Challenges in Diagnosis and Therapeutic Approach of Acute on Chronic Liver Failure—A Review of Current Evidence
Abstract
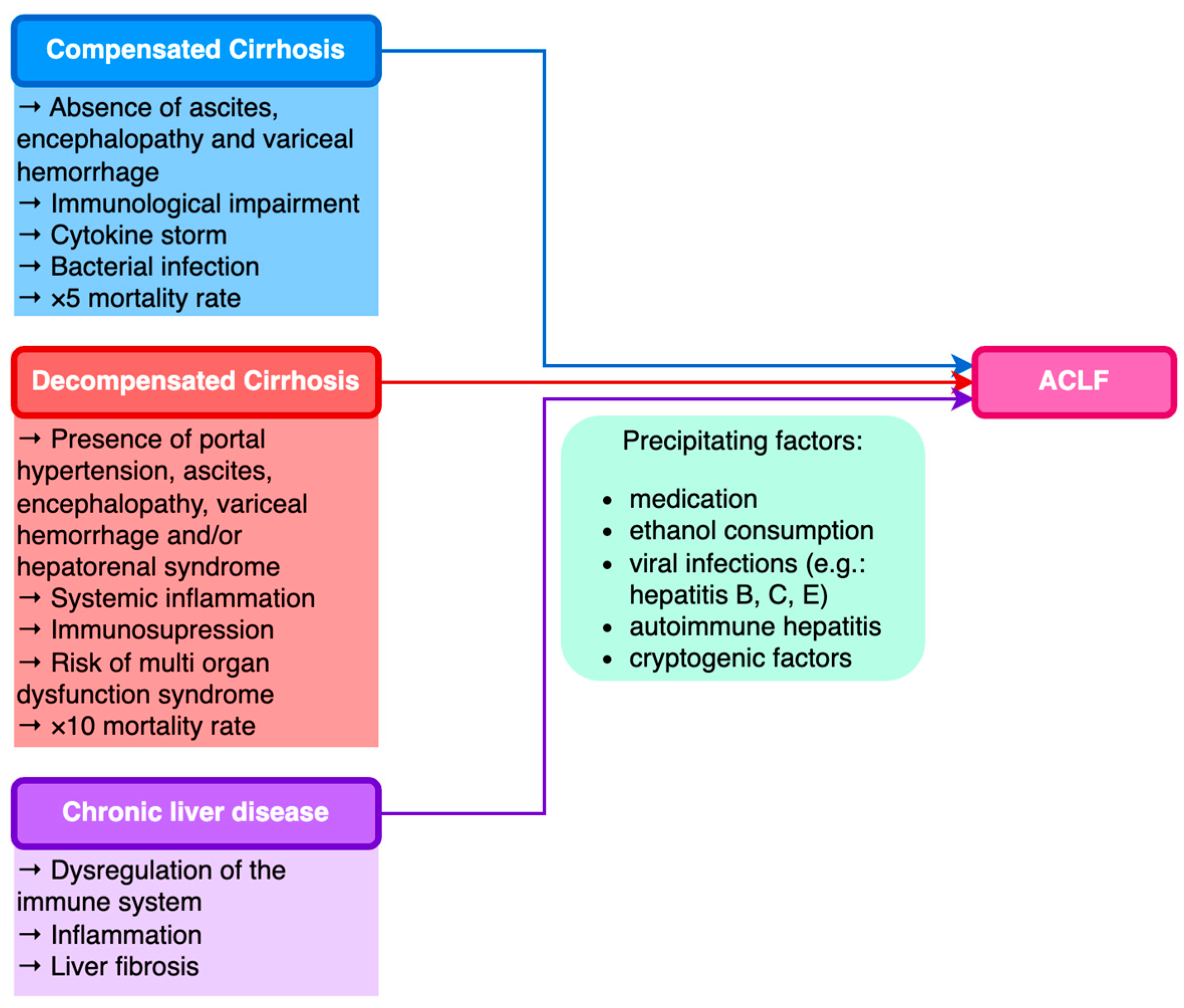
1. Introduction
2. Etiological Factors of ACLF
3. Pathophysiological Hypotheses in ACLF
3.1. Systemic Inflammation
3.2. Metabolic Dysfunction
4. The Involvement of the Gut Microbiome in ACLF
5. Biological Evaluation and Imaging in ACLF
6. ACLF Severity Classification
7. Management of ACLF
7.1. Management of Viral Hepatitis-Related ACLF
7.2. Management of Complications
7.3. Novel Therapeutic Strategies
7.3.1. Granulocyte Colony-Stimulating Factor (GCSF)
7.3.2. Mesenchymal Stem Cell Transplantation
7.3.3. Inhibition of Toll-like Receptor 4 (TLR-4); TAK-242
7.3.4. Oxysterol Sulfates: 25-Hydroxycholesterol 3-Sulfate (25HC3S)
7.3.5. Recombinant Alkaline Phosphatase
7.3.6. Mitofusin-2
7.3.7. Statins
7.3.8. N-Acetylcysteine
7.4. Liver Support and Transplantation
8. Prognosis of ACLF
9. Prevention of ACLF
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Leary, J.G.; Reddy, K.R.; Garcia-Tsao, G.; Biggins, S.W.; Wong, F.; Fallon, M.B.; Subramanian, R.M.; Kamath, P.S.; Thuluvath, P.; Vargas, H.E.; et al. NACSELD Acute-on-Chronic Liver Failure (NACSELD-ACLF) Score Predicts 30-Day Survival in Hospitalized Patients with Cirrhosis. Hepatology 2018, 67, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-Chronic Liver Failure: Consensus Recommendations of the Asian Pacific Association for the Study of the Liver (APASL): An Update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kedarisetty, C.K.; Abbas, Z.; Amarapurkar, D.; Bihari, C.; Chan, A.C.; Chawla, Y.K.; Dokmeci, A.K.; Garg, H.; Ghazinyan, H.; et al. Acute-on-Chronic Liver Failure: Consensus Recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 2014, 8, 453–471. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients with Acute Decompensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; O’Leary, J.G.; Lai, J.C.; Wong, F.; Long, M.D.; Wong, R.J.; Kamath, P.S. Acute-on-Chronic Liver Failure Clinical Guidelines. Off. J. Am. Coll. Gastroenterol. ACG 2022, 117, 225. [Google Scholar] [CrossRef]
- Engelmann, C.; Thomsen, K.L.; Zakeri, N.; Sheikh, M.; Agarwal, B.; Jalan, R.; Mookerjee, R.P. Validation of CLIF-C ACLF Score to Define a Threshold for Futility of Intensive Care Support for Patients with Acute-on-Chronic Liver Failure. Crit. Care 2018, 22, 254. [Google Scholar] [CrossRef]
- Engelmann, C.; Berg, T. Clinical Practice Guidelines for Acute-on-Chronic Liver Failure: Are We Ready for Reaching Global Consensus? Hepatobiliary Surg. Nutr. 2023, 12, 239–243. [Google Scholar] [CrossRef]
- Trebicka, J.; Reiberger, T.; Laleman, W. Gut-Liver Axis Links Portal Hypertension to Acute-on-Chronic Liver Failure. Visc. Med. 2018, 34, 270–275. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, D.J. Acute-on-Chronic Liver Failure. Clin. Mol. Hepatol. 2013, 19, 349–359. [Google Scholar] [CrossRef]
- Gawande, A.; Gupta, G.K.; Gupta, A.; Wanjari, S.J.; Goel, V.; Rathore, V.; Bhardwaj, H.; Nijhawan, S. Acute-on-Chronic Liver Failure: Etiology of Chronic and Acute Precipitating Factors and Their Effect on Mortality. J. Clin. Exp. Hepatol. 2019, 9, 699–703. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.-Y.; Zhang, N.; Li, S.-T.; Zeng, B.; Pavesi, M.; Amorós, À.; Mookerjee, R.P.; Xia, Q.; Xue, F.; et al. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci. Rep. 2016, 6, 25487. [Google Scholar] [CrossRef]
