An Updated Review on Monkeypox Viral Disease: Emphasis on Genomic Diversity
Abstract
1. Introduction
Genetic Variability
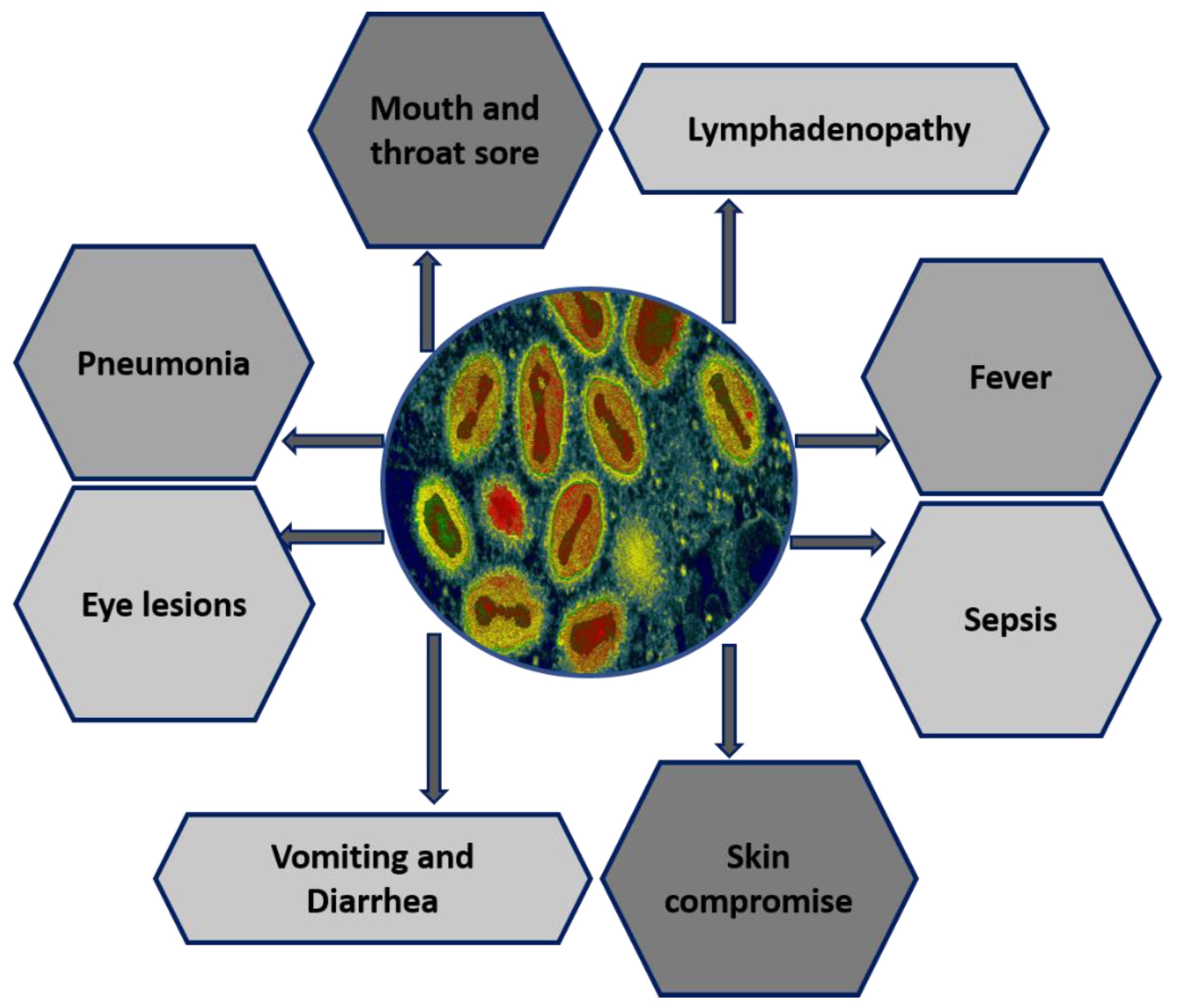
2. Geographical Range and Progressive Epidemiology of MPXV
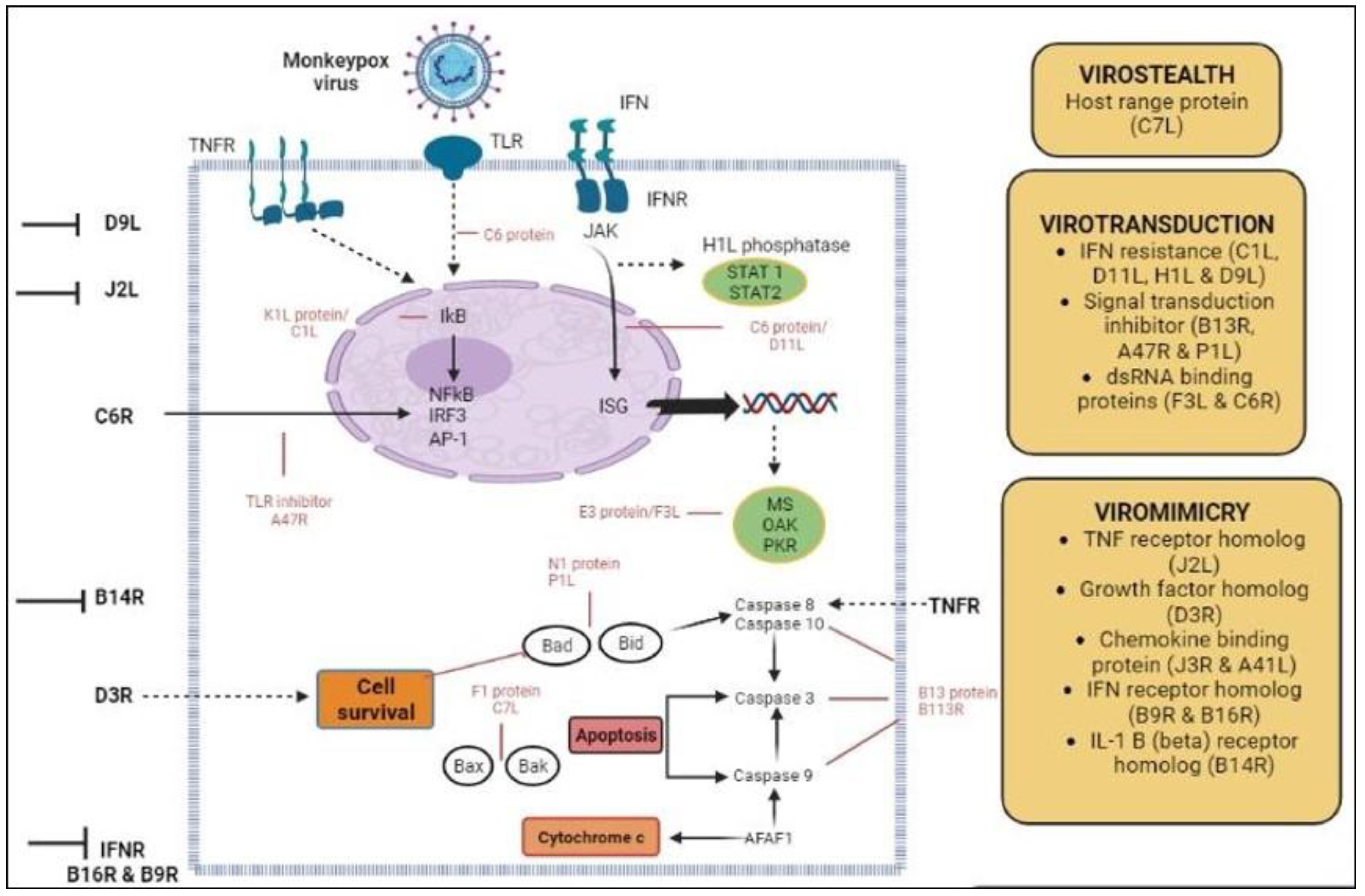
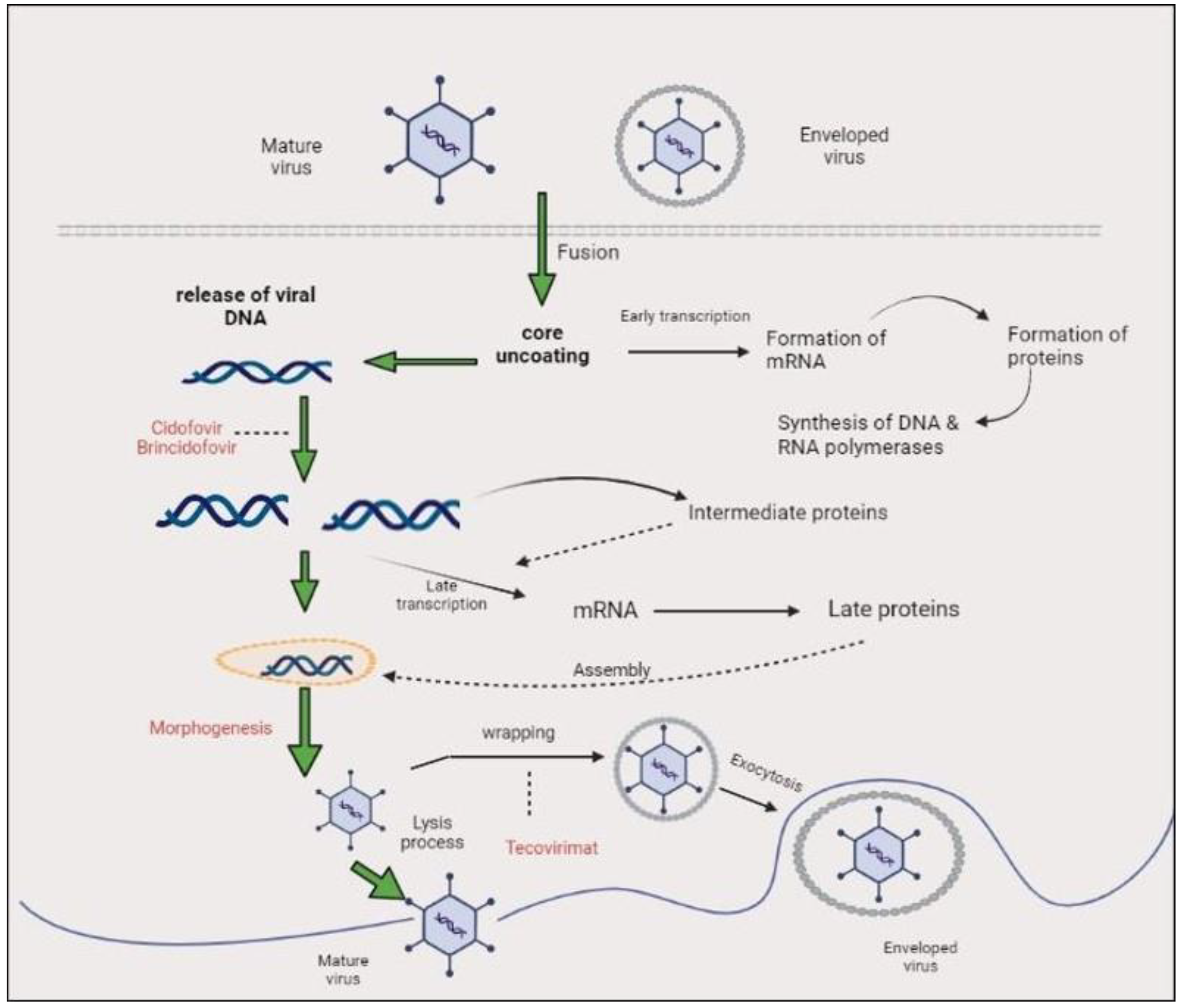
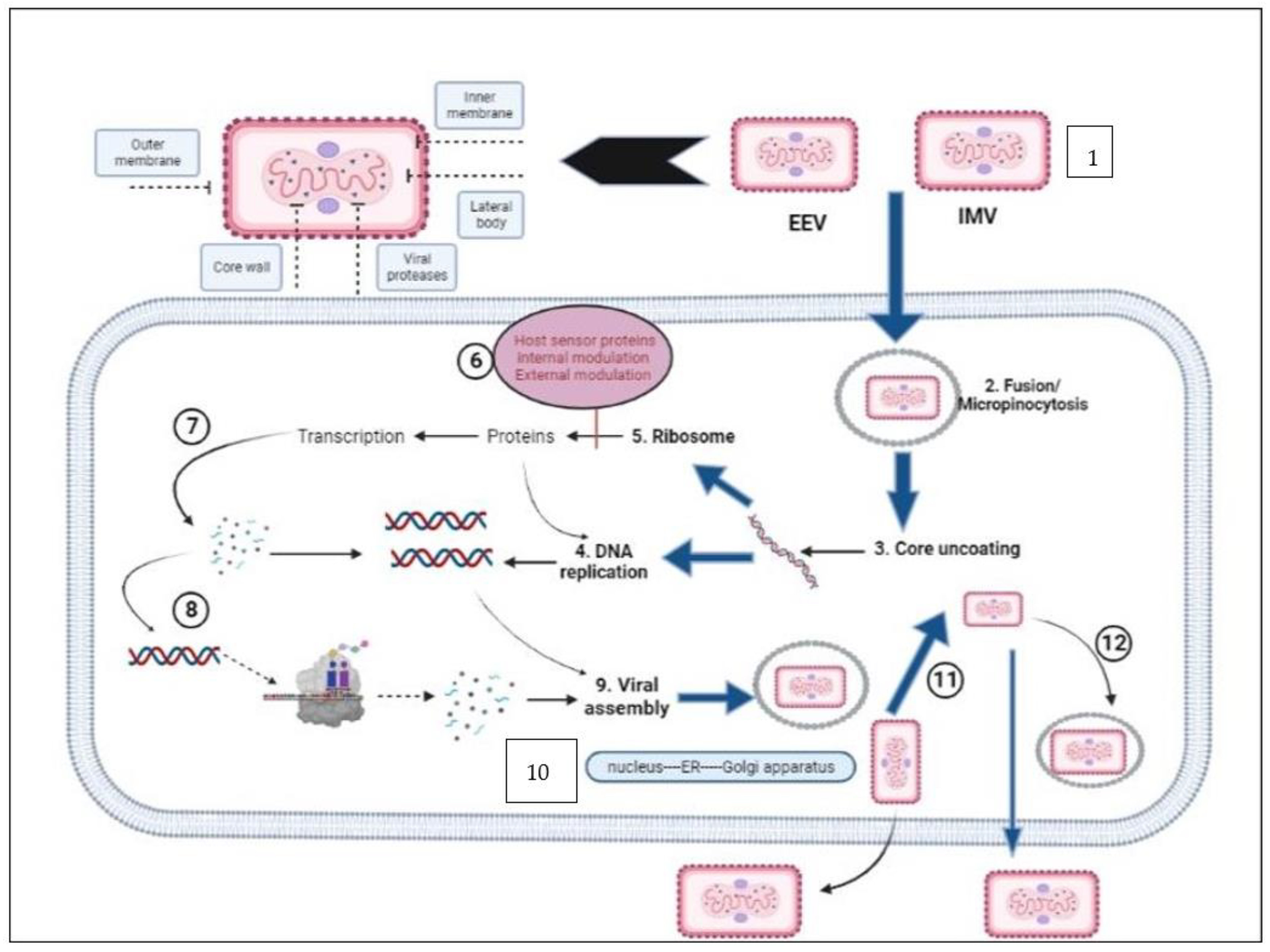
3. Genome Organization, Replication Cycle, and Morphology
Clades of Monkeypox
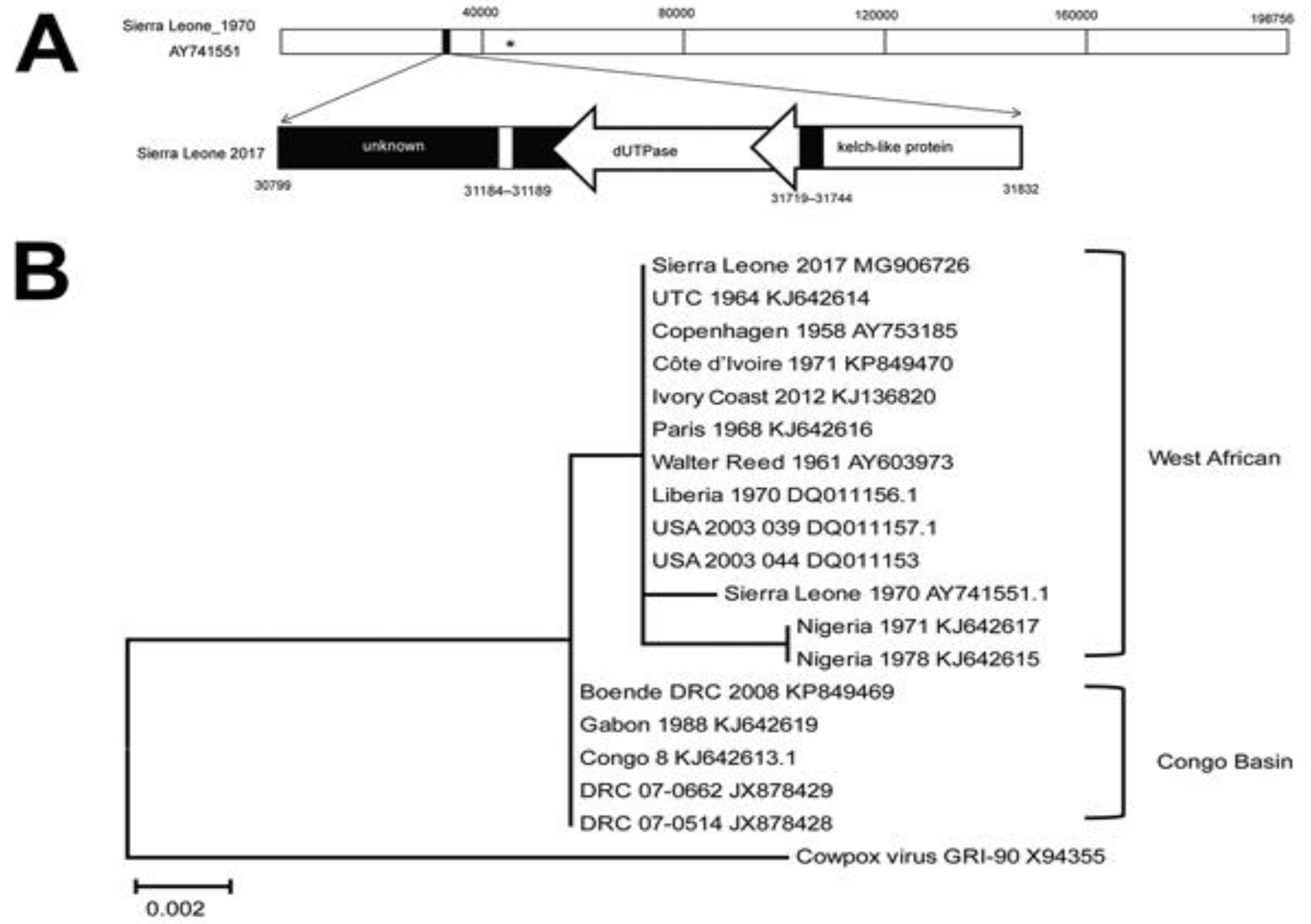
4. Phylogenetic Analysis of MPXV
Nigerian Phylogeny
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Forni, D.; Molteni, C.; Cagliani, R.; Sironi, M. Geographic Structuring and Divergence Time Frame of Monkeypox Virus in the Endemic Region. J. Infect. Dis. 2022, 227, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: A re-emergent threat to humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.; Abu Serhan, H.; Mahmoud, M.A.; Rodriguez-Morales, A.J.; Sah, R. Ophthalmic manifestations of monkeypox virus. Eye 2022, 37, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Dhama, K.; Chakraborty, C. Recently spreading human monkeypox virus infection and its transmission during COVID-19 pandemic period: A travelers’ prospective. Travel Med. Infect. Dis. 2022, 49, 102398. [Google Scholar] [CrossRef]
- Xiang, Y.; White, A. Monkeypox virus emerges from the shadow of its more infamous cousin: Family biology matters. Emerg. Microbes Infect. 2022, 11, 1768–1777. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral loads in clinical samples of men with monkeypox virus infection: A French case series. Lancet Infect. Dis. 2023, 23, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Haider, F.; Khan, S.; Ahmad, I.; Ahmed, N.; Imran, M.; Rashid, S.; Ren, Z.-G.; Khattak, S.; Ji, X.-Y. Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review. Life 2023, 13, 522. [Google Scholar] [CrossRef]
- Adnan, N.; Haq, Z.; Malik, A.; Mehmood, A.; Ishaq, U.; Faraz, M.; Malik, J.; Mehmoodi, A. Human monkeypox virus: An updated review. Medicine 2022, 101, e30406. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Alshahrani, N.Z.; Alzahrani, F.; Alarifi, A.M.; Algethami, M.R.; Alhumam, M.N.; Ayied, H.A.M.; Awan, A.Z.; Almutairi, A.F.; Bamakhrama, S.A.; Almushari, B.S.; et al. Assessment of Knowledge of Monkeypox Viral Infection among the General Population in Saudi Arabia. Pathogens 2022, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Al-Musa, A.; Chou, J.; LaBere, B. The resurgence of a neglected orthopoxvirus: Immunologic and clinical aspects of monkeypox virus infections over the past six decades. Clin. Immunol. 2022, 243, 109108. [Google Scholar] [CrossRef] [PubMed]
- Claro, I.M.; Romano, C.M.; Candido, D.D.S.; de Lima, E.L.; Lindoso, J.A.L.; Ramundo, M.S.; Moreira, F.R.R.; Barra, L.A.C.; Borges, L.M.S.; Medeiros, L.A.; et al. Shotgun metagenomic sequencing of the first case of monkeypox virus in Brazil, 2022. Rev. Do Inst. De Med. Trop. De São Paulo 2022, 64. [Google Scholar] [CrossRef] [PubMed]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Atkinson, B.; Gould, S.; Spencer, A.; Onianwa, O.; Furneaux, J.; Grieves, J.; Summers, S.; Crocker-Buqué, T.; Fletcher, T.; Bennett, A.; et al. Monkeypox virus contamination in an office-based workplace environment. J. Hosp. Infect. 2022, 130, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Dhama, K. Evolution, epidemiology, geographical distribution, and mutational landscape of newly emerging monkeypox virus. Geroscience 2022, 44, 2895–2911. [Google Scholar] [CrossRef]
- Yong, S.E.F.; Ng, O.T.; Ho, Z.J.M.; Mak, T.M.; Marimuthu, K.; Vasoo, S.; Yeo, T.W.; Ng, Y.K.; Cui, L.; Ferdous, Z. Imported Monkeypox, Singapore. Emerg. Infect. Dis. 2020, 26, 1826. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Alonso-Cadenas, J.A.; Roguera-Sopena, M.; Lorusso, N.; Miguel, L.G.S.; Calvo, C. Monkeypox virus infections in children in Spain during the first months of the 2022 outbreak. Lancet Child Adolesc. Health 2022, 6, e22–e23. [Google Scholar] [CrossRef]
