Pharmacological Proposal Approach to Managing Chronic Pain Associated with COVID-19
Abstract
1. Introduction
2. Chronic Pain Associated with COVID-19
- Painful manifestations associated with vaccines.
- Pain in the acute phase of the disease and in palliative care.
- Chronic Post-COVID Pain (CPCoP).
- The impact of the pandemic on the chronic pain population.
2.1. Painful Manifestations Associated with COVID-19 Vaccines
2.2. Pain in the Acute Phase of the Disease and in Palliative Care
2.3. Chronic Post-COVID Pain (CPCoP)
2.4. Impact of the Pandemic on the Chronic Pain Population
3. Discussion
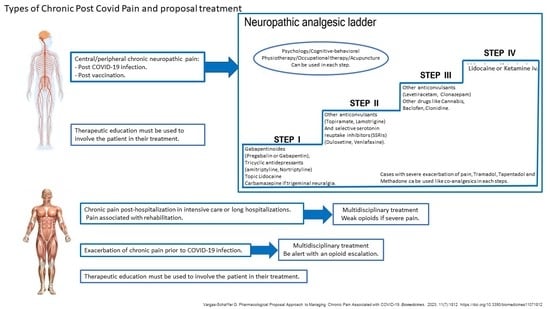
Treatment of Chronic Pain Associated with COVID-19
- The importance of taking the medication regularly and as directed.
- Dose increases should be made gradually to avoid adverse reactions.
- The possibility of successive trials if the effect of co-analgesics is unsuccessful.
- The probability of having to take more than one drug (multimodality).
- The prolonged use of these molecules in the case of improvement.
4. Future Directions
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 11 November 2021).
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. 2021. Available online: https://www.who.int/publications/i/item/WHO/2019-nCoV/Post_COVID-19_condition/Clinical_case_definition/2021.1 (accessed on 20 May 2023).
- Mandal, S.; Barnett, J.; E Brill, S.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; E Hillman, T.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2020, 76, 396–398. [Google Scholar] [CrossRef]
- Yelin, D.; Wirtheim, E.; Vetter, P.; Kalil, A.C.; Bruchfeld, J.; Runold, M.; Guaraldi, G.; Mussini, C.; Gudiol, C.; Pujol, M.; et al. Long-term consequences of COVID-19: Research needs. Lancet Infect. Dis. 2020, 20, P1115–P1117. [Google Scholar] [CrossRef]
- Davido, B.; Seang, S.; Tubinan, R.; de Truchis, P. Post COVID-19 chronic symptoms: A postinfectious entity. Clin. Microbiol. Infect. 2020, 26, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression, and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A’court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Global Burden of Disease Long COVID Collaborators. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- The PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med. 2022, 10, P761–P775. [Google Scholar] [CrossRef] [PubMed]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xiong, W.; Mu, J.; Zhang, Q.; Zhang, H.; Zou, L.; Li, W.; He, L.; Sander, J.W.; Zhou, D. The potential neurological effect of the COVID-19 vaccines: A review. Acta Neurol. Scand. 2021, 144, 3–12. [Google Scholar] [CrossRef]
- Narasimhalu, K.; Lee, W.C.; Salkade, P.R.; De Silva, D.A. Trigeminal and cervical radiculitis after tozinameran vaccination against COVID-19. BMJ Case Rep. 2021, 14, e242344. [Google Scholar] [CrossRef] [PubMed]
- Dionne, A.; Sperotto, F.; Chamberlain, S.; Baker, A.L.; Powell, A.J.; Prakash, A.; Castellanos, D.A.; Saleeb, S.F.; de Ferranti, S.D.; Newburger, J.W.; et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021, 6, 1446–1450. [Google Scholar] [CrossRef]
- Finsterer, J. SARS-CoV-2 vaccinations are unsafe for those experiencing post-vaccination Guillain-Barre syndrome. Ann. Med. Surg. 2021, 68, 102584. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Gelbenegger, G.; Cacioppo, F.; Firbas, C.; Jilma, B. Rhabdomyolysis following Ad26.COV2. S COVID-19 vaccination. Vaccines 2021, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cai, Y.; Chen, Y.; Williams, A.P.; Gao, Y.; Zeng, J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines 2021, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, D.; Bottaccioli, A.G.; Bottaccioli, F. Letter to the editor: Kim, S.-W., Su, K.-P. Using psychoneuroimmunity against COVID-19. Brain Behav. Immun. 2020, 87, 170–171. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334, Erratum in: Ther. Deliv. 2016, 7, 411. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front. Immunol. 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Micallef, J.; Soeiro, T.; Jonville-Béra, A.P. French society of pharmacology, therapeutics (SFPT). Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie 2020, 75, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.L.; Samet, J.; Franz, C.K.; Hsieh, C.; Nguyen, C.V.; Horbinski, C.; Deshmukh, S. Musculoskeletal involvement of COVID-19: Review of imaging. Skelet. Radiol. 2021, 50, 1763–1773. [Google Scholar] [CrossRef]
- European Medicines Association. EMA Gives Advice on the Use of Non-Steroidal Anti-Inflammatories for COVID-19. 2020. Available online: https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19 (accessed on 20 May 2023).
- DrDrożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Majewski, P.; Rotter, I.; Kotfis, K. COVID-19: Pain management in patients with SARS-CoV-2 infection—Molecular mechanisms, challenges, and perspectives. Brain Sci. 2020, 10, 465. [Google Scholar] [CrossRef]
- Madhok, J.; Mihm, F.G. Rethinking sedation during prolonged mechanical ventilation for COVID-19 respiratory failure. Anesth. Analg. 2020, 131, e123–e124. [Google Scholar] [CrossRef] [PubMed]
- Hanidziar, D.; Bittner, E. Sedation of mechanically ventilated COVID-19 patients: Challenges and special considerations. Anesth. Analg. 2020, 131, e40–e41. [Google Scholar] [CrossRef]
- Janssen, D.J.A.; Ekström, M.; Currow, D.C.; Johnson, M.J.; Maddocks, M.; Simonds, A.K.; Tonia, T.; Marsaa, K. COVID-19: Guidance on palliative care from a European Respiratory Society international task force. Eur. Respir. J. 2020, 56, 2002583. [Google Scholar] [CrossRef]
- Janssen, D.J.A. Palliative care in COVID-19. Curr. Opin. Support. Palliat. Care 2021, 15, 199–204. [Google Scholar] [CrossRef]
- Scherer, J.S.; Qian, Y.; Rau, M.E.; Soomro, Q.H.; Sullivan, R.; Linton, J.; Zhong, J.; Chodosh, J.; Charytan, D.M. Utilization of palliative care for patients with COVID-19 and acute kidney injury during a COVID-19 surge. Clin. J. Am. Soc. Nephrol. 2022, 17, 342–349. [Google Scholar] [CrossRef]
- Brankovic, M.; Jeon, H.; Markovic, N.; Choi, C.; Adam, S.; Ampey, M.; Pergament, K.; Chyn, E.T.Y. Palliative care of COVID-19 patients with do-not-resuscitate status in underrepresented minorities. Eur. J. Clin. Investig. 2022, 53, e13889. [Google Scholar] [CrossRef]
- Hasson, F.; Slater, P.; Fee, A.; McConnell, T.; Payne, S.; Finlay, D.-A.; McIlfatrick, S. The impact of COVID-19 on out-of-hours adult hospice care: An online survey. BMC Palliat. Care 2022, 21, 94. [Google Scholar] [CrossRef]
- Giraldo, G.S.P.; Ali, S.T.; Kang, A.K.; Patel, T.R.; Budhiraja, S.; Gaelen, J.I.; Lank, G.K.; Clark, J.R.; Mukherjee, S.; Singer, T.; et al. Neurologic manifestations of long COVID differ based on acute COVID-19 severity. Ann. Neurol 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular presentations in patients with COVID-19. Neurol. Sci. 2020, 41, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.M.; Su, X.; Wang, X.Q. Pain Symptoms in Patients with Coronavirus Disease (COVID-19): A Literature Review. J. Pain Res. 2021, 14, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Beydon, M.; Chevalier, K.; Al Tabaa, O.; Hamroun, S.; Delettre, A.-S.; Thomas, M.; Herrou, J.; Riviere, E.; Mariette, X. Myositis as a manifestation of SARS-CoV-2. Ann. Rheum. Dis. 2020, 80, e42. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef]
- Yapici-Eser, H.; Koroglu, Y.E.; Oztop-Cakmak, O.; Keskin, O.; Gursoy, A.; Gursoy-Ozdemir, Y. Neuropsychiatric symptoms of COVID-19 explained by SARS-CoV-2 proteins’ mimicry of human protein interactions. Front. Hum. Neurosci. 2021, 15, 656313. [Google Scholar] [CrossRef] [PubMed]
- Vasilevska, V.; Guest, P.C.; Bernstein, H.-G.; Schroeter, M.L.; Geis, C.; Steiner, J. Molecular mimicry of NMDA receptors may contribute to neuropsychiatric symptoms in severe COVID-19 cases. J. Neuroinflamm. 2021, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, T.; Crewther, S. Post-COVID 19 Neurological Syndrome (PCNS); A novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2021, 419, 117179. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome. ERJ Open Res. 2020, 6, 00542–02020. [Google Scholar] [CrossRef]
- Chatkoff, D.K.; Leonard, M.T.; Najdi, R.R.; Cruga, B.; Forsythe, A.; Bourgeau, C.; Easton, H. A brief survey of the COVID-19 pandemic’s impact on the chronic pain experience. Pain Manag. Nurs. 2022, 23, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Choinière, M.; Pagé, M.G.; Lacasse, A.; Dassieu, L.; Thompson, J.M.; Janelle-Montcalm, A.; Dorais, M.; Nguefackb, H.L.N.; Hudspith, M.; Moor, G.; et al. Impact of the COVID-19 pandemic on Canadian Armed Forces Veterans who live with chronic pain. JMVFH 2021, 7, 92–105. [Google Scholar] [CrossRef]