- Yin, S.; Wang, S.J.; Gu, W.Y.; Zhang, Y.; Chen, L.Y.; Li, H. Risk of Different Precipitating Events for Progressing to Acute-on-Chronic Liver Failure in HBV-Related Cirrhotic Patients. J. Dig. Dis. 2017, 18, 292–301. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Y.; Hu, Y.; Wu, W.; Yang, Q.; Zheng, M.; Zhang, S.; Xu, Z.; Wu, Y.; Yan, H.; et al. Acute-on-chronic Liver Failure Precipitated by Hepatic Injury Is Distinct from That Precipitated by Extrahepatic Insults. Hepatology 2015, 62, 232. [Google Scholar] [CrossRef]
- Kumar, M.; Chauhan, R.; Gupta, N.; Hissar, S.; Sakhuja, P.; Sarin, S.K. Spontaneous Increases in Alanine Aminotransferase Levels in Asymptomatic Chronic Hepatitis B Virus-Infected Patients. Gastroenterology 2009, 136, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, A.; Almeida, J.A.; Chawla, Y.K.; Fan, S.T.; Garg, H.; de Silva, H.J.; Hamid, S.S.; Jalan, R.; Komolmit, P.; et al. Acute-on-Chronic Liver Failure: Consensus Recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol. Int. 2009, 3, 269–282. [Google Scholar] [CrossRef]
- Wan, Z.; Wu, Y.; Yi, J.; You, S.; Liu, H.; Sun, Z.; Zhu, B.; Zang, H.; Li, C.; Liu, F.; et al. Combining Serum Cystatin C with Total Bilirubin Improves Short-Term Mortality Prediction in Patients with HBV-Related Acute-On-Chronic Liver Failure. PLoS ONE 2015, 10, e0116968. [Google Scholar] [CrossRef] [PubMed]
- Radha Krishna, Y.; Saraswat, V.A.; Das, K.; Himanshu, G.; Yachha, S.K.; Aggarwal, R.; Choudhuri, G. Clinical Features and Predictors of Outcome in Acute Hepatitis A and Hepatitis E Virus Hepatitis on Cirrhosis. Liver Int. 2009, 29, 392–398. [Google Scholar] [CrossRef]
- Agrawal, S.; Rana, B.S.; Mitra, S.; Duseja, A.; Das, A.; Dhiman, R.K.; Chawla, Y. A Case of Acute-on-Chronic Liver Failure (ACLF) Due to An Uncommon Acute And Chronic Event. J. Clin. Exp. Hepatol. 2018, 8, 95–97. [Google Scholar] [CrossRef]
- Kahraman, A.; Miller, M.; Gieseler, R.K.; Gerken, G.; Scolaro, M.J.; Canbay, A. Non-Alcoholic Fatty Liver Disease in HIV-Positive Patients Predisposes for Acute-on-Chronic Liver Failure: Two Cases. Eur. J. Gastroenterol. Hepatol. 2006, 18, 101. [Google Scholar] [CrossRef]
- Duseja, A.; Chawla, Y.K.; Dhiman, R.K.; Kumar, A.; Choudhary, N.; Taneja, S. Non-Hepatic Insults Are Common Acute Precipitants in Patients with Acute on Chronic Liver Failure (ACLF). Dig. Dis. Sci. 2010, 55, 3188–3192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ke, W.; Xie, J.; Zhao, Z.; Xie, D.; Gao, Z. Comparison of Effects of Hepatitis E or A Viral Superinfection in Patients with Chronic Hepatitis B. Hepatol. Int. 2010, 4, 615–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, J.; Guo, D.; Gao, D.; Huang, W.; Li, Z.; Jia, B. Clinical Analysis of Patients Suffering from Chronic Hepatitis B Superinfected with Other Hepadnaviruses. J. Med. Virol. 2016, 88, 1003–1009. [Google Scholar] [CrossRef]
- Beisel, C.; Addo, M.M.; Schulze zur Wiesch, J. Seroconversion of HBsAG Coincides with Hepatitis A Super-Infection: A Case Report. World J. Clin. Cases 2020, 8, 1651–1655. [Google Scholar] [CrossRef]