- Atkinson, B.; Burton, C.; Pottage, T.; Thompson, K.; Ngabo, D.; Crook, A.; Pitman, J.; Summers, S.; Lewandowski, K.; Furneaux, J.; et al. Infection-competent monkeypox virus contamination identified in domestic settings following an imported case of monkeypox into the UK. Environ. Microbiol. 2022, 24, 4561–4569. [Google Scholar] [CrossRef]
- Feng, J.; Xue, G.; Cui, X.; Du, B.; Feng, Y.; Cui, J.; Zhao, H.; Gan, L.; Fan, Z.; Fu, T.; et al. Development of a Loop-Mediated Isothermal Amplification Method for Rapid and Visual Detection of Monkeypox Virus. Microbiol. Spectr. 2022, 10, e02714-22. [Google Scholar] [CrossRef]
- Lampejo, T. Risks of monkeypox virus infection in children. J. Med. Virol. 2022, 95, e28080. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Sallustio, A.; Centrone, F.; Casulli, D.; Accogli, M.; Saracino, A.; Foti, C.; Grandolfo, M.; Buccoliero, G.B.; Vitale, V. Monkeypox Virus Infections in Southern Italy: Is There a Risk for Community Spread? Int. J. Environ. Res. Public Health 2022, 19, 11719. [Google Scholar] [CrossRef] [PubMed]
- Meaney-Delman, D.M.; Galang, R.R.; Petersen, B.W.; Jamieson, D.J. A Primer on Monkeypox Virus for Obstetrician–Gynecologists: Diagnosis, Prevention, and Treatment. Obstet. Gynecol. 2022, 140, 391. [Google Scholar] [CrossRef]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef]
- Mariën, J.; Laudisoit, A.; Patrono, L.; Baelo, P.; Vredendaal, R.v.; Musaba, P.; Gembu, G.; Mande, C.; Ngoy, S.; Mussaw, M. Monkeypox viruses circulate in distantly-related small mammal species in the Democratic Republic of the Congo. Researchsquare 2021, 25. [Google Scholar] [CrossRef]
- Gould, S.; Atkinson, B.; Onianwa, O.; Spencer, A.; Furneaux, J.; Grieves, J.; Taylor, C.; Milligan, I.; Bennett, A.; Fletcher, T.; et al. Air and surface sampling for monkeypox virus in a UK hospital: An observational study. Lancet Microbe 2022, 3, e904–e911. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Carroll, D.S.; Karem, K.L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr. Opin. Virol. 2012, 2, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Kalil, M.N.A.; Yusof, W.; Abu Bakar, M.A.; Sjahid, A.S.; Hassan, R.; Fauzi, M.H.; Yean, C.Y. A Performance Assessment Study of Different Clinical Samples for Rapid COVID-19 Antigen Diagnosis Tests. Diagnostics 2022, 12, 847. [Google Scholar] [CrossRef]
- Ajose, O.A. Comparative Study of Variola and Varicella in Nigeria; University of Glasgow: Scotland, UK, 1939. [Google Scholar]
- Kampf, G. Efficacy of biocidal agents and disinfectants against the monkeypox virus and other orthopoxviruses. J. Hosp. Infect. 2022, 127, 101–110. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, M.; Shin, H.; Choi, C.-H.; Choi, M.-M.; Kim, J.W.; Yi, H.; Yoo, C.-K.; Rhie, G.-E. Isolation and identification of monkeypox virus MPXV-ROK-P1-2022 from the first case in the Republic of Korea. Osong Public Health Res. Perspect. 2022, 13, 308–311. [Google Scholar] [CrossRef]
- Liu, J.; Mucker, E.M.; Chapman, J.L.; Babka, A.M.; Gordon, J.M.; Bryan, A.V.; Raymond, J.L.W.; Bell, T.M.; Facemire, P.R.; Goff, A.J.; et al. Retrospective detection of monkeypox virus in the testes of nonhuman primate survivors. Nat. Microbiol. 2022, 7, 1980–1986. [Google Scholar] [CrossRef]
- Hoffmann, C.; Jessen, H.; Wyen, C.; Grunwald, S.; Noe, S.; Teichmann, J.; Krauss, A.; Kolarikal, H.; Scholten, S.; Schuler, C.; et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: A large outbreak cohort in Germany. HIV Med. 2022, 24, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, J.; Zhang, L.; Yan, S.; Li, D.; Zhang, C.; Lai, A.; Su, S. Laboratory diagnostics for monkeypox: An overview of sensitivities from various published tests. Travel Med. Infect. Dis. 2022, 49, 102425. [Google Scholar] [CrossRef]
