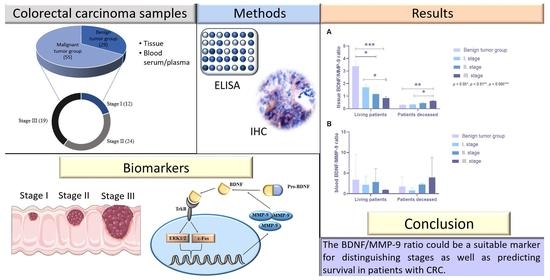
Stage-Dependent Levels of Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase 9 in the Prognosis of Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material
3. Methods
4. Results
4.1. Kaplan–Meier Survival Analysis
4.2. Analyses of MMP-9 and BDNF Using ELISA
4.2.1. Tissue MMP-9 in Benign/Malignant Tumor Groups
4.2.2. Serum MMP-9 in Benign/Malignant Tumor Groups
4.2.3. BDNF in Benign/Malignant Tumor Groups
4.3. Analyses of MMP-9 and BDNF Using Immunohistochemical Staining
4.3.1. Determination of Tissue MMP-9 Expression by IHC
4.3.2. BDNF/MMP-9 Ratios
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Fang, L.; Yang, Z.; Zhang, M.; Meng, M.; Feng, J.; Chen, C. Clinical characteristics and survival analysis of colorectal cancer in China: A retrospective cohort study with 13,328 patients from southern China. Gastroenterol. Rep. 2021, 9, 571–582. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.; Mathai, M.; Zulli, A. A synopsis of modern–day colorectal cancer: Where we stand. BBA Rev. Cancer 2022, 2, 188699. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [Green Version]
- Heiss, J.A.; Brenner, H. Epigenome-wide discovery and evaluation of leukocyte DNA methylation markers for the detection of colorectal cancer in a screening setting. Clin. Epigenetics 2017, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Vatandoost, N.; Ghanbari, J.; Mojaver, M.; Avan, A.; Ghayour-Mobarhan, M.; Nedaeinia, R.; Salehi, R. Early detection of colorectal cancer: From conventional methods to novel biomarkers. J. Cancer Res. Clin. Oncol. 2016, 142, 341–351. [Google Scholar] [CrossRef]
- Nikolaou, S.; Shengyang, Q.; Fiorentino, F.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. A systemic review of blood diagnostic markers in colorectal cancer. Tech. Coloproctol. 2018, 22, 481–498. [Google Scholar] [CrossRef] [Green Version]
- Maslankova, J.; Vecurkovska, I.; Rabajdova, M.; Katuchova, J.; Kicka, M.; Gayova, M.; Katuch, V. Regulation of transforming growth factor-β signaling as a therapeutic approach to treating colorectal cancer. World J. Gastroenterol. 2022, 8, 4744–4761. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Guren, M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019, 4, 894–895. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Ha, S.-E.; Wu, M.; Zogg, H.; Ronkon, C.F.; Lee, M.-Y.; Ro, S. Extracellular matrix biomarkers in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 9185. [Google Scholar] [CrossRef]
- Serra, R. Matrix Metalloproteinases in Health and Disease. Biomolecules 2020, 10, 1138. [Google Scholar] [CrossRef]
- Bassiouni, W.; Ali, M.A.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Pezeshkian, Z.; Nobili, S.; Peyravian, N.; Shojaee, B.; Nazari, H.; Soleimani, H.; Asadzadeh-Aghdaei, H.; Ashrafian Bonab, M.; Nazemalhosseini-Mojarad, E.; Mini, E. Insights into the Role of Matrix Metalloproteinases in Precancerous Conditions and in Colorectal Cancer. Cancers 2021, 13, 6226. [Google Scholar] [CrossRef]
- Said, A.H.; Raufman, J.P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Lan, Y.; Li, E.; Li, J.; Deng, Q.; Deng, X. Diagnostic values of MMP-7, MMP-9, MMP-11, TIMP-1, TIMP-2, CEA, and CA19-9 in patients with colorectal cancer. J. Int. Med. Res. 2021, 49, 1–11. [Google Scholar] [CrossRef]
- Wattanawongdon, W.; Bartpho, T.S.; Tongtawee, T. Expression of Matrix Metalloproteinase-7 Predicts Poor Prognosis in Gastric Cancer. Biomed Res. Int. 2022, 2022, 2300979. [Google Scholar] [CrossRef]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Girondo, M.; Rodríguez-Berrocal, F.J.; Cubiella, J.; Castro, I.; Hernández, V.; Martínez-Zorzano, V.S. Serum matrix metalloproteinase-9 in colorectal cancer family-risk population screening. Sci. Rep. 2015, 5, 13030. [Google Scholar] [CrossRef] [Green Version]