- Spada, E.; Genovese, D.; Tosti, M.E.; Mariano, A.; Cuccuini, M.; Proietti, L.; Giuli, C.D.; Lavagna, A.; Crapa, G.E.; Morace, G.; et al. An Outbreak of Hepatitis A Virus Infection with a High Case-Fatality Rate among Injecting Drug Users. J. Hepatol. 2005, 43, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Lefilliatre, P.; Villeneuve, J.-P. Fulminant Hepatitis A in Patients with Chronic Liver Disease. Can. J. Public Health 2000, 91, 168–170. [Google Scholar] [CrossRef]
- Sagnelli, E.; Coppola, N.; Pisaturo, M.; Pisapia, R.; Onofrio, M.; Sagnelli, C.; Catuogno, A.; Scolastico, C.; Piccinino, F.; Filippini, P. Clinical and Virological Improvement of Hepatitis B Virus—Related or Hepatitis C Virus—Related Chronic Hepatitis with Concomitant Hepatitis A Virus Infection. Clin. Infect. Dis. 2006, 42, 1536–1543. [Google Scholar] [CrossRef]
- Deterding, K.; Tegtmeyer, B.; Cornberg, M.; Hadem, J.; Potthoff, A.; Böker, K.H.W.; Tillmann, H.L.; Manns, M.P.; Wedemeyer, H. Hepatitis A Virus Infection Suppresses Hepatitis C Virus Replication and May Lead to Clearance of HCV. J. Hepatol. 2006, 45, 770–778. [Google Scholar] [CrossRef]
- Cacopardo, B.; Nunnari, G.; Nigro, L. Clearance of HCV RNA Following Acute Hepatitis A Superinfection. Dig. Liver Dis. 2009, 41, 371–374. [Google Scholar] [CrossRef]
- Kashyap, P.; Deka, M.; Medhi, S.; DUTTA, S.; Kashyap, K.; Kumari, N. Association of Toll-like Receptor 4 with Hepatitis A Virus Infection in Assam. Acta Virol. 2018, 62, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Rubicz, R.; Yolken, R.; Drigalenko, E.; Carless, M.A.; Dyer, T.D.; Kent Jr, J.; Curran, J.E.; Johnson, M.P.; Cole, S.A.; Fowler, S.P.; et al. Genome-Wide Genetic Investigation of Serological Measures of Common Infections. Eur. J. Hum. Genet. 2015, 23, 1544–1548. [Google Scholar] [CrossRef]
- Kumar, A.; Saraswat, V.A. Hepatitis E and Acute-on-Chronic Liver Failure. J. Clin. Exp. Hepatol. 2013, 3, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Son, H.J.; Lee, S.S.; Jeon, H.; Cho, J.-K.; Kim, H.J.; Cha, R.R.; Lee, J.M.; Kim, H.J.; Jung, W.T.; et al. Acute Hepatitis E Virus Superinfection Increases Mortality in Patients with Cirrhosis. BMC Infect. Dis. 2022, 22, 62. [Google Scholar] [CrossRef]
- Kmush, B.; Wierzba, T.; Krain, L.; Nelson, K.; Labrique, A.B. Epidemiology of Hepatitis E in Low- and Middle-Income Countries of Asia and Africa. Semin. Liver Dis. 2013, 33, 015–029. [Google Scholar] [CrossRef] [PubMed]
- Artru, F.; Louvet, A.; Ruiz, I.; Levesque, E.; Labreuche, J.; Ursic-Bedoya, J.; Lassailly, G.; Dharancy, S.; Boleslawski, E.; Lebuffe, G.; et al. Liver Transplantation in the Most Severely Ill Cirrhotic Patients: A Multicenter Study in Acute-on-Chronic Liver Failure Grade 3. J. Hepatol. 2017, 67, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Matsumoto, N.; Ishii, T.; Arima, S.; Shibuya, S.; Honda, M.; Sasaki-Tanaka, R.; Masuzaki, R.; Kanezawa, S.; Nishizawa, T.; et al. Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare. Viruses 2023, 15, 183. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Saisho, H. Acute Hepatitis C Virus Infection, 1986-2001: A Rare Cause of Fulminant Hepatitis in Chiba, Japan. Hepatogastroenterology 2004, 51, 556–558. [Google Scholar]
- Liang, T.J.; Jeffers, L.; Reddy, R.K.; Silva, M.O.; Cheinquer, H.; Findor, A.; De Medina, M.; Yarbough, P.O.; Reyes, G.R.; Schiff, E.R. Fulminant or Subfulminant Non-A, Non-B Viral Hepatitis: The Role of Hepatitis C and E Viruses. Gastroenterology 1993, 104, 556–562. [Google Scholar] [CrossRef]