- Badenoch, J.B.; Conti, I.; Rengasamy, E.R.; Watson, C.J.; Butler, M.; Hussain, Z.; Carter, B.; Rooney, A.G.; Zandi, M.S.; Lewis, G.; et al. Neurological and psychiatric presentations associated with human monkeypox virus infection: A systematic review and meta-analysis. Eclinicalmedicine 2022, 52, 101644. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, S.; Vitale, F.; Geremia, A.; Tramuto, F.; Maida, C.M.; Sciuto, A.; Coco, C.; Manuele, R.; Frasca, E.; Frasca, M. First case of monkeypox virus, SARS-CoV-2 and HIV co-infection. J. Infect. 2022, 86, e21–e23. [Google Scholar] [CrossRef] [PubMed]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic Variability of Monkeypox Virus among Humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239. [Google Scholar] [CrossRef]
- Shchelkunov, S.; Totmenin, A.; Safronov, P.; Mikheev, M.; Gutorov, V.; Ryazankina, O.; Petrov, N.; Babkin, I.; Uvarova, E.; Sandakhchiev, L.; et al. Analysis of the Monkeypox Virus Genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef]
- Campbell, J.A.; Trossman, D.S.; Yokoyama, W.M.; Carayannopoulos, L.N. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J. Exp. Med. 2007, 204, 1311–1317. [Google Scholar] [CrossRef]
- Happi, C.; Adetifa, I.; Mbala, P.; Njouom, R.; Nakoune, E.; Happi, A.; Ndodo, N.; Ayansola, O.; Mboowa, G.; Bedford, T.; et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022, 20, e3001769. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Nandi, S.S.; Mohapatra, R.K.; Dhama, K.; Agoramoorthy, G. Appearance and re-appearance of zoonotic disease during the pandemic period: Long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Vet. Q. 2022, 42, 119–124. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: An observational analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Davidson, W.B.; Curns, A.T.; Conover, C.S.; Huhn, G.; Davis, J.P.; Wegner, M.; Croft, D.R.; Newman, A.; Obiesie, N.N.; et al. Spectrum of Infection and Risk Factors for Human Monkeypox, United States, 2003. Emerg. Infect. Dis. 2007, 13, 1332–1339. [Google Scholar] [CrossRef]
- Lucar, J.; Roberts, A.; Saardi, K.M.; Yee, R.; Siegel, M.O.; Palmore, T.N. Monkeypox Virus–Associated Severe Proctitis Treated With Oral Tecovirimat: A Report of Two Cases. Ann. Intern. Med. 2022, 175, 1626–1627. [Google Scholar] [CrossRef]
- E Hernandez, L.; Jadoo, A.; Kirsner, R.S. Human monkeypox virus infection in an immunocompromised man: Trial with tecovirimat. Lancet 2022, 400, e8. [Google Scholar] [CrossRef]
- Cohen, J.; Powderly, W.G.; Opal, S.M. Infectious Diseases E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ma, Y.; Chen, M.; Bao, Y.; Song, S. MPoxVR: A comprehensive genomic resource for monkeypox virus variant surveillance. Innovation 2022, 3, 100296. [Google Scholar] [CrossRef]
- Hutson, C.L.; Nakazawa, Y.J.; Self, J.; Olson, V.A.; Regnery, R.L.; Braden, Z.; Weiss, S.; Malekani, J.; Jackson, E.; Tate, M.; et al. Laboratory Investigations of African Pouched Rats (Cricetomys gambianus) as a Potential Reservoir Host Species for Monkeypox Virus. PLoS Negl. Trop. Dis. 2015, 9, e0004013. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, pathogenesis, treatment and prevention. Signal Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef]
- Keasey, S.; Pugh, C.; Tikhonov, A.; Chen, G.; Schweitzer, B.; Nalca, A.; Ulrich, R.G. Proteomic Basis of the Antibody Response to Monkeypox Virus Infection Examined in Cynomolgus Macaques and a Comparison to Human Smallpox Vaccination. PLoS ONE 2010, 5, e15547. [Google Scholar] [CrossRef]
- Song, H.; Josleyn, N.; Janosko, K.; Skinner, J.; Reeves, R.K.; Cohen, M.; Jett, C.; Johnson, R.; Blaney, J.E.; Bollinger, L.; et al. Monkeypox Virus Infection of Rhesus Macaques Induces Massive Expansion of Natural Killer Cells but Suppresses Natural Killer Cell Functions. PLoS ONE 2013, 8, e77804. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. 2019, 33, 1027–1043. [Google Scholar] [CrossRef]
- Hutson, C.L.; Lee, K.N.; Abel, J.; Carroll, D.S.; Montgomery, J.M.; Olson, V.A.; Li, Y.; Davidson, W.; Hughes, C.; Dillon, M. Monkeypox zoonotic associations: Insights from laboratory evaluation of animals associated with the multi-state us outbreak. Am. J. Trop. Med. Hyg. 2007, 76, 757–768. [Google Scholar] [CrossRef]
