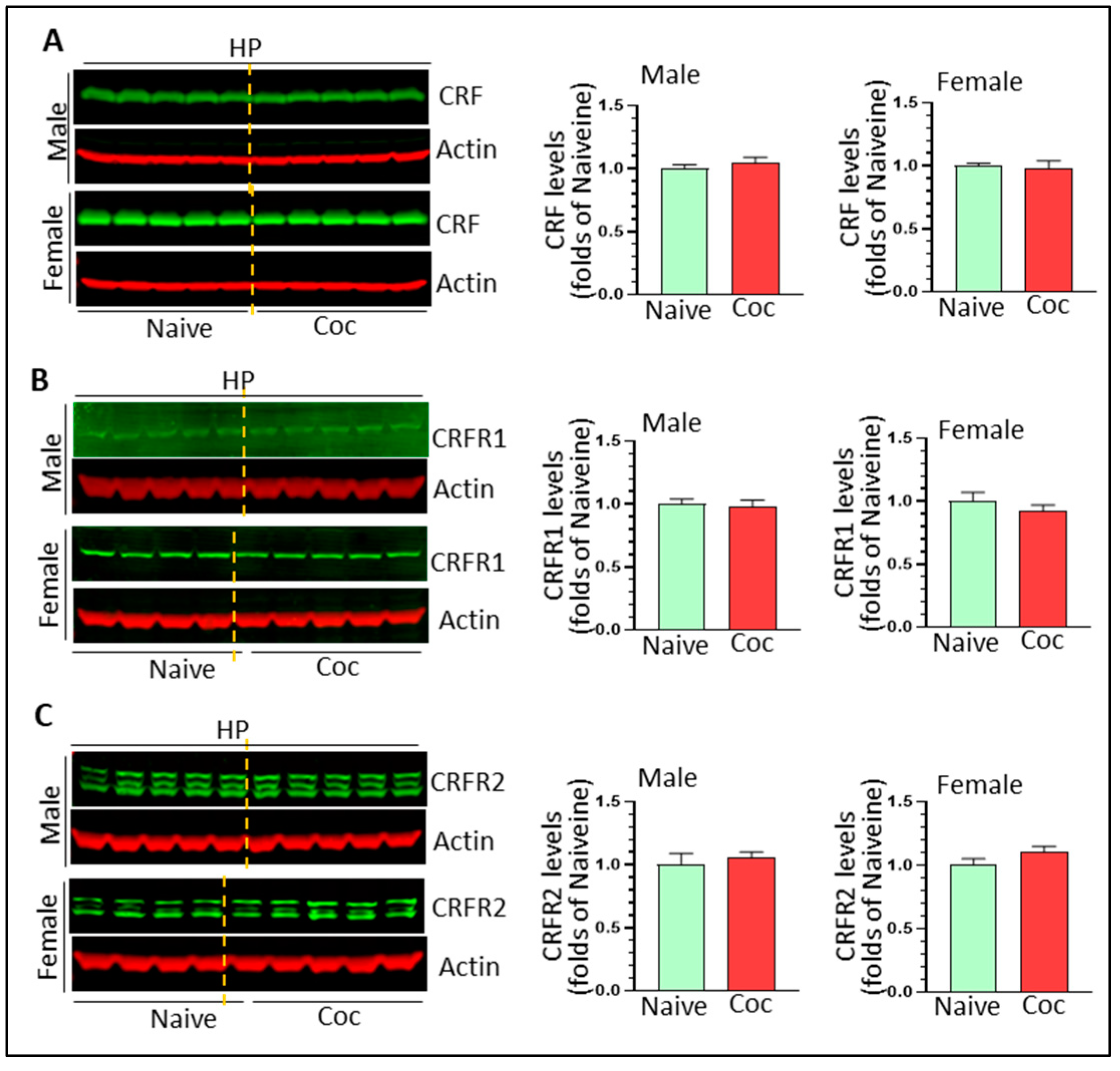
3.4. The Effects of Cocaine on CRF Signaling and NF-κB Levels in Rat Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedegaard, H.; Bastian, B.A.; Trinidad, J.P.; Spencer, M.; Warner, M. Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2011–2016. Natl. Vital Stat. Rep. 2018, 67, 1–14. [Google Scholar]
- Tanabe, J.; York, P.; Krmpotich, T.; Miller, D.; Dalwani, M.; Sakai, J.T.; Mikulich-Gilbertson, S.K.; Thompson, L.; Claus, E.; Banich, M.; et al. Insula and orbitofrontal cortical morphology in substance dependence is modulated by sex. AJNR Am. J. Neuroradiol. 2013, 34, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Chou, S.P.; Saha, T.D.; Pickering, R.P.; Kerridge, B.T.; Ruan, W.J.; Huang, B.; Jung, J.; Zhang, H.; Fan, A.; et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017, 74, 911–923. [Google Scholar] [CrossRef]
- Mayo, L.M.; Paul, E.; DeArcangelis, J.; Van Hedger, K.; de Wit, H. Gender differences in the behavioral and subjective effects of methamphetamine in healthy humans. Psychopharmacology 2019, 236, 2413–2423. [Google Scholar] [CrossRef] [Green Version]
- Lejuez, C.W.; Bornovalova, M.A.; Reynolds, E.K.; Daughters, S.B.; Curtin, J.J. Risk factors in the relationship between gender and crack/cocaine. Exp. Clin. Psychopharmacol. 2007, 15, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Quinones-Jenab, V.; Jenab, S. Influence of sex differences and gonadal hormones on cocaine addiction. ILAR J. 2012, 53, 14–22. [Google Scholar] [CrossRef]
- Back, S.E.; Brady, K.T.; Jackson, J.L.; Salstrom, S.; Zinzow, H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology 2005, 180, 169–176. [Google Scholar] [CrossRef]
- VanHouten, J.P.; Rudd, R.A.; Ballesteros, M.F.; Mack, K.A. Drug Overdose Deaths among Women Aged 30–64 Years—United States, 1999–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1–5. [Google Scholar] [CrossRef]
- Clark, K.H.; Wiley, C.A.; Bradberry, C.W. Psychostimulant abuse and neuroinflammation: Emerging evidence of their interconnection. Neurotox. Res. 2013, 23, 174–188. [Google Scholar] [CrossRef]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.; Guo, M.; Niu, F.; Yang, L.; Callen, S.E.; Buch, S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J. Neuroinflamm. 2016, 13, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.L.; Liao, K.; Periyasamy, P.; Yang, L.; Cai, Y.; Callen, S.E.; Buch, S. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 2015, 11, 995–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.H.; Lutjens, R.; van der Stap, L.D.; Lekic, D.; Romano-Spica, V.; Morales, M.; Koob, G.F.; Repunte-Canonigo, V.; Sanna, P.P. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc. Natl. Acad. Sci. USA 2005, 102, 11533–11538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piechota, M.; Korostynski, M.; Solecki, W.; Gieryk, A.; Slezak, M.; Bilecki, W.; Ziolkowska, B.; Kostrzewa, E.; Cymerman, I.; Swiech, L.; et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010, 11, R48. [Google Scholar] [CrossRef] [Green Version]