- Jain, A.; Kar, P.; Madan, K.; Das, U.P.; Budhiraja, S.; Gopalkrishna, V.; Sharma, J.K.; Das, B.C. Hepatitis C Virus Infection in Sporadic Fulminant Viral Hepatitis in North India: Cause or Co-Factor? Eur. J. Gastroenterol. Hepatol. 1999, 11, 1231–1237. [Google Scholar] [CrossRef]
- Maheshwari, A.; Ray, S.; Thuluvath, P.J. Acute Hepatitis C. Lancet 2008, 372, 321–332. [Google Scholar] [CrossRef]
- Rao, A.; Rule, J.A.; Cerro-Chiang, G.; Stravitz, R.T.; McGuire, B.M.; Lee, G.; Fontana, R.J.; Lee, W.M. Role of Hepatitis C Infection in Acute Liver Injury/Acute Liver Failure in North America. Dig. Dis. Sci. 2023, 68, 304–311. [Google Scholar] [CrossRef]
- Younis, B.B.; Arshad, R.; Khurhsid, S.; Masood, J.; Nazir, F.; Tahira, M. Fulminant Hepatic Failure (FHF) Due to Acute Hepatitis C. Pak. J. Med. Sci. 2015, 31, 1009–1011. [Google Scholar] [CrossRef]
- Sagnelli, E.; Pisaturo, M.; Stanzione, M.; Messina, V.; Alessio, L.; Sagnelli, C.; Starace, M.; Pasquale, G.; Coppola, N. Clinical Presentation, Outcome, and Response to Therapy among Patients with Acute Exacerbation of Chronic Hepatitis C. Clin. Gastroenterol. Hepatol. 2013, 11, 1174–1180.e11. [Google Scholar] [CrossRef]
- Thiel, A.M.; Rissland, J.; Lammert, F.; Casper, M. Acute liver failure as a rare case of a frequent disease. Z. Gastroenterol. 2018, 56, 255–258. [Google Scholar] [CrossRef]
- Trebicka, J.; Amoros, A.; Pitarch, C.; Titos, E.; Alcaraz-Quiles, J.; Schierwagen, R.; Deulofeu, C.; Fernandez-Gomez, J.; Piano, S.; Caraceni, P.; et al. Addressing Profiles of Systemic Inflammation Across the Different Clinical Phenotypes of Acutely Decompensated Cirrhosis. Front. Immunol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological Bacterial Translocation in Liver Cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef]
- Trebicka, J.; Gu, W.; Ibáñez-Samaniego, L.; Hernández-Gea, V.; Pitarch, C.; Garcia, E.; Procopet, B.; Giráldez, Á.; Amitrano, L.; Villanueva, C.; et al. Rebleeding and Mortality Risk Are Increased by ACLF but Reduced by Pre-Emptive TIPS. J. Hepatol. 2020, 73, 1082–1091. [Google Scholar] [CrossRef]
- Costa, D.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.; Paternostro, R.; Schwabl, P.; Scheiner, B.; Stättermayer, A.F.; Pinter, M.; et al. Systemic Inflammation Increases across Distinct Stages of Advanced Chronic Liver Disease and Correlates with Decompensation and Mortality. J. Hepatol. 2021, 74, 819–828. [Google Scholar] [CrossRef]
- Hernández-Gea, V.; Procopet, B.; Giráldez, Á.; Amitrano, L.; Villanueva, C.; Thabut, D.; Ibañez-Samaniego, L.; Silva-Junior, G.; Martinez, J.; Genescà, J.; et al. Preemptive-TIPS Improves Outcome in High-Risk Variceal Bleeding: An Observational Study. Hepatology 2019, 69, 282–293. [Google Scholar] [CrossRef]
- Kumar, R.; Kerbert, A.J.C.; Sheikh, M.F.; Roth, N.; Calvao, J.A.F.; Mesquita, M.D.; Barreira, A.I.; Gurm, H.S.; Ramsahye, K.; Mookerjee, R.P.; et al. Determinants of Mortality in Patients with Cirrhosis and Uncontrolled Variceal Bleeding. J. Hepatol. 2021, 74, 66–79. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Gerbes, A.L.; Labenz, J.; Appenrodt, B.; Dollinger, M.; Gundling, F.; Gülberg, V.; Holstege, A.; Lynen-Jansen, P.; Steib, C.J.; Trebicka, J.; et al. Aktualisierte S2k-Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) „Komplikationen der Leberzirrhose“: AWMF-Nr.: 021-017. Z. Gastroenterol. 2019, 57, 611–680. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Kunz, D.; Yagmur, E.; Timmer-Stranghöner, A.; Vidacek, D.; Siewert, E.; Bach, J.; Geier, A.; Purucker, E.A.; Gressner, A.M.; et al. Patients with Acute on Chronic Liver Failure Display ‘Sepsis-like’ Immune Paralysis. J. Hepatol. 2005, 42, 195–201. [Google Scholar] [CrossRef]