- Altindis, M.; Puca, E.; Shapo, L. Diagnosis of monkeypox virus–An overview. Travel Med. Infect. Dis. 2022, 50, 102459. [Google Scholar] [CrossRef]
- Luna, N.; Ramírez, A.L.; Muñoz, M.; Ballesteros, N.; Patiño, L.H.; Castañeda, S.A.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.; Ramírez, J.D. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel Med. Infect. Dis. 2022, 49, 102402. [Google Scholar] [CrossRef]
- Mahmoud, A.; Nchasi, G. Monkeypox virus: A zoonosis of concern. J. Med. Virol. 2022, 95, e27968. [Google Scholar] [CrossRef]
- Gigante, C.M.; Korber, B.; Seabolt, M.H.; Wilkins, K.; Davidson, W.; Rao, A.K.; Zhao, H.; Smith, T.G.; Hughes, C.M.; Minhaj, F.; et al. Multiple lineages of monkeypox virus detected in the United States, 2021–2022. BioRxiv 2022, 378, 560–565. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R. Monkeypox outbreak—Nine states, May 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 764. [Google Scholar] [CrossRef]
- Luciani, L.; Lapidus, N.; Amroun, A.; Falchi, A.; Souksakhone, C.; Mayxay, M.; Dubot-Pérès, A.; Villarroel, P.M.S.; Diarra, I.; Koita, O. Susceptibility to monkeypox virus infection: Seroprevalence of orthopoxvirus in 4 population samples; France, Bolivia, Laos and Mali. medRxiv 2022, 62. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shang, J.; Weng, S.; Aliyari, S.R.; Ji, C.; Cheng, G.; Wu, A. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J. Med. Virol. 2022, 95, e28036. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-S.; Lin, C.-Y.; Urbina, A.N.; Wang, W.-H.; Assavalapsakul, W.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; Yu, M.-L.; Wang, S.-F. The first case of monkeypox virus infection detected in Taiwan: Awareness and preparation. Int. J. Infect. Dis. 2022, 122, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, L.; Che, D. Monkeypox virus under COVID-19: Caution for sexual transmission—Correspondence. Int. J. Surg. 2022, 104, 106768. [Google Scholar] [CrossRef] [PubMed]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Le Pluart, D.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Shapovalova, V. Monkeypox virus–new challenges of modernity: Experimental organizational and legal, clinical and pharmacological studies. SSP Mod. Pharm. Med. 2022, 2, 3. [Google Scholar] [CrossRef]
- Thakur, V.; Thakur, P.; Srivastava, S.; Kumar, P. Monkeypox virus (MPX) in humans a concern: Trespassing the global boundaries—Correspondence. Int. J. Surg. 2022, 104, 106703. [Google Scholar] [CrossRef]
- See, K.C. Vaccination for Monkeypox Virus Infection in Humans: A Review of Key Considerations. Vaccines 2022, 10, 1342. [Google Scholar] [CrossRef]
- Vusirikala, A.; Charles, H.; Balasegaram, S.; Macdonald, N.; Kumar, D.; Barker-Burnside, C.; Cumiskey, K.; Dickinson, M.; Watson, M.; Olufon, O.; et al. Epidemiology of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022. Emerg. Infect. Dis. 2022, 28, 2082–2086. [Google Scholar] [CrossRef]
- Shaheen, N.; Diab, R.A.; Meshref, M.; Shaheen, A.; Ramadan, A.; Shoib, S. Is there a need to be worried about the new monkeypox virus outbreak? A brief review on the monkeypox outbreak. Ann. Med. Surg. 2022, 81, 104396. [Google Scholar] [CrossRef]
- Pfeiffer, J.A. High-contact object and surface contamination in a household of persons with monkeypox virus infection—Utah, June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1092. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Ahmadabad, R.A.; Sahab-Negah, S. Monkeypox virus from neurological complications to neuroinvasive properties: Current status and future perspectives. J. Neurol. 2022, 270, 101–108. [Google Scholar] [CrossRef]
- Zahid, M.; Ahmed, S.H.; Waseem, S.; Shaikh, T.G.; Ahmed, K.A.H.M.; Ullah, I. Monkeypox virus: A tale of disparity between the wealthy and low-to-middle income nations. Ann. Med. Surg. 2022, 80, 104286. [Google Scholar] [CrossRef]
- Sah, R.; Abdelaal, A.; Asija, A.; Basnyat, S.; Sedhai, Y.R.; Ghimire, S.; Sah, S.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Monkeypox virus containment: The application of ring vaccination and possible challenges. J. Travel Med. 2022, 29, taac085. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Farfour, E.; Zucman, D. Monkeypox virus: A novel sexually transmitted disease? A case report from France. Travel Med. Infect. Dis. 2022, 49, 102394. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Morgan, C.N.; Whitehill, F.; Doty, J.B.; Schulte, J.; Matheny, A.; Stringer, J.; Delaney, L.J.; Esparza, R.; Rao, A.K.; McCollum, A.M. Environmental Persistence of Monkeypox Virus on Surfaces in Household of Person with Travel-Associated Infection, Dallas, Texas, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1982–1989. [Google Scholar] [CrossRef]
- Pastula, D.M.; Tyler, K.L. An Overview of Monkeypox Virus and Its Neuroinvasive Potential. Ann. Neurol. 2022, 92, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Sv, P.; Ittamalla, R. What concerns the general public the most about monkeypox virus?—A text analytics study based on Natural Language Processing (NLP). Travel Med. Infect. Dis. 2022, 49, 102404. [Google Scholar] [CrossRef]
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.O.; Syed, M.A.; Tariq, R.; Mansoor, S. Multicounty outbreak of monkeypox virus—Challenges and recommendations. J. Med. Virol. 2022, 95, e27966. [Google Scholar] [CrossRef]
- Wawina-Bokalanga, T.; Sklenovska, N.; Vanmechelen, B.; Bloemen, M.; Vergote, V.; Laenen, L.; Andre, E.; Van Ranst, M.; Muyembe, J.-J.T.; Maes, P. An accurate and rapid Real-time PCR approach for human Monkeypox virus diagnosis. medRxiv 2022. [Google Scholar] [CrossRef]
- Petersen, B.; Damon, I. Smallpox, monkeypox, and other poxvirus infections. In Goldman-Cecil Medicine, 26th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Shafaati, M.; Zandi, M. Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med. Infect. Dis. 2022, 49, 102414. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Schulte, J.; Chen, T.-H.; Hughes, C.M.; Davidson, W.; Neff, J.M.; Markarian, M.; Delea, K.C.; Wada, S.; Liddell, A. Monkeypox in a traveler returning from Nigeria—Dallas, Texas, July 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 509. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: A prospective observational cohort study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, B.; Muñoz-Gómez, A.; Sanchiz, A.; Orviz, E.; Valls-Carbo, A.; Sagastagoitia, I.; Ayerdi, O.; Martín, R.; Puerta, T.; Vera, M.; et al. Monitoring monkeypox virus in saliva and air samples in Spain: A cross-sectional study. Lancet Microbe 2023, 4, e21–e28. [Google Scholar] [CrossRef]
- Spicknall, I.H.; Pollock, E.D.; Clay, P.A.; Oster, A.M.; Charniga, K.; Masters, N.; Nakazawa, Y.J.; Rainisch, G.; Gundlapalli, A.V.; Gift, T.L. Modeling the Impact of Sexual Networks in the Transmission of Monkeypox Virus among Gay, Bisexual, and Other Men Who Have Sex with Men—United States. 2022; Volume 71. Available online: https://stacks.cdc.gov/view/cdc/120698 (accessed on 12 May 2023).
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Nörz, D.; Brehm, T.T.; Tang, H.T.; Grewe, I.; Hermanussen, L.; Matthews, H.; Pestel, J.; Degen, O.; Günther, T.; Grundhoff, A.; et al. Clinical characteristics and comparison of longitudinal qPCR results from different specimen types in a cohort of ambulatory and hospitalized patients infected with monkeypox virus. J. Clin. Virol. 2022, 155, 105254. [Google Scholar] [CrossRef]
- Peter, O.J.; Kumar, S.; Kumari, N.; Oguntolu, F.A.; Oshinubi, K.; Musa, R. Transmission dynamics of Monkeypox virus: A mathematical modelling approach. Model. Earth Syst. Environ. 2022, 8, 3423–3434. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical Manifestations of Human Monkeypox Influenced by Route of Infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Abbas, S.; Rehan, S.T.; Hasan, M.M. Monkeypox virus: A future scourge to the Pakistani Healthcare system. Ann. Med. Surg. 2022, 79, 103978. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.R.; Lee, M.; Shin, H.; Kim, J.-W.; Choi, M.-M.; Kim, Y.M.; Kim, J.; Na, H.K. The First Case of Monkeypox in the Republic of Korea. J. Korean Med. Sci. 2022, 37, e224. [Google Scholar] [CrossRef] [PubMed]