- Northcutt, A.L.; Hutchinson, M.R.; Wang, X.; Baratta, M.V.; Hiranita, T.; Cochran, T.A.; Pomrenze, M.B.; Galer, E.L.; Kopajtic, T.A.; Li, C.M.; et al. DAT isn’t all that: Cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatry 2015, 20, 1525–1537. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Kunitachi, S.; Iyo, M.; Hashimoto, K. The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol. Biochem. Behav. 2012, 101, 303–306. [Google Scholar] [CrossRef]
- Chen, H.; Manev, H. Effects of minocycline on cocaine sensitization and phosphorylation of GluR1 receptors in 5-lipoxygenase deficient mice. Neuropharmacology 2011, 60, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Vallender, E.J.; Goswami, D.B.; Shinday, N.M.; Westmoreland, S.V.; Yao, W.D.; Rowlett, J.K. Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol. Depend. 2017, 175, 9–23. [Google Scholar] [CrossRef]
- Little, K.Y.; Ramssen, E.; Welchko, R.; Volberg, V.; Roland, C.J.; Cassin, B. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 2009, 168, 173–180. [Google Scholar] [CrossRef]
- Kohno, M.; Link, J.; Dennis, L.E.; McCready, H.; Huckans, M.; Hoffman, W.F.; Loftis, J.M. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol. Biochem. Behav. 2019, 179, 34–42. [Google Scholar] [CrossRef]
- Catale, C.; Bussone, S.; Lo Iacono, L.; Carola, V. Microglial alterations induced by psychoactive drugs: A possible mechanism in substance use disorder? Semin. Cell Dev. Biol. 2019, 94, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Bachtell, R.K.; Jones, J.D.; Heinzerling, K.G.; Beardsley, P.M.; Comer, S.D. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol. Depend. 2017, 180, 156–170. [Google Scholar] [CrossRef]
- Song, N.; Li, T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front. Immunol. 2018, 9, 2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Fabi, C.; Bellet, M.M.; Costantini, C.; Nunziangeli, L.; Romani, L.; Brancorsini, S. Epigenetic Mechanisms of Inflammasome Regulation. Int. J. Mol. Sci. 2020, 21, 5758. [Google Scholar] [CrossRef]
- Guan, Y.; Han, F. Key Mechanisms and Potential Targets of the NLRP3 Inflammasome in Neurodegenerative Diseases. Front. Integr. Neurosci. 2020, 14, 37. [Google Scholar] [CrossRef]
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.; Cooper, M.A.; O’Neill, L.A.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav. Immun. 2017, 61, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, W.; Shinohara, M.L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol. 2017, 27, 213–219. [Google Scholar] [CrossRef]
- Cao, S.; Shrestha, S.; Li, J.; Yu, X.; Chen, J.; Yan, F.; Ying, G.; Gu, C.; Wang, L.; Chen, G. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci. Rep. 2017, 7, 2417. [Google Scholar] [CrossRef] [Green Version]