- Murnane, M.J.; Cai, J.; Shuja, S.; McAneny, D.; Klepeis, V.; Willett, J.B. Active MMP-2 effectively identifies the presence of colorectal cancer. Int. J. Cancer 2009, 125, 2893–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, A.G.; Holloway, R.W.; Miller, V.A.; Waisman, D.M. Plasmin and Plasminogen System in the Tumor Microenvironment: Implications for Cancer Diagnosis, Prognosis, and Therapy. Cancers 2021, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Gonias, S.L.; Zampieri, C. Plasminogen Receptors in Human Malignancies: Effects on Prognosis and Feasibility as Targets for Drug Development. Curr. Drug Targets 2020, 21, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.A.; Buckley, B.J.; Ranson, M. The Urokinase Plasminogen Activation System in Pancreatic Cancer: Prospective Diagnostic and Therapeutic Targets. Biomolecules 2022, 12, 152. [Google Scholar] [CrossRef]
- Kudelski, J.; Tokarzewicz, A.; Gudowska-Sawczuk, M.; Mroczko, B.; Chłosta, P.; Bruczko-Goralewska, M.; Mitura, P.; Młynarczyk, G. The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer. Biomedicines 2023, 11, 956. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [Green Version]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.; Senior, R.M.; Shibuya, M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am. J. Physiol. Liver Physiol. 2019, 316, G278–G290. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrkowicz, M.; Janoska-Jazdzik, M.; Koweszko, T.; Szulc, A. The Influence of Psychotherapy on Peripheral Brain-Derived Neurotrophic Factor Concentration Levels and Gene Methylation Status: A Systematic Review. J. Clin. Med. 2021, 10, 4424. [Google Scholar] [CrossRef] [PubMed]
- Brunetto de Farias, C.; Rosemberg, D.B.; Heinen, T.E.; Koehler-Santos, P.; Abujamra, A.L.; Kapczinski, F.; Brunetto, A.L.; Ashton-Prolla, P.; Meurer, L.; Reis Bogo, M.; et al. BDNF/TrkB content and interaction with gastrin-releasing peptide receptor blockade in colorectal cancer. Oncology 2010, 79, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Perraud, A.; Jauberteau, M.-O.; Mathonnet, M. Tropomyosin-related kinase B/brain derived-neurotrophic factor signaling pathway as a potential therapeutic target for colorectal cancer. World J. Gastroenterol. 2016, 22, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, M.; Oziębło, D.; Ołdak, M.; Rejmak, E.; Kaczmarek, L.; Skarżyński, H. Longitudinal Changes in BDNF and MMP-9 Protein Plasma Levels in Children after Cochlear Implantation. Int. J. Mol. Sci. 2023, 24, 3714. [Google Scholar] [CrossRef]
- Savic, G.; Stevanovic, I.; Mihajlovic, D.; Jurisevic, M.; Gajovic, N.; Jovanovic, I.; Ninkovic, M. MMP-9/BDNF ratio predicts more severe COVID-19 outcomes. Int. J. Med. Sci. 2022, 19, 1903–1911. [Google Scholar] [CrossRef]
- Niculescu, D.; Michaelsen-Preusse, K.; Güner, Ü.; van Dorland, R.; Wierenga, C.J.; Lohmann, C. A BDNF-Mediated Push-Pull Plasticity Mechanism for Synaptic Clustering. Cell Rep. 2018, 24, 2063–2074. [Google Scholar] [CrossRef] [Green Version]
- Kiskova, T.; Ekmekcioglu, C.; Garajova, M.; Orendas, P.; Bojkova, B.; Bobrov, N.; Jäger, W.; Kassayova, M.; Thalhammer, T. A combination of resveratrol and melatonin exerts chemopreventive effects in N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Eur. J. Cancer Prev. 2012, 21, 163–170. [Google Scholar] [CrossRef]
- Ahmed, S.; Johnson, K.; Ahmed, O.; Iqbal, N. Advances in the management of colorectal cancer: From biology to treatment. Int. J. Color. Dis. 2014, 29, 1031–1042. [Google Scholar] [CrossRef]