- Mahmud, N.; Kaplan, D.E.; Taddei, T.H.; Goldberg, D.S. Incidence and Mortality of Acute-on-Chronic Liver Failure Using Two Definitions in Patients with Compensated Cirrhosis. Hepatology 2019, 69, 2150–2163. [Google Scholar] [CrossRef]
- Jalan, R.; Yurdaydin, C.; Bajaj, J.S.; Acharya, S.K.; Arroyo, V.; Lin, H.-C.; Gines, P.; Kim, W.R.; Kamath, P.S. Toward an Improved Definition of Acute-on-Chronic Liver Failure. Gastroenterology 2014, 147, 4–10. [Google Scholar] [CrossRef]
- Wlodzimirow, K.A.; Eslami, S.; Abu-Hanna, A.; Nieuwoudt, M.; Chamuleau, R.A.F.M. A Systematic Review on Prognostic Indicators of Acute on Chronic Liver Failure and Their Predictive Value for Mortality. Liver Int. 2013, 33, 40–52. [Google Scholar] [CrossRef]
- Singer, C.E.; Vasile, C.M.; Popescu, M.; Popescu, A.I.S.; Marginean, I.C.; Iacob, G.A.; Popescu, M.D.; Marginean, C.M. Role of Iron Deficiency in Heart Failure—Clinical and Treatment Approach: An Overview. Diagnostics 2023, 13, 304. [Google Scholar] [CrossRef]
- Avolio, A.W.; Gaspari, R.; Teofili, L.; Bianco, G.; Spinazzola, G.; Soave, P.M.; Paiano, G.; Francesconi, A.G.; Arcangeli, A.; Nicolotti, N.; et al. Postoperative Respiratory Failure in Liver Transplantation: Risk Factors and Effect on Prognosis. PLoS ONE 2019, 14, e0211678. [Google Scholar] [CrossRef]
- Mattos, D.Â.Z.; Mattos, D.A.A. Letter to the Editor: Acute-on-Chronic Liver Failure: Conceptual Divergences. Hepatology 2019, 70, 1076. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jansen, C.; Jimenez, C.; et al. PREDICT Identifies Precipitating Events Associated with the Clinical Course of Acutely Decompensated Cirrhosis. J. Hepatol. 2021, 74, 1097–1108. [Google Scholar] [CrossRef]
- Clària, J.; Stauber, R.E.; Coenraad, M.J.; Moreau, R.; Jalan, R.; Pavesi, M.; Amorós, À.; Titos, E.; Alcaraz-Quiles, J.; Oettl, K.; et al. Systemic Inflammation in Decompensated Cirrhosis: Characterization and Role in Acute-on-Chronic Liver Failure. Hepatology 2016, 64, 1249–1264. [Google Scholar] [CrossRef]
- Thabut, D.; Tazi, K.A.; Bonnefont-Rousselot, D.; Aller, M.; Farges, O.; Guimont, M.C.; Tellier, Z.; Guichard, C.; Ogier-Denis, E.; Poynard, T.; et al. High-Density Lipoprotein Administration Attenuates Liver Proinflammatory Response, Restores Liver Endothelial Nitric Oxide Synthase Activity, and Lowers Portal Pressure in Cirrhotic Rats. Hepatology 2007, 46, 1893–1906. [Google Scholar] [CrossRef]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-Chronic Liver Failure: An Update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef]
- Clària, J.; Arroyo, V.; Moreau, R. The Acute-on-Chronic Liver Failure Syndrome, or When the Innate Immune System Goes Astray. J. Immunol. 2016, 197, 3755–3761. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Kamath, P.S.; Jalan, R.; Ginès, P.; Nevens, F.; Fernández, J.; To, U.; García-Tsao, G.; Schnabl, B. Acute-on-Chronic Liver Failure in Cirrhosis. Nat. Rev. Dis. Prim. 2016, 2, 1–18. [Google Scholar] [CrossRef]