- Karan, A.; Styczynski, A.R.; Huang, C.; Sahoo, M.K.; Srinivasan, K.; Pinsky, B.A.; Salinas, J.L. Human Monkeypox without Viral Prodrome or Sexual Exposure, California, USA, 2022. Emerg. Infect. Dis. 2022, 28, 2121–2123. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.; Ly, H. The potential risks posed by inter- and intraspecies transmissions of monkeypox virus. Virulence 2022, 13, 1681–1683. [Google Scholar] [CrossRef]
- Chelsky, Z.L.; Dittmann, D.; Blanke, T.; Chang, M.; Vormittag-Nocito, E.; Jennings, L.J. Validation Study of a Direct Real-Time PCR Protocol for Detection of Monkeypox Virus. J. Mol. Diagn. 2022, 24, 1155–1159. [Google Scholar] [CrossRef]
- Dashraath, P.; Nielsen-Saines, K.; Mattar, C.; Musso, D.; Tambyah, P.; Baud, D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet 2022, 400, 21–22. [Google Scholar] [CrossRef]
- Alakunle, E.F.; Okeke, M.I. Monkeypox virus: A neglected zoonotic pathogen spreads globally. Nat. Rev. Genet. 2022, 20, 507–508. [Google Scholar] [CrossRef]
- Alshahrani, N.Z.; Algethami, M.R.; Alarifi, A.M.; Alzahrani, F.; Alshehri, E.A.; Alshehri, A.M.; Sheerah, H.A.; Abdelaal, A.; Sah, R.; Rodriguez-Morales, A.J. Knowledge and Attitude Regarding Monkeypox Virus among Physicians in Saudi Arabia: A Cross-Sectional Study. Vaccines 2022, 10, 2099. [Google Scholar] [CrossRef]
- Batéjat, C.; Grassin, Q.; Feher, M.; Hoinard, D.; Vanhomwegen, J.; Manuguerra, J.-C. Heat inactivation of monkeypox virus. J. Biosaf. Biosecurity 2022, 4, 121–123. [Google Scholar] [CrossRef]
- Giacomelli, A.; Moschese, D.; Pozza, G.; Casalini, G.; Cossu, M.V.; Rizzardini, G.; Antinori, S. Route of monkeypox viral inoculum as a determinant of atypical clinical presentation. J. Med. Virol. 2022, 95, e28112. [Google Scholar] [CrossRef] [PubMed]
- Ferré, V.M.; Bachelard, A.; Zaidi, B.M.; Armand-Lefevre, L.; Descamps, D.; Charpentier, C.; Ghosn, J. Detection of Monkeypox Virus in Anorectal Swabs From Asymptomatic Men Who Have Sex With Men in a Sexually Transmitted Infection Screening Program in Paris, France. Ann. Intern. Med. 2022, 175, 1491–1492. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: An observational cohort study. Clin. Microbiol. Infect. 2022, 29, 233–239. [Google Scholar] [CrossRef]
- Delaney, K.P. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission—United States, August 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1126. [Google Scholar] [CrossRef]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef] [PubMed]
- Uwishema, O.; Adekunbi, O.; Peñamante, C.A.; Bekele, B.K.; Khoury, C.; Mhanna, M.; Nicholas, A.; Adanur, I.; Dost, B.; Onyeaka, H. The burden of monkeypox virus amidst the COVID-19 pandemic in Africa: A double battle for Africa. Ann. Med. Surg. 2022, 80, 104197. [Google Scholar] [CrossRef]
- Petersen, E.; Zumla, A.; Hui, D.; Blumberg, L.; Valdoleiros, S.; Amao, L.; Ntoumi, F.; Asogun, D.; Simonsen, L.; Haider, N.; et al. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int. J. Infect. Dis. 2022, 122, 569–571. [Google Scholar] [CrossRef]
- Vouga, M.; Nielsen-Saines, K.; Dashraath, P.; Baud, D. The monkeypox outbreak: Risks to children and pregnant women. Lancet Child Adolesc. Health 2022, 6, 751–753. [Google Scholar] [CrossRef]
- Yang, Q.; Xia, D.; Syed, A.A.S.; Wang, Z.; Shi, Y. Highly accurate protein structure prediction and drug screen of monkeypox virus proteome. J. Infect. 2022, 86, 66–117. [Google Scholar] [CrossRef]
- Mucker, E.M.; Shamblin, J.D.; Raymond, J.L.; Twenhafel, N.A.; Garry, R.F.; Hensley, L.E. Effect of Monkeypox Virus Preparation on the Lethality of the Intravenous Cynomolgus Macaque Model. Viruses 2022, 14, 1741. [Google Scholar] [CrossRef]
- Veintimilla, C.; Catalán, P.; Alonso, R.; de Viedma, D.G.; Pérez-Lago, L.; Palomo, M.; Cobos, A.; Aldamiz-Echevarria, T.; Muñoz, P. The relevance of multiple clinical specimens in the diagnosis of monkeypox virus, Spain, June 2022. Eurosurveillance 2022, 27, 2200598. [Google Scholar] [CrossRef] [PubMed]
- Remichkova, M. Poxviruses: Smallpox vaccine, its complications and chemotherapy. Virus Adapt. Treat. 2010, 2, 41–46. [Google Scholar] [CrossRef]
- Smith, G.L.; Murphy, B.J.; Law, M. Vaccinia virus motility. Annu. Rev. Microbiol. 2003, 57, 323–342. [Google Scholar] [CrossRef]
- Ola, P. Monkeypox is the manifestation of spectral diseases and not of monkeypox virus transmission. 2022. [Google Scholar] [CrossRef]
- Wolfe, M.K.; Duong, D.; Hughes, B.; Chan-Herur, V.; White, B.; Boehm, A. Detection of monkeypox viral DNA in a routine wastewater monitoring program. medRxiv 2022. [Google Scholar] [CrossRef]
- Wurtzer, S.; Levert, M.; Dhenain, E.; Boni, M.; Tournier, J.N.; Londinsky, N.; Lefranc, A.; Ferraris, O.; Moulin, L.; Sig, O. First Detection of Monkeypox Virus Genome in Sewersheds in France. medRxiv 2022, 9, 991–996. [Google Scholar] [CrossRef]
- Wei, Q. Is China ready for monkeypox? Anim. Model. Exp. Med. 2022, 5, 397. [Google Scholar] [CrossRef]
- Shida, H.; Tanabe, K.; Matsumoto, S. Mechanism of virus occlusion into A-type inclusion during poxvirus infection. Virology 1977, 76, 217–233. [Google Scholar] [CrossRef]
- Okeke, M.I.; Adekoya, O.A.; Moens, U.; Tryland, M.; Traavik, T.; Nilssen, O. Comparative sequence analysis of A-type inclusion (ATI) and P4c proteins of orthopoxviruses that produce typical and atypical ATI phenotypes. Virus Genes 2009, 39, 200–209. [Google Scholar] [CrossRef]
- de Jonge, E.F.; Peterse, C.M.; Koelewijn, J.M.; van der Drift, A.-M.R.; van der Beek, R.F.; Nagelkerke, E.; Lodder, W.J. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci. Total Environ. 2022, 852, 158265. [Google Scholar] [CrossRef]
- Yadav, P.D.; Reghukumar, A.; Sahay, R.R.; Sudeep, K.; Shete, A.M.; Raman, A.; Vk, P.; Abraham, P.; Benson, R.; Sm, S.; et al. First two cases of Monkeypox virus infection in travellers returned from UAE to India, July 2022. J. Infect. 2022, 85, e145–e148. [Google Scholar] [CrossRef] [PubMed]
- Boesecke, C.; Monin, M.B.; van Bremen, K.; Schlabe, S.; Hoffmann, C. Severe monkeypox-virus infection in undiagnosed advanced HIV infection. Infection 2022, 50, 1633–1634. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, P.B.; Huggins, J.W.; Ibrahim, M.S.; Lawler, J.V.; Martin, J.W. Smallpox and related orthopoxviruses. In Medical Aspects of Biological Warfare; TMM Publications, Office of The Surgeon General: Washington, DC, USA, 2007; pp. 215–240. [Google Scholar]
- de Nicolas-Ruanes, B.; Vivancos, M.; Azcarraga-Llobet, C.; Moreno, A.; Rodriguez-Dominguez, M.; Berna-Rico, E.; Garcia-Mouronte, E.; Carron-Herrero, A.; McGee, A.; Galan, J.; et al. Monkeypox virus case with maculopapular exanthem and proctitis during the Spanish outbreak in 2022. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e658–e660. [Google Scholar] [CrossRef]
- Hoff, N.; Doshi, R.; Colwell, B.; Kebela-Illunga, B.; Mukadi, P.; Mossoko, M.; Spencer, D.; Muyembe-Tamfum, J.-J.; Okitolonda-Wemakoy, E.; Lloyd-Smith, J.; et al. Evolution of a Disease Surveillance System: An Increase in Reporting of Human Monkeypox Disease in the Democratic Republic of the Congo, 2001–2013. Int. J. Trop. Dis. Health 2017, 25, 35885. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.C.; Wang, C.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus Genome Evolution: The Role of Gene Loss. Viruses 2010, 2, 1933–1967. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Lopera, J.G.; Falendysz, E.A.; Rocke, T.E.; Osorio, J.E. Attenuation of monkeypox virus by deletion of genomic regions. Virology 2015, 475, 129–138. [Google Scholar] [CrossRef]
- Ye, F.; Song, J.; Zhao, L.; Zhang, Y.; Xia, L.; Zhu, L.; Kamara, I.L.; Ren, J.; Wang, W.; Tian, H.; et al. Molecular Evidence of Human Monkeypox Virus Infection, Sierra Leone. Emerg. Infect. Dis. 2019, 25, 1220–1222. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Mauldin, M.R.; Emerson, G.L.; Reynolds, M.G.; Lash, R.R.; Gao, J.; Zhao, H.; Li, Y.; Muyembe, J.-J.; Kingebeni, P.M.; et al. A Phylogeographic Investigation of African Monkeypox. Viruses 2015, 7, 2168–2184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]

| Animals Infected by Mpox | Geographical Location | Detection Technique | References |
|---|---|---|---|
| Gambian pouched rats | African territory | Viral isolation and PCR | [50] |
| Monkeys (Sooty mangabey) | Côte d’Ivoire | Molecular-testing PCR | [51] |
| Macaques (Cynomolgus) | Singapore/Copenhagen | Isolation of virus | [52] |
| Macaques (Rhesus) | Copenhagen | Antibody testing | [53] |
| Opossums | South America | Viral isolation and PCR | [54] |
| Monkeys (Asian) | Copenhagen | Isolation of virus | [55] |
| Hedgehogs (African) | Africa | Viral isolation, PCR, and antibody detection | [55] |
| Sun squirrels | Zaire | Detection of antibody | [56] |
| Woodchucks | USA | Viral isolation and PCR | |
| Jerboas | Illinois, USA | Viral isolation, PCR, and antibody detection | |
| Shot-tailed opossums | USA | Viral isolation, PCR, and antibody detection | |
| Giant anteaters (Myrmecophaga tridactyla) | Rotterdam | Isolation of virus | [57] |
| Porcupines (Atherurus africanus) | Zaire | Viral isolation and PCR | |
| Elephant shrews | DRC | Serological test | |
| Prairie dogs | USA | Viral isolation and PCR | |
| Rope squirrels | Zaire | Viral isolation and PCR | |
| Domestic pigs | DRC | Serological test | |
| Dormice (African) | USA | Viral isolation and PCR |
| Country | Duration | Cases Reported | Mortality |
|---|---|---|---|
| DRC | 1970 | 1 | 100% |
| DRC | 1981–1986 | 338 | 9.8% |
| DRC | 1996–1997 | 92 | 3.3% |
| DRC | 2001–2013 | 17,186 | 2.46% |
| DRC | 2017 | 88 | 6.3% |
| Sudan | 2005 | 37 | 0 |
| Cameron | 1989 | 1 | 0 |
| Nigeria | 1971 | 2 | 0 |
| Nigeria | 1978 | 1 | 0 |
| Nigeria | 2017–2018 | 228 | 2.6% |
| Gabon | 1991 | 9 | 0 |
| Sierra Leone | 1970–1971 | 4 | 0 |
| USA | 2003 | 47 | 0 |
| Central African Republic | 2015–2018 | 104 | 8.3% |
| Character | Smallpox | Monkeypox | Varicella | References |
|---|---|---|---|---|
| Viral cycle completion | 28 days | 28 days | 21 days | [87,89,95] |
| Incubation time | 2 weeks | 2 weeks | 3 weeks | |
| Lesion inflammatory cycle | 2–4 weeks | 2–4 weeks | 1–3 weeks | |
| Body temperature | >40 °C | 38.5–40.5 °C | 38 ± 8 °C | |
| Lymphadenopathy | Often | Often | Rare | |
| Lesion | Centrifugal | Centrifugal | Centripetal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaan, A.A.; Alasiri, N.A.; Aljeldah, M.; Alshukairiis, A.N.; AlMusa, Z.; Alfouzan, W.A.; Abuzaid, A.A.; Alamri, A.A.; Al-Afghani, H.M.; Al-baghli, N.; et al. An Updated Review on Monkeypox Viral Disease: Emphasis on Genomic Diversity. Biomedicines 2023, 11, 1832. https://doi.org/10.3390/biomedicines11071832
Rabaan AA, Alasiri NA, Aljeldah M, Alshukairiis AN, AlMusa Z, Alfouzan WA, Abuzaid AA, Alamri AA, Al-Afghani HM, Al-baghli N, et al. An Updated Review on Monkeypox Viral Disease: Emphasis on Genomic Diversity. Biomedicines. 2023; 11(7):1832. https://doi.org/10.3390/biomedicines11071832
Chicago/Turabian StyleRabaan, Ali A., Nada A. Alasiri, Mohammed Aljeldah, Abeer N. Alshukairiis, Zainab AlMusa, Wadha A. Alfouzan, Abdulmonem A. Abuzaid, Aref A. Alamri, Hani M. Al-Afghani, Nadira Al-baghli, and et al. 2023. "An Updated Review on Monkeypox Viral Disease: Emphasis on Genomic Diversity" Biomedicines 11, no. 7: 1832. https://doi.org/10.3390/biomedicines11071832
APA StyleRabaan, A. A., Alasiri, N. A., Aljeldah, M., Alshukairiis, A. N., AlMusa, Z., Alfouzan, W. A., Abuzaid, A. A., Alamri, A. A., Al-Afghani, H. M., Al-baghli, N., Alqahtani, N., Al-baghli, N., Almoutawa, M. Y., Mahmoud Alawi, M., Alabdullah, M., Bati, N. A. A., Alsaleh, A. A., Tombuloglu, H., Arteaga-Livias, K., ... Imran, M. (2023). An Updated Review on Monkeypox Viral Disease: Emphasis on Genomic Diversity. Biomedicines, 11(7), 1832. https://doi.org/10.3390/biomedicines11071832