- Dassieu, L.; Pagé, M.G.; Lacasse, A.; Laflamme, M.; Perron, V.; Janelle-Montcalm, A.; Hudspith, M.; Moor, G.; Sutton, K.; Thompson, J.M.; et al. Chronic pain experience and health inequities during the COVID-19 pandemic in Canada: Qualitative findings from the chronic pain & COVID-19 pan-Canadian study. Int. J. Equity Health 2021, 20, 147. [Google Scholar]
- El-Tallawy, S.N.; Nalamasu, R.; Pergolizzi, J.V.; Gharibo, C. Pain management during the COVID-19 pandemic. Pain Ther. 2020, 9, 453–466. [Google Scholar] [CrossRef]
- Correia, J.C.; Waqas, A.; Aujoulat, I.; Davies, M.J.; Assal, J.-P.; Golay, A.; Pataky, Z. Evolution of therapeutic patient education: A systematic scoping review and scientometric analysis. Int. J. Environ. Res. Public Health 2022, 19, 6128. [Google Scholar] [CrossRef]
- Vargas-Schaffer, G.; Cogan, J. Patient therapeutic education: Placing the patient at the centre of the WHO analgesic ladder. Can. Fam. Physician 2014, 60, 235–241. [Google Scholar]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Shinu, P.; Morsy, M.A.; Nair, A.B.; Al Mouslem, A.K.; Venugopala, K.N.; Goyal, M.; Bansal, M.; Jacob, S.; Deb, P.K. Novel therapies for the treatment of neuropathic pain: Potential and pitfalls. J. Clin. Med. 2022, 11, 3002. [Google Scholar] [CrossRef]
- Balanaser, M.; Carley, M.; Baron, R.; Finnerup, N.B.; Moore, R.A.; Rowbotham, M.C.; Chaparro, L.E.; Gilron, I. Combination pharmacotherapy for the treatment of neuropathic pain in adults: Systematic review and meta-analysis. Pain 2023, 164, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019, 20 (Suppl. S1), S2–S12. [Google Scholar] [CrossRef]
- Vargas-Schaffer, G.; Steverman, A.; Potvin, V. Monitoring pharmacological treatment in patients with chronic noncancer pain. Cureus 2021, 13, e20358. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Kalso, E.A. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014, 4, CD005451. [Google Scholar] [CrossRef]
- Birse, F.; Derry, S.; Moore, R.A. Phenytoin for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 2019, CD009485. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.; Derry, S.; Wiffen, P.J.; Moore, R.A. Clonazepam for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 2019, CD009486. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Lunn, M.P.T. Levetiracetam for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014, 7, CD010943. [Google Scholar] [CrossRef]
- Moore, R.A.; Wiffen, P.J.; Derry, S.; Toelle, T.; Rice, A.S. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014, 4, CD007938. [Google Scholar]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst. Rev. 2019, 1, CD007076. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Lamotrigine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2013, 2019, CD006044. [Google Scholar] [CrossRef]
- Chaparro, L.E.; Wiffen, P.J.; Moore, R.A.; Gilron, I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, 2020, CD008943. [Google Scholar] [CrossRef]
- Pereira, J.E.G.; Pereira, L.F.G.; Linhares, R.M.; Bersot, C.D.A.; Aslanidis, T.; Ashmawi, H.A. Efficacy and safety of ketamine in the treatment of neuropathic pain: A systematic review and meta-analysis of randomized controlled trials. J. Pain Res. 2022, 15, 1011–1037. [Google Scholar] [CrossRef]
- Van Velzen, M.; Dahan, J.D.; van Dorp, E.L.; Mogil, J.S.; Hooijmans, C.R.; Dahan, A. Efficacy of ketamine in relieving neuropathic pain: A systematic review and meta-analysis of animal studies. Pain 2021, 162, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Wien, P.J.; Moore, R.A.; Quinlan, J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014, 2014, CD010958. [Google Scholar] [CrossRef]