- Lilley, E.J.; Cooper, Z.; Schwarze, M.L.; Mosenthal, A.C. Palliative Care in Surgery: Defining the Research Priorities. J. Palliat. Med. 2017, 20, 702–709. [Google Scholar] [CrossRef]
- Petersen, S.H.; Harling, H.; Kirkeby, L.T.; Wille-Jørgensen, P.; Mocellin, S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst. Rev. 2012, 2012, CD004078. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, S.M.; Galjart, B.; Verhoef, C.; Slooter, G.D.; Koopman, M.; Verhoeven, R.H.A.; de Wilt, J.H.W.; van Erning, F.N. Disease recurrence after colorectal cancer surgery in the modern era: A population-based study. Int. J. Color. Dis. 2021, 36, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Glynne-Jones, R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017, 18, e354–e363. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.F.; Wang, L.; Ran, J.C.; Wang, H.; Liu, C.C.; Zhang, H.Z.; Yang, L.; Shi, S.S.; Jiang, L.M.; Fan, J.H.; et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: Overall design and results from a multicenter retrospective epidemiologic survey. Cancer 2021, 127, 1880–1893. [Google Scholar] [CrossRef]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef]
- Jonsson, A.; Hjalmarsson, C.; Falk, P.; Ivarsson, M.L. Stability of matrix metalloproteinase-9 as biological marker in colorectal cancer. Med. Oncol. 2018, 35, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Tang, F.; Zhang, B.; Zhao, Y.; Feng, J.; Rao, Z. Matrix metalloproteinase-9 overexpression is closely related to poor prognosis in patients with colon cancer. World J. Surg. Oncol. 2014, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroczko, B.; Groblewska, M.; Okulczyk, B.; Kedra, B.; Szmitkowski, M. The diagnostic value of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) determination in the sera of colorectal adenoma and cancer patients. Int. J. Color. Dis. 2010, 25, 1177–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabás, L.; Hritz, I.; István, G.; Tulassay, Z.; Herszényi, L. The Behavior of MMP-2, MMP-7, MMP-9, and Their Inhibitors TIMP-1 and TIMP-2 in Adenoma-Colorectal Cancer Sequence. Dig. Dis. 2021, 39, 217–224. [Google Scholar] [CrossRef]
- Blondy, S.; Christou, N.; David, V.; Verdier, M.; Jauberteau, M.O.; Mathonnet, M.; Perraud, A. Neurotrophins and their involvement in digestive cancers. Cell Death Dis. 2019, 10, 123. [Google Scholar] [CrossRef] [Green Version]
- Radin, D.P.; Patel, P. BDNF: An Oncogene or Tumor Suppressor? Anticancer Res. 2017, 37, 3983–3990. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Liu, B.; Ji, R.; Jiang, X.; Yan, X.; Xin, Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 2019, 17, 2031–2039. [Google Scholar] [CrossRef] [Green Version]
- Doranish, S.; Atali, S.; Ray, R.; Al Khashali, H.; Coleman, K.L.; Guthrie, J.; Heyl, D.; Evans, H.G. Differences in the Relative Abundance of ProBDNF and Mature BDNF in A549 and H1299 Human Lung Cancer Cell Media. Int. J. Mol. Sci. 2021, 22, 7059. [Google Scholar] [CrossRef]
- Frisch, S.M.; Schaller, M.; Cieply, B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J. Cell. Sci. 2013, 126, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Brierley, G.V.; Priebe, I.K.; Purins, L.; Fung, K.Y.; Tabor, B.; Lockett, T.; Nice, E.; Gibbs, P.; Tie, J.; McMurrick, P.; et al. Serum concentrations of brain-derived neurotrophic factor (BDNF) are decreased in colorectal cancer patients. Cancer Biomark. 2013, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Junior, V.; Fernandes, G.M.M.; Oliveira-Cucolo, J.G.; Pavarino, E.C.; Goloni-Bertollo, E.M. Role of Tropomyosin-related kinase B receptor and brain-derived neurotrophic factor in cancer. Cytokine 2020, 136, 155270. [Google Scholar] [CrossRef]