- Kubes, P.; Mehal, W.Z. Sterile Inflammation in the Liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Csak, T.; Satishchandran, A.; Kodys, K.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Szabo, G. STING-IRF3 Pathway Links Endoplasmic Reticulum Stress with Hepatocyte Apoptosis in Early Alcoholic Liver Disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16544–16549. [Google Scholar] [CrossRef]
- Ganeshan, K.; Nikkanen, J.; Man, K.; Leong, Y.A.; Sogawa, Y.; Maschek, J.A.; Van Ry, T.; Chagwedera, D.N.; Cox, J.E.; Chawla, A. Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell 2019, 177, 399–413.e12. [Google Scholar] [CrossRef]
- López-Vicario, C.; Checa, A.; Urdangarin, A.; Aguilar, F.; Alcaraz-Quiles, J.; Caraceni, P.; Amorós, A.; Pavesi, M.; Gómez-Cabrero, D.; Trebicka, J.; et al. Targeted Lipidomics Reveals Extensive Changes in Circulating Lipid Mediators in Patients with Acutely Decompensated Cirrhosis☆. J. Hepatol. 2020, 73, 817–828. [Google Scholar] [CrossRef]
- Van Wyngene, L.; Vandewalle, J.; Libert, C. Reprogramming of Basic Metabolic Pathways in Microbial Sepsis: Therapeutic Targets at Last? EMBO Mol. Med. 2018, 10, e8712. [Google Scholar] [CrossRef]
- Moreau, R.; Clària, J.; Aguilar, F.; Fenaille, F.; Lozano, J.J.; Junot, C.; Colsch, B.; Caraceni, P.; Trebicka, J.; Pavesi, M.; et al. Blood Metabolomics Uncovers Inflammation-Associated Mitochondrial Dysfunction as a Potential Mechanism Underlying ACLF. J. Hepatol. 2020, 72, 688–701. [Google Scholar] [CrossRef]
- Gómez-Hurtado, I.; Such, J.; Sanz, Y.; Francés, R. Gut Microbiota-Related Complications in Cirrhosis. World J. Gastroenterol. 2014, 20, 15624–15631. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Gosalbes, M.J.; Abellan, J.J.; Durbán, A.; Pérez-Cobas, A.E.; Latorre, A.; Moya, A. Metagenomics of Human Microbiome: Beyond 16s RDNA. Clin. Microbiol. Infect. 2012, 18 (Suppl. S4), 47–49. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Doré, J.; Simrén, M.; Buttle, L.; Guarner, F. Hot Topics in Gut Microbiota. United Eur. Gastroenterol. J. 2013, 1, 311–318. [Google Scholar] [CrossRef]
- Acharya, C.; Bajaj, J.S. Altered Microbiome in Patients With Cirrhosis and Complications. Clin. Gastroenterol. Hepatol. 2019, 17, 307–321. [Google Scholar] [CrossRef]
- Schwenger, K.J.; Clermont-Dejean, N.; Allard, J.P. The Role of the Gut Microbiome in Chronic Liver Disease: The Clinical Evidence Revised. JHEP Rep. 2019, 1, 214–226. [Google Scholar] [CrossRef]
- Duseja, A.; Chawla, Y.K. Obesity and NAFLD: The Role of Bacteria and Microbiota. Clin. Liver Dis. 2014, 18, 59–71. [Google Scholar] [CrossRef]
- Artzner, T.; Michard, B.; Weiss, E.; Barbier, L.; Noorah, Z.; Merle, J.-C.; Paugam-Burtz, C.; Francoz, C.; Durand, F.; Soubrane, O.; et al. Liver Transplantation for Critically Ill Cirrhotic Patients: Stratifying Utility Based on Pretransplant Factors. Am. J. Transpl. 2020, 20, 2437–2448. [Google Scholar] [CrossRef]
- Inherited Metabolic Liver Disease Collaboration Group, Chinese Society of Hepatology, Chinese Medical Association. [Guidelines for the diagnosis and treatment of hepatolenticular degeneration (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi 2022, 30, 9–20. [Google Scholar] [CrossRef]
- Steve, R.J.; Gnanadurai, F.J.; Anantharam, R.; Jeyaseelan, V.; Zachariah, U.G.; Goel, A.; Chundamannil, E.E.; Abraham, P. Expanded Diagnostic Approach to Hepatitis E Virus Detection in Patients with Acute-on-Chronic Liver Failure: A Pilot Study. Indian J. Med. Microbiol. 2018, 36, 391–396. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. EASL-CLIF Consortium CANONIC Study Acute-on-Chronic Liver Failure: A New Syndrome That Will Re-Classify Cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef]
- Xie, F.; Yan, L.; Lu, J.; Zheng, T.; Shi, C.; Ying, J.; Shen, R.; Yang, J. Effects of Nucleoside Analogue on Patients with Chronic Hepatitis B-Associated Liver Failure: Meta-Analysis. PLoS ONE 2013, 8, e54773. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; O’Leary, J.G.; Reddy, K.R.; Wong, F.; Biggins, S.W.; Patton, H.; Fallon, M.B.; Garcia-Tsao, G.; Maliakkal, B.; Malik, R.; et al. Survival in Infection-Related Acute-on-Chronic Liver Failure Is Defined by Extrahepatic Organ Failures. Hepatology 2014, 60, 250–256. [Google Scholar] [CrossRef]
- Sargenti, K.; Prytz, H.; Nilsson, E.; Bertilsson, S.; Kalaitzakis, E. Bacterial Infections in Alcoholic and Nonalcoholic Liver Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1080–1086. [Google Scholar] [CrossRef]
- Solà, E.; Solé, C.; Simón-Talero, M.; Martín-Llahí, M.; Castellote, J.; Garcia-Martínez, R.; Moreira, R.; Torrens, M.; Márquez, F.; Fabrellas, N.; et al. Midodrine and Albumin for Prevention of Complications in Patients with Cirrhosis Awaiting Liver Transplantation. A Randomized Placebo-Controlled Trial. J. Hepatol. 2018, 69, 1250–1259. [Google Scholar] [CrossRef]
- O’Brien, A.; Kamath, P.S.; Trotter, J. MACHT—Outpatient Albumin Infusions Do Not Prevent Complications of Cirrhosis in Patients on the Liver Transplant Waiting List. J. Hepatol. 2018, 69, 1217–1218. [Google Scholar] [CrossRef]
- Caraceni, P.; Riggio, O.; Angeli, P.; Alessandria, C.; Neri, S.; Foschi, F.G.; Levantesi, F.; Airoldi, A.; Boccia, S.; Svegliati-Baroni, G.; et al. Long-Term Albumin Administration in Decompensated Cirrhosis (ANSWER): An Open-Label Randomised Trial. Lancet 2018, 391, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Freemantle, N.; Forrest, E.; Kallis, Y.; Ryder, S.D.; Wright, G.; Portal, A.J.; Becares Salles, N.; Gilroy, D.W.; O’Brien, A. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N. Engl. J. Med. 2021, 384, 808–817. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Skene, S.S.; Shabir, Z.; Maini, A.; Sylvestre, Y.; Bennett, K.; Bevan, S.; O’Beirne, J.; Forrest, E.; Portal, J.; et al. Administration of Albumin Solution Increases Serum Levels of Albumin in Patients With Chronic Liver Failure in a Single-Arm Feasibility Trial. Clin. Gastroenterol. Hepatol. 2018, 16, 748–755.e6. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Stravitz, R.T. Intensive Care Unit Management of Patients with Liver Failure. Clin. Liver Dis. 2014, 18, 957–978. [Google Scholar] [CrossRef]
- Olson, J.C.; Wendon, J.A.; Kramer, D.J.; Arroyo, V.; Jalan, R.; Garcia-Tsao, G.; Kamath, P.S. Intensive Care of the Patient with Cirrhosis. Hepatology 2011, 54, 1864–1872. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN Guideline on Clinical Nutrition in Liver Disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines on Nutrition in Chronic Liver Disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Moreno, C.; Deltenre, P.; Senterre, C.; Louvet, A.; Gustot, T.; Bastens, B.; Hittelet, A.; Piquet, M.-A.; Laleman, W.; Orlent, H.; et al. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology 2016, 150, 903–910.e8. [Google Scholar] [CrossRef]
- Yang, J.; Sun, H.; Liu, Q. The Comparative Efficacy and Safety of Entecavir and Lamivudine in Patients with HBV-Associated Acute-on-Chronic Liver Failure: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 5802674. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Hu, C.; Chen, Y.; Zhang, R.; Fu, S.; Zhou, M.; Gao, Z.; Fu, M.; Yan, T.; Yang, Y.; et al. Short-Term and Long-Term Safety and Efficacy of Tenofovir Alafenamide, Tenofovir Disoproxil Fumarate and Entecavir Treatment of Acute-on-Chronic Liver Failure Associated with Hepatitis B. BMC Infect. Dis. 2021, 21, 567. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, G.; Chen, X.; Zhang, H.; Jiang, D.; Yang, G. Initial Combination Anti-Viral Therapy with Lamivudine and Adefovir Dipivoxil Decreases Short-Term Fatality Rate of Hepatitis-B-Virus-Related Acute-on-Chronic Liver Failure. Virol. J. 2015, 12, 97. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.Y.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific Clinical Practice Guidelines on the Management of Hepatitis B: A 2015 Update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, J.; Shao, L.; Xin, J.; Jiang, L.; Zhou, Q.; Shi, D.; Jiang, J.; Sun, S.; Jin, L.; et al. Development of Diagnostic Criteria and a Prognostic Score for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Gut 2018, 67, 2181–2191. [Google Scholar] [CrossRef]
- Zhao, R.-H.; Shi, Y.; Zhao, H.; Wu, W.; Sheng, J.-F. Acute-on-Chronic Liver Failure in Chronic Hepatitis B: An Update. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Sandahl, T.D.; Kazankov, K.; George, J.; Grønbæk, H. Liver-Related Effects of Chronic Hepatitis C Antiviral Treatment. World J. Gastroenterol. 2020, 26, 2931–2947. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; Hezode, C.; Trinh, R.; Kowdley, K.V.; Zeuzem, S.; Agarwal, K.; Shiffman, M.L.; Wedemeyer, H.; Berg, T.; Yoshida, E.M.; et al. ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis. N. Engl. J. Med. 2014, 370, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; Schiff, E.R.; Vierling, J.M.; Landis, C.; Fontana, R.J.; Yang, R.; McPhee, F.; Hughes, E.A.; Noviello, S.; Swenson, E.S. Daclatasvir with Sofosbuvir and Ribavirin for Hepatitis C Virus Infection with Advanced Cirrhosis or Post-Liver Transplantation Recurrence. Hepatology 2016, 63, 1493–1505. [Google Scholar] [CrossRef]
- Pungpapong, S.; Aqel, B.; Leise, M.; Werner, K.T.; Murphy, J.L.; Henry, T.M.; Ryland, K.; Chervenak, A.E.; Watt, K.D.; Vargas, H.E.; et al. Multicenter Experience Using Simeprevir and Sofosbuvir with or without Ribavirin to Treat Hepatitis C Genotype 1 after Liver Transplant. Hepatology 2015, 61, 1880–1886. [Google Scholar] [CrossRef]
- Ferenci, P.; Bernstein, D.; Lalezari, J.; Cohen, D.; Luo, Y.; Cooper, C.; Tam, E.; Marinho, R.T.; Tsai, N.; Nyberg, A.; et al. ABT-450/r-Ombitasvir and Dasabuvir with or without Ribavirin for HCV. N. Engl. J. Med. 2014, 370, 1983–1992. [Google Scholar] [CrossRef]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained Virologic Response to Interferon-Free Therapies Ameