Association of Blood Levels of Vitamin D and Its Binding Protein with Clinical Phenotypes of Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Biochemical Analysis
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Study Population
3.2. Clinical Features
3.3. Serum 25(OH)D and DBP Levels
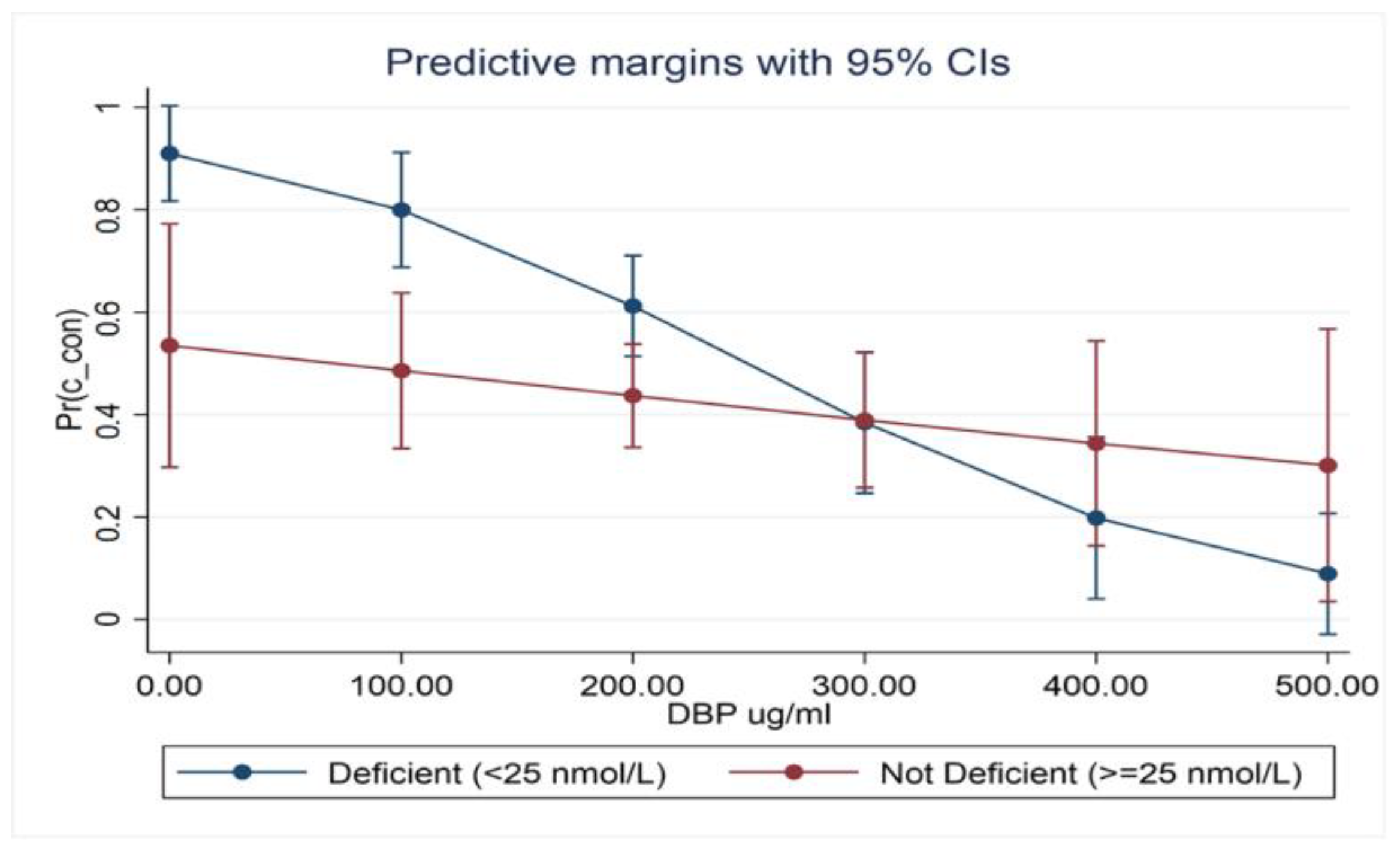
- There was an interaction between 25(OH)D and DBP on MS status in severely deficient (25(OH)D < 25 nmol/L) newly diagnosed (drug-naïve) patients.
- Low serum 25(OH)D with normal DBP had no significant impact on MS status (OR 1.02; 0.54–1.92; p = 0.994); low serum DBP showed a better, albeit non-significant, interaction (OR 1.56; 0.87–2.82; p = 0.139).
- The low 25(OH)D and DBP combination demonstrated a significant association with MS (OR 2.67; 1.35–5.29; p = 0.005).
- Serum 25(OH)D was within strata of DBP and appeared to be more strongly associated with MS when DBP levels were reduced (OR 1.71; 0.86–3.40, p = 126). This relationship is best demonstrated in the marginal probabilities diagram (Figure 1).
3.4. Vitamin D and DBP Levels in Different MS Clinical Phenotypes
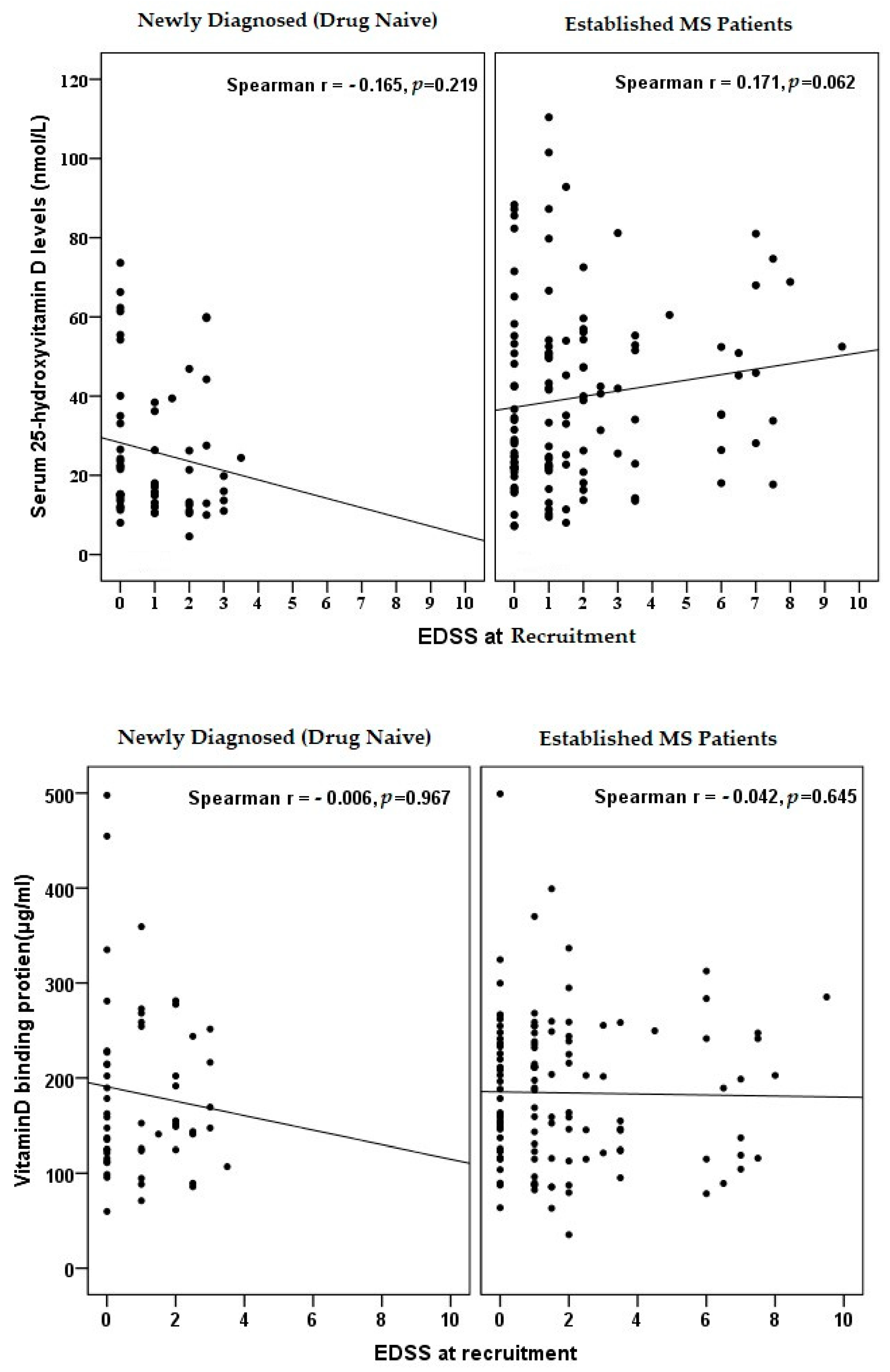
3.5. Correlation of Vitamin D and DBP with Disease Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshinker, B.G. Medical progress: Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef]
- Simon, K.C.; Munger, K.I.; Yang, X.; Ascherio, A. Polymorphism in vitamin D metabolism related genes and risk of multiple sclerosis. Mult. Scler. 2010, 16, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson Jr., S.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Pittas, F.; Tremlett, H.; Dwyer, T.; Gies, P.; van der Mei, I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann. Neurol. 2010, 68, 193–203. [Google Scholar] [CrossRef]
- Van der Mei, I.A.; Ponsonby, A.L.; Dwyer, T.; Blizzard, L.; Taylor, B.V.; Kilpatrick, T.; Butzkueven, H.; McMichael, A.J. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J. Neurol. 2007, 254, 581–590. [Google Scholar] [CrossRef]
- Mowry, E.M.; Waubant, E.; McCulloch, C.E.; Okuda, D.T.; Evangelista, A.A.; Lincoln, R.R.; Gourraud, P.A.; Brenneman, D.; Owen, M.C.; Qualley, P.; et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann. Neurol. 2012, 72, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.; Cook, N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol. Metab. 2000, 11, 320–327. [Google Scholar] [CrossRef]
- Chun, R.F. New perspectives on vitamin D binding protein. Cell Biochem. Func. 2012, 30, 445–456. [Google Scholar] [CrossRef]
- Qin, Z.; Qin, Y.; Liu, S. Alteration of DBP levels in CSF of patients with MS by proteomics analysis. Cell. Mol. Neurobiol. 2009, 29, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bai, S.; Qin, Z.; Yang, Y.; Cui, Y.; Qin, Y. Quantitative proteomic analysis of the cerebrospinal fluid of patients with multiple sclerosis. J. Cell. Mol. Med. 2009, 13, 1586–1603. [Google Scholar] [CrossRef] [Green Version]
- Niino, M.; Fukazawa, T.; Miyazaki, Y.; Minami, N.; Tashiro, J.; Amino, I.; Nonaka, T.; Fujiki, N.; Doi, S.; Kikuchi, S. Association of Vitamin D levels in Japanese patients with Multiple Sclerosis. Clin. Exp. Neuroimmunol. 2013, 4, 193–200. [Google Scholar] [CrossRef]
- Grut, V.; Biström, M.; Salzer, J.; Stridh, P.; Lindam, A.; Alonso-Magdalena, L.; Andersen, O.; Jons, D.; Gunnarsson, M.; Vrethem, M.; et al. Free vitamin D3 index and vitamin D-binding protein in multiple sclerosis: A presymptomatic case-control study. Eur. J. Neurol. 2022, 29, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Omidifar, A.; Varzandi, T.; Salehnezhad, T.; Sahraian, M.A. Reduction in circulating vitamin D binding protein in patients with multiple sclerosis. BMC Neurol. 2021, 21, 168. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qin, Z.; Zhu, Y.; Li, Y.; Qin, Y.; Jing, Y.; Liu, S. Vitamin D-binding protein in cerebrospinal fluid is associated with multiple sclerosis progression. Mol. Neurobiol. 2013, 47, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Damoiseaux, J.; Hupperts, R. Circulating vitamin D binding protein levels are not associated with relapses or with vitamin D status in multiple sclerosis. Mult. Scler. 2014, 20, 433–437. [Google Scholar] [CrossRef]
- Aktürk, T.; Turan, Y.; Tanik, N.; Karadağ, M.E.; Sacmaci, H.; Inan, L.E. Vitamin D, vitamin D binding protein, vitamin D receptor levels and cardiac dysautonomia in patients with multiple sclerosis: A cross-sectional study. Arq Neuropsiquiatr. 2019, 77, 848–854. [Google Scholar] [CrossRef]
- Rinaldi, A.O.; Sanseverino, I.; Purificato, C.; Cortese, A.; Mechelli, R.; Francisci, S.; Salvetti, M.; Millefiorini, E.; Gessani, S.; Gauzzi, M.C. Increased circulating levels of vitamin D binding protein in MS patients. Toxins 2015, 7, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacholder, S.; Silverman, D.T.; McLaughlin, J.K.; Mandel, J.S. Selection of controls in case-control studies. III. Design options. Am. J. Epidemiol. 1992, 135, 1042–1050. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis. Revision to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the healthy european paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Al-Shammri, S.N.; Hanna, M.G.; Chattopadhyay, A.; Akanji, A.O. Sociocultural and Demographic Risk Factors for the Development of Multiple Sclerosis in Kuwait: A Case-Control Study. PLoS ONE 2015, 10, e0132106. [Google Scholar] [CrossRef] [Green Version]
- Al-Din, A.S.N.; Khogali, M.; Poser, C.M.; Al-Nassar, K.E.; Shakir, R.; Hussain, J.; Behbahani, K.; Chadha, G. Epidemiology of multiple sclerosis in Arabs in Kuwait—a comparative study between Kuwaitis and Palestinians. J. Neurol. Sci. 1990, 100, 137–141. [Google Scholar] [CrossRef]
- Al Shubaili, A.F.; Alramzy, K.; Ayyad, Y.M.; Gerish, Y. Epidemiology of multiple sclerosis in Kuwait: New trends in incidence and prevalence. Eur. Neurol. 2005, 53, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Alroughani, R.; Ahmed, S.; Behbahani, R.; Khan, R.; Thussu, A.; Alexander, K.J.; Ashkanani, A.; Nagarajan, V.; Al-Hashel, J. Inreasing prevalence and incidence rates of multiple sclerosis in Kuwait. Mult. Scler. 2014, 20, 543–547. [Google Scholar] [CrossRef]
- Kurtzke, J.F. MS epidemiology worldwide. One view of current status. Acta Neurol. Scand. 1995, 91 (Suppl. 161), 23–33. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Stolzenberg-Solomon, R.Z.; Kopp, W.; Rager, H.; Virtamo, J.; Albanes, D. Impact of Circulating Vitamin D Binding Protein Levels on the Association between 25-Hydroxyvitamin D and Pancreatic Cancer Risk: A Nested Case–Control Study. Cancer Res. 2012, 72, 1190–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D (3). Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef] [Green Version]
- Gauzzi, M.C. Vitamin D-binding protein and multiple sclerosis: Evidence, controversies, and needs. Mult. Scler. 2018, 24, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Lehmensiek, V.; Sussmuth, S.D.; Tauscher, G.; Brettschneider, J.; Felk, S.; Gillardon, F.; Tumani, H. Cerebrospinal fluid proteome profile in multiple sclerosis. Mult. Scler. 2007, 13, 840–849. [Google Scholar] [CrossRef] [PubMed]

| Controls (n) | Patients with MS (n) | p * | p $ | p # | |||
|---|---|---|---|---|---|---|---|
| All | Established Patients | Newly Diagnosed (Drug-Naïve) Patients | |||||
| Number (n) | 146 | 195 | 134 | 61 | |||
| Age (in years) mean (SD) | 33.8 (9.5) | 33.2 (10.4) | 34.4 (11.0) | 30.8 (8.3) | 0.589 b | 0.665 b | 0.029 b |
| Gender | 0.526 a | 0.672 a | 0.466 a | ||||
| Male | 52 (35.6) | 76 (39.0) | 51 (38.1) | 25 (41.0) | |||
| Female | 94 (64.4) | 119 (61.0) | 83 (61.9) | 36 (59.0) | |||
| BMI (kg/m2) | 28.7 (10) | 28.6 (12.5) | 28.8 (11.5) | 28.2 (10.5) | 0.359 c | 0.241 c | 0.933 c |
| 25–29.9 (overweight) | 50 (36.8) | 59 (31.9) | 39 (30.5) | 20 (35.1) | |||
| >30 (Obese) | 49 (36.0) | 62 (33.5) | 42 (32.8) | 20 (35.1) | |||
| Daily direct sunlight exposure (minutes) | <0.001 a | <0.001 a | 0.008 a | ||||
| <10 | 36 (26.1) | 62 (31.8) | 37 (27.6) | 25 (41.0) | |||
| 10–15 | 32 (23.2) | 93 (47.7) | 72 (53.7) | 21 (34.4) | |||
| 15–30 | 35 (25.4) | 25 (12.8) | 17 (12.7) | 8 (13.1) | |||
| >30 | 35 (23.4) | 15 (7.7) | 8 (6.0) | 7 (11.5) | |||
| Mode of dressing | 0.654 a | 0.980 a | 0.026 a | ||||
| Traditional/Western (Exposed Face/Arms | 112 (83.0) | 158 (81.0) | 116 (86.6) | 42 (68.9) | |||
| Fully covered eyes/hands Exposed | 23 (17.0) | 37 (19.0) | 18 (13.4) | 19 (31.1) | |||
| MS Subtype | |||||||
| Relapsing–remitting (n, %) | - | 166 (85.2) | 105 (78.4) | 61 (100) | |||
| Secondary progressive (n, %) | - | 26 (13.3) | 26 (19.4) | - | |||
| Primary progressive (n, %) | - | 3 (1.5) | 3 (2.2) | - | |||
| Age at recruitment year (median *) | - | 32 (27–38) | 33 (27–41) | 31 (26–36) | |||
| Age at diagnosis, year (median *) | - | 28 (21–34) | 27 (20–33) | 31 (26–36) | |||
| Age at onset, year, (median *) | - | 27 (20–32) | 26 (19–32) | 29 (22–33) | |||
| Duration of disease from onset of symptoms, year (median *) | - | 3 (1–8) | 5 (2–10) | 2 (1–7) | |||
| EDSS score (median *) | - | 1.5 (0–2.5) | 1.5 (0–3) | 1 (0–2) | |||
| Annualised relapse rate (median *) | 3 (1–5) | 4 (2–7) | 0.1 (0.1–0.2) | ||||
| Controls (n) | Patients with MS (n) | p a | p b | p c | p d | |||
|---|---|---|---|---|---|---|---|---|
| All | Established MS | Newly Diagnosed (Drug-Naïve) | ||||||
| n | 146 | 195 | 134 | 61 | ||||
| 25(OH)D (nmol/L) | 28.4 (18.0–50.4) | 27.3 (17.0–50.8) | 34.5 (22.1–52.7) | 18.9 (13.0–35.9) | 0.82 | 0.081 | 0.001 | <0.001 |
| DBP (µg/mL) | 236 (152–288) | 163 (123–241) | 183 (123–241) | 152 (117–235) | <0.001 | <0.001 | <0.001 | 0.401 |
| Healthy Controls | Relapsing-Remitting | p a | Secondary Progressive | p b | p c | |
|---|---|---|---|---|---|---|
| All Subjects (n) | 146 | 166 | 26 | |||
| Subjects on Vitamin D supplement | 33 | 60 | 16 | |||
| 25(OH)D (nmol/L) | 28.4 (18.0–50.4) | 25.7 (16.0–47.8) | 0.387 | 45.5 (22.0–59.2) | 0.046 | 0.025 |
| DBP (µg/mL) | 236 (152–288) | 162 (123–239) | <0.001 | 194 (127–246) | 0.049 | 0.508 |
| Subjects not on Vitamin D supplement | n = 112 | n = 101 | n = 10 | |||
| 25(OH)D (nmol/L) | 26.2 (16.7–35.0) | 22.1 (13.0–36.3) | 0.051 | 35.4 (19.4–64.7) | 0.056 | 0.029 |
| DBP (µg/mL) | 246 (161–299) | 159 (120–248) | <0.001 | 247 (222–271) | 0.801 | 0.011 |
| Controls | MS Relapse | p a | MS Remission | p b | p c | |
|---|---|---|---|---|---|---|
| N | 146 | 36 | 25 | |||
| 25(OH)D (nmol/L) | 25.4 (16.3–33.5) | 16.0 (12.5–26.5) | 0.028 | 22.5 (14.1–47.2) | 0.198 | 0.058 |
| DBP (µg/mL) | 247 (165–303) | 155 (113–244) | <0.001 | 151 (118–233) | 0.001 | 0.318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shammri, S.; Chattopadhyay, A.; Hanah, M.G.; Doi, S.; Akanji, A. Association of Blood Levels of Vitamin D and Its Binding Protein with Clinical Phenotypes of Multiple Sclerosis. Biomedicines 2023, 11, 1808. https://doi.org/10.3390/biomedicines11071808
Al-Shammri S, Chattopadhyay A, Hanah MG, Doi S, Akanji A. Association of Blood Levels of Vitamin D and Its Binding Protein with Clinical Phenotypes of Multiple Sclerosis. Biomedicines. 2023; 11(7):1808. https://doi.org/10.3390/biomedicines11071808
Chicago/Turabian StyleAl-Shammri, Suhail, Arpita Chattopadhyay, Magdy Girgis Hanah, Suhail Doi, and Abayomi Akanji. 2023. "Association of Blood Levels of Vitamin D and Its Binding Protein with Clinical Phenotypes of Multiple Sclerosis" Biomedicines 11, no. 7: 1808. https://doi.org/10.3390/biomedicines11071808
APA StyleAl-Shammri, S., Chattopadhyay, A., Hanah, M. G., Doi, S., & Akanji, A. (2023). Association of Blood Levels of Vitamin D and Its Binding Protein with Clinical Phenotypes of Multiple Sclerosis. Biomedicines, 11(7), 1808. https://doi.org/10.3390/biomedicines11071808