- Chivero, E.T.; Guo, M.L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chivero, E.T.; Thangaraj, A.; Tripathi, A.; Periyasamy, P.; Guo, M.L.; Buch, S. NLRP3 Inflammasome Blockade Reduces Cocaine-Induced Microglial Activation and Neuroinflammation. Mol. Neurobiol. 2021, 58, 2215–2230. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Chivero, E.T.; Callen, S.E.; Buch, S. NLRP3 Inflammasome Is Involved in Cocaine-Mediated Potentiation on Behavioral Changes in CX3CR1-Deficient Mice. J. Pers. Med. 2021, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Guyon, A.; Morselli, L.L.; Balbo, M.L.; Tasali, E.; Leproult, R.; L’Hermite-Baleriaux, M.; Van Cauter, E.; Spiegel, K. Effects of Insufficient Sleep on Pituitary-Adrenocortical Response to CRH Stimulation in Healthy Men. Sleep 2017, 40, zsx064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Lenker, K.P.; Basta, M.; Bixler, E.O.; Chrousos, G.P. Hypothalamic-pituitary-adrenal (HPA) axis response to exogenous corticotropin-releasing hormone (CRH) is attenuated in men with chronic insomnia. J. Sleep Res. 2022, 31, e13526. [Google Scholar] [CrossRef]
- Makino, S.; Gold, P.W.; Schulkin, J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994, 640, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Itoga, C.A.; Chen, Y.; Fateri, C.; Echeverry, P.A.; Lai, J.M.; Delgado, J.; Badhon, S.; Short, A.; Baram, T.Z.; Xu, X. New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J. Comp. Neurol. 2019, 527, 2474–2487. [Google Scholar] [CrossRef] [Green Version]
- Pomrenze, M.B.; Millan, E.Z.; Hopf, F.W.; Keiflin, R.; Maiya, R.; Blasio, A.; Dadgar, J.; Kharazia, V.; De Guglielmo, G.; Crawford, E.; et al. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front. Neurosci. 2015, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Spangler, R.; Ho, A.; Kreek, M.J. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res. Mol. Brain Res. 2003, 114, 73–79. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Logrip, M.L.; Koob, G.F. Corticotropin releasing factor: A key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014, 35, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Carrette, L.L.G.; de Guglielmo, G.; Kallupi, M.; Maturin, L.; Brennan, M.; Boomhower, B.; Conlisk, D.; Sedighim, S.; Tieu, L.; Fannon, M.J.; et al. The Cocaine and Oxycodone Biobanks, Two Repositories from Genetically Diverse and Behaviorally Characterized Rats for the Study of Addiction. eNeuro 2021, 8, ENEURO-0033. [Google Scholar] [CrossRef]
- Guo, L.; Reed, K.M.; Carter, A.; Cheng, Y.; Roodsari, S.K.; Martinez Pineda, D.; Wellman, L.L.; Sanford, L.D.; Guo, M.L. Sleep-Disturbance-Induced Microglial Activation Involves CRH-Mediated Galectin 3 and Autophagy Dysregulation. Cells 2022, 12, 160. [Google Scholar] [CrossRef]
- Xu, W.; Bai, F.; Tummalapalli, C.M.; Miller, D.D.; Middaugh, L.; Boggan, W.O. The interactive effects of cocaine/gender on immune function in mice. An observation of in vivo acute cocaine exposure. Int. J. Immunopharmacol. 1997, 19, 333–340. [Google Scholar] [CrossRef]
- Kenny, P.J.; Hoyer, D.; Koob, G.F. Animal Models of Addiction and Neuropsychiatric Disorders and Their Role in Drug Discovery: Honoring the Legacy of Athina Markou. Biol. Psychiatry 2018, 83, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Giuliano, C.; Belin, D. Addictive behaviour in experimental animals: Prospects for translation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calipari, E.S.; Godino, A.; Peck, E.G.; Salery, M.; Mervosh, N.L.; Landry, J.A.; Russo, S.J.; Hurd, Y.L.; Nestler, E.J.; Kiraly, D.D. Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat. Commun. 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Li, H.; Siemsen, B.M.; Healey, K.L.; Tran, P.K.; Woronoff, N.; Boger, H.A.; Kalivas, P.W.; Reissner, K.J. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol. Psychiatry 2016, 80, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Brandi, E.; Torres-Garcia, L.; Svanbergsson, A.; Haikal, C.; Liu, D.; Li, W.; Li, J.Y. Brain region-specific microglial and astrocytic activation in response to systemic lipopolysaccharides exposure. Front. Aging Neurosci. 2022, 14, 910988. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.W.; Kim, K.T. Region-Specific Characteristics of Astrocytes and Microglia: A Possible Involvement in Aging and Diseases. Cells 2022, 11, 1902. [Google Scholar] [CrossRef]
- Healy, L.M.; Zia, S.; Plemel, J.R. Towards a definition of microglia heterogeneity. Commun. Biol. 2022, 5, 1114. [Google Scholar] [CrossRef]
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 2018, 172, 500–516.e16. [Google Scholar] [CrossRef] [Green Version]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Loram, L.C.; Sholar, P.W.; Taylor, F.R.; Wiesler, J.L.; Babb, J.A.; Strand, K.A.; Berkelhammer, D.; Day, H.E.; Maier, S.F.; Watkins, L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 2012, 37, 1688–1699. [Google Scholar] [CrossRef] [Green Version]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017, 65, 1504–1520. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chen, Y.W.; Tsai, S.F.; Wu, S.N.; Shih, Y.H.; Jiang-Shieh, Y.F.; Yang, T.T.; Kuo, Y.M. Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K(+) channel. Sci. Rep. 2016, 6, 22864. [Google Scholar] [CrossRef] [Green Version]
- Murtaj, V.; Belloli, S.; Di Grigoli, G.; Pannese, M.; Ballarini, E.; Rodriguez-Menendez, V.; Marmiroli, P.; Cappelli, A.; Masiello, V.; Monterisi, C.; et al. Age and Sex Influence the Neuro-inflammatory Response to a Peripheral Acute LPS Challenge. Front. Aging Neurosci. 2019, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Hitzemann, R.; Bergeson, S.E.; Berman, A.E.; Bubier, J.A.; Chesler, E.J.; Finn, D.A.; Hein, M.; Hoffman, P.; Holmes, A.; Kisby, B.R.; et al. Sex Differences in the Brain Transcriptome Related to Alcohol Effects and Alcohol Use Disorder. Biol. Psychiatry 2022, 91, 43–52. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Marcos, M.; Torres, J.L.; Costa-Alba, P.; Garcia-Garcia, F.; Laso, F.J.; Guerri, C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol. 2017, 22, 1829–1841. [Google Scholar] [CrossRef]
- Finn, D.A.; Hashimoto, J.G.; Cozzoli, D.K.; Helms, M.L.; Nipper, M.A.; Kaufman, M.N.; Wiren, K.M.; Guizzetti, M. Binge Ethanol Drinking Produces Sexually Divergent and Distinct Changes in Nucleus Accumbens Signaling Cascades and Pathways in Adult C57BL/6J Mice. Front. Genet. 2018, 9, 325. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Rainville, J.R.; Williams, K.; Lile, J.A.; Hodes, G.E.; Vassoler, F.M.; Turner, J.R. Sexually dimorphic neuroimmune response to chronic opioid treatment and withdrawal. Neuropharmacology 2021, 186, 108469. [Google Scholar] [CrossRef]
- Cannella, L.A.; Andrews, A.M.; Razmpour, R.; McGary, H.; Corbett, C.B.; Kahn, J.; Ramirez, S.H. Reward and immune responses in adolescent females following experimental traumatic brain injury. Behav. Brain Res. 2020, 379, 112333. [Google Scholar] [CrossRef]
- Haas, A.L.; Peters, R.H. Development of substance abuse problems among drug-involved offenders. Evidence for the telescoping effect. J. Subst. Abuse 2000, 12, 241–253. [Google Scholar] [CrossRef]
- Gutzeit, O.; Segal, L.; Korin, B.; Iluz, R.; Khatib, N.; Dabbah-Assadi, F.; Ginsberg, Y.; Fainaru, O.; Ross, M.G.; Weiner, Z.; et al. Progesterone Attenuates Brain Inflammatory Response and Inflammation-Induced Increase in Immature Myeloid Cells in a Mouse Model. Inflammation 2021, 44, 956–964. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Salinas, I.; Fender, E.; Sengelaub, D.R.; Wellman, C.L. Gonadal hormones differentially regulate sex-specific stress effects on glia in the medial prefrontal cortex. J. Neuroendocrinol. 2019, 31, e12762. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Dempsey, R.E.; Roodsari, S.K.; Shuboni-Mulligan, D.D.; George, O.; Sanford, L.D.; Guo, M.-L. Cocaine Regulates NLRP3 Inflammasome Activity and CRF Signaling in a Region- and Sex-Dependent Manner in Rat Brain. Biomedicines 2023, 11, 1800. https://doi.org/10.3390/biomedicines11071800
Cheng Y, Dempsey RE, Roodsari SK, Shuboni-Mulligan DD, George O, Sanford LD, Guo M-L. Cocaine Regulates NLRP3 Inflammasome Activity and CRF Signaling in a Region- and Sex-Dependent Manner in Rat Brain. Biomedicines. 2023; 11(7):1800. https://doi.org/10.3390/biomedicines11071800
Chicago/Turabian StyleCheng, Yan, Rachael Elizabeth Dempsey, Soheil Kazemi Roodsari, Dorela D. Shuboni-Mulligan, Olivier George, Larry D. Sanford, and Ming-Lei Guo. 2023. "Cocaine Regulates NLRP3 Inflammasome Activity and CRF Signaling in a Region- and Sex-Dependent Manner in Rat Brain" Biomedicines 11, no. 7: 1800. https://doi.org/10.3390/biomedicines11071800
APA StyleCheng, Y., Dempsey, R. E., Roodsari, S. K., Shuboni-Mulligan, D. D., George, O., Sanford, L. D., & Guo, M.-L. (2023). Cocaine Regulates NLRP3 Inflammasome Activity and CRF Signaling in a Region- and Sex-Dependent Manner in Rat Brain. Biomedicines, 11(7), 1800. https://doi.org/10.3390/biomedicines11071800