| Number of Patients | ||||
|---|---|---|---|---|
| Benign Tumor Group | Malignant Tumor Group | |||
| Stage I | Stage II | Stage III | ||
| Sex | ||||
| Males Females | 12 | 7 | 18 | 15 |
| 17 | 5 | 6 | 4 | |
| Tumor localization | ||||
| Colon | 20 | 7 | 16 | 14 |
| Rectum | 9 | 5 | 8 | 5 |
| Findings | ||||
| Hemorrhoids | 4 | - | - | - |
| Diverticulitis | 5 | - | - | - |
| Adenomas | 20 | - | - | - |
| Adenocarcinomas | - | 12 | 24 | 19 |
| Survival | ||||
| Living patients | 26 | 6 | 19 | 7 |
| Deceased patients | 3 | 6 | 5 | 12 |
| Tissue MMP-9 Levels (ng/mL) | Serum MMP-9 Levels (ng/mL) | Plasma BDNF Levels (ng/mL) | |
|---|---|---|---|
| Benign tumor group | |||
| Females Males p | 277.68 (4.39–886.19) 114.27 (3.91–586.69) >0.05 | 46.08 (3.83–197.64) 506.48 (6.80–1046.05) >0.05 | 24.34 (4.74–55.74) 11.27 (5.12–29.74) >0.05 |
| Malignant tumor group | |||
| Females Males p | 355.04 (4.94–1038.41) 287.6 (4.04–1155.27) >0.05 | 20.63 (3.85–82.55) 283.33 (4.38–1173.02) >0.05 | 49.66 (0.89–120.68) 43.32 (1.49–142.11) >0.05 |
| Benign tumor group (both sexes) | 176.09 (4.35–886.19) | 297.21 (3.83–1046.05) | 23.39 (4.74–65.74) |
| Stage I | |||
| Living patients Patients deceased p | 279.16 (11.84–869.28) 200.21 (60.38–239.041) >0.05 | 320.56 (31.70–1173.02) 29.41 (3.85–50.73) >0.05 | 67.98 (37.92–120.68) 37.16 (3.89–70.29) >0.05 |
| Stage II | |||
| Living patients Patients deceased p | 549.48 (4.06–868.17) 24.67 (19.18–33.02) 0.04 * | 94.86 (5.28–364.07) no data >0.05 | 42.59 (11.24–67.74) 35.74 (4.25–96.54) >0.05 |
| Stage III | |||
| Living patients Patients deceased p | 233.14 (46.37–272.14) 254.61 (4.04–784.15) >0.05 | 9.70 (1 patient) 46.64 (4.38–245.86) >0.05 | 5.34 (2.58–9.18) 51.26 (3.33–142.12) 0.008 ** |
| IHC BDNF | IHC MMP-9 | |
|---|---|---|
| Benign tumor group | 101 (98–103) | 97 (89–104) |
| Stage I | ||
| Living patients Patients deceased p | 188 (155–208) 106 (102–116) 0.004 ** | 111 (99–115) 314 (282–335) 0.004 ** |
| Stage II | ||
| Living patients Patients deceased p | 143 (128–151) 121 (115–130) 0.0051 ** | 125 (110–130) 275 (262–285) 0.002 ** |
| Stage III | ||
| Living patients Patients deceased p | 109 (101–118) 151 (145–160) 0.002 ** | 132 (117–145) 242 (228–251) 0.004 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Večurkovská, I.; Mašlanková, J.; Tomečková, V.; Kaťuchová, J.; Kisková, T.; Fröhlichová, L.; Mareková, M.; Stupák, M. Stage-Dependent Levels of Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase 9 in the Prognosis of Colorectal Cancer. Biomedicines 2023, 11, 1839. https://doi.org/10.3390/biomedicines11071839
Večurkovská I, Mašlanková J, Tomečková V, Kaťuchová J, Kisková T, Fröhlichová L, Mareková M, Stupák M. Stage-Dependent Levels of Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase 9 in the Prognosis of Colorectal Cancer. Biomedicines. 2023; 11(7):1839. https://doi.org/10.3390/biomedicines11071839
Chicago/Turabian StyleVečurkovská, Ivana, Jana Mašlanková, Vladimíra Tomečková, Jana Kaťuchová, Terézia Kisková, Lucia Fröhlichová, Mária Mareková, and Marek Stupák. 2023. "Stage-Dependent Levels of Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase 9 in the Prognosis of Colorectal Cancer" Biomedicines 11, no. 7: 1839. https://doi.org/10.3390/biomedicines11071839
APA StyleVečurkovská, I., Mašlanková, J., Tomečková, V., Kaťuchová, J., Kisková, T., Fröhlichová, L., Mareková, M., & Stupák, M. (2023). Stage-Dependent Levels of Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase 9 in the Prognosis of Colorectal Cancer. Biomedicines, 11(7), 1839. https://doi.org/10.3390/biomedicines11071839