- Serednicki, W.T.; Wrzosek, A.; Woron, J.; Garlicki, J.; Dobrogowski, J.; Jakowicka-Wordliczek, J.; Wordliczek, J.; Zajaczkowska, R. Topical clonidine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2022, 2022, CD010967. [Google Scholar] [CrossRef]
- Kumar, A.; Maitra, S.; Khanna, P.; Baidya, D.K. Clonidine for management of chronic pain: A brief review of the current evidence. Saudi J. Anaesth. 2014, 8, 92–96. [Google Scholar] [CrossRef]
- Derry, S.; Rice, A.S.C.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 2021, CD007393. [Google Scholar] [CrossRef]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 2020, CD012182. [Google Scholar] [CrossRef]
- Duehmke, R.M.; Hollingshead, J.; Cornblath, D.R. Tramadol for neuropathic pain. Cochrane Database Syst. Rev. 2006, 3, CD003726. [Google Scholar] [CrossRef]
- Gaskell, H.; Derry, S.; Stannard, C.; Moore, R.A. Oxycodone for neuropathic pain in adults. Cochrane Database Syst. Rev. 2016, 2016, CD010692. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 depressive symptoms: Epidemiology, pathophysiology, and pharmacological treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Benros, M.E.; Klein, R.S.; Vinkers, C.H. How COVID-19 shaped mental health: From infection to pandemic effects. Nat. Med. 2022, 28, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhanizadeh, A.; Nikbakht, F. Investigating the potential mechanisms of depression induced-by COVID-19 infection in patients. J. Clin. Neurosci. 2021, 91, 283–287. [Google Scholar] [CrossRef]
- Ricci, L.; Villegente, J.; Loyal, D.; Ayav, C.; Kivits, J.; Rat, A. Tailored patient therapeutic educational interventions: A patient-centred communication model. Health Expect. 2022, 25, 276–289. [Google Scholar] [CrossRef]
- Bombard, Y.; Baker, G.R.; Orlando, E.; Fancott, C.; Bhatia, P.; Casalino, S.; Onate, K.; Denis, J.-L.; Pomey, M.-P. Engaging patients to improve quality of care: A systematic review. Implementation Sci. 2018, 13, 98. [Google Scholar] [CrossRef]
- Pantelic, M.; Ziauddeen, N.; Boyes, M.; O’hara, M.E.; Hastie, C.; Alwan, N.A. Long COVID stigma: Estimating burden and validating scale in a UK-based sample. PLoS ONE 2022, 17, e0277317. [Google Scholar] [CrossRef] [PubMed]
- Van de Vyver, J.; Leite, A.C.; Alwan, N.A. Navigating the social identity of long covid. BMJ 2021, 375, n2933. [Google Scholar] [CrossRef]
- FitzGerald, C.; Hurst, S. Implicit bias in healthcare professionals: A systematic review. BMC Med. Ethics 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Mai, H.; Kapoor, S.; Puelles, V.; Czogalla, J.; Schaedler, J.; Vering, J.; Delbridge, C.; Steinke, H.; Frenzel, H.; et al. SARS-CoV-2 spike protein accumulation in the skull-meninges-brain axis: Potential implications for long-term neurological complications in post-COVID-19. bioRxiv 2023. [Google Scholar] [CrossRef]
- Krasemann, S.; Dittmayer, C.; von Stillfried, S.; Meinhardt, J.; Heinrich, F.; Hartmann, K.; Pfefferle, S.; Thies, E.; von Manitius, R.; Aschman, T.A.D.; et al. Assessing and improving the validity of COVID-19 autopsy studies—A multicentre approach to establish essential standards for immunohistochemical and ultrastructural analyses. eBioMedicine 2022, 83, 104193. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Bosworth, M.; King, S.; Pouwels, K.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of long COVID in people infected with SARS-CoV-2 after two doses of a COVID-19 vaccine: Community-based, matched cohort study. medRxiv 2022. preprint. [Google Scholar] [CrossRef]




| Alteration of cranial nerves and peripheral nerves. | Craneal nerf dysfunction. Polyradiculitis/Polyneuropathy. Anosmia. Dysgeusia. Tinnitus. Sensorineural hearing loss. Blurred vision. |
| Movement disorders | Parkinsonism. Status epilepticus. Cerebellar dysfunction. Nonspecific movement disorder. Gait disturbances. Seizure. |
| Other neurological disorders | Encephalitis/Encephalopathy. Ischemic or hemorrhagic Stroke. Headache. Dysautonomia. Fatigue. |
| Cognitive and psychological disturbances | Brain fog. Short-term memory deficit. Attention deficit. Depression and/or anxiety. Post-traumatic stress disorder. Obsessive-compulsive symptomatology. |
| Anticonvulsant | Classification | Mechanism of Action | Recommended Doses in Monotherapy | Maximum Doses |
|---|---|---|---|---|
| Gabapentin Pregabalin | Ion channel modulators (reduce neuronal excitability) | Calcium ion channels. Decreasing the density of pre-synaptic voltage-gated calcium channels and subsequent release of excitatory neurotransmitter | 300 mg/day 150 mg/day | 3600 mg/day 600 mg/day |
| Topiramate | Multiple mechanisms of action. increasing GABA activity and inhibiting glutamate activity, topiramate blocks neuronal excitability | Canales de iones sódicos receptores GABAA, NMDA AMPA/kainato | 100–200 mg/day | 1000 mg/day |
| Carbamazepine Oxcarbazepine Lamotrigine Phenytoin | Ion channel modulators (reduce neuronal excitability) | Sodium ion channels Selectively binds and inhibits voltage-gated sodium channels, stabilizing presynaptic neuronal membranes and inhibiting presynaptic glutamate and aspartate release. Stabilizing the inactive state of the sodium channel and prolonging the neuronal refractory period | 100–200 mg/day 600 mg/day 100–200 mg/day 200–400 mg/day | 1200 mg/day 2400 mg/day 600–700 mg/day 500–600 mg/day |
| Levetiracetam | Modulators of the presynaptic junction | Modulation of synaptic neurotransmitter release through binding to the synaptic vesicle protein SV2A in the brain | 250 mg/day | 1500 mg/day |
| Clonazepam Diazepam | GABAergic transmission enhancers (potentiate inhibitory neurotransmission) | GABA-A receptors action. | 0.5–1 mg/day 5–10 mg/day | 2–4 mg/day 15–20 mg/day |
| DRUGS | NNT 50% Decrease in Pain Intensity | Pathologies Where They Are Most Used |
|---|---|---|
| Carbamazepine | 1.7 | Trigeminal neuralgia |
| Phenytoin | 2.1 | Refractory NP |
| Clonazepam | 4 | Refractory NP |
| Topiramate | 5.29 | Headaches |
| Levetiracetam | 4–10 | Refractory NP |
| Gabapentin | 6 8 | Diabetic neuropathy Post herpetic neuralgia and others NP |
| Pregabaline | 3.8–6.9 8.2 5.9 11.1 | Diabetic neuropathy Post herpetic neuralgia and others NP |
| Lamotrigine | 8.3 | Central NP |
| Other drugs used to treat NP | ||
| Baclofen | 1.4 (2–2.5) | Atypical trigeminal neuralgia Refractory NP |
| Ketamine | 3–5 >10 | Refractory NP Depression |
| Lidocaine | 4–10 | Topical application Post herpetic neuralgia. HIV neuropathy Cancer-related neuropathy Refractory NP |
| Clonidine | 4–10 | Diabetic neuropathy Post herpetic neuralgia. HIV neuropathy Cancer-related neuropathy Refractory NP |
| Capsaicin | 8.8 (10–12) | Topical application Peripheral neuropathic pain Post herpetic neuralgia. HIV neuropathy Refractory NP |
| Cannabis | NNTB (30–50) 20–25 | Low quality studies |
| Opioids | ||
| Tramadol and Tapentadol | 4.4 | NP |
| Metadona | 0 | Very limited data for NP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Schaffer, G. Pharmacological Proposal Approach to Managing Chronic Pain Associated with COVID-19. Biomedicines 2023, 11, 1812. https://doi.org/10.3390/biomedicines11071812
Vargas-Schaffer G. Pharmacological Proposal Approach to Managing Chronic Pain Associated with COVID-19. Biomedicines. 2023; 11(7):1812. https://doi.org/10.3390/biomedicines11071812
Chicago/Turabian StyleVargas-Schaffer, Grisell. 2023. "Pharmacological Proposal Approach to Managing Chronic Pain Associated with COVID-19" Biomedicines 11, no. 7: 1812. https://doi.org/10.3390/biomedicines11071812
APA StyleVargas-Schaffer, G. (2023). Pharmacological Proposal Approach to Managing Chronic Pain Associated with COVID-19. Biomedicines, 11(7), 1812. https://doi.org/10.3390/biomedicines11071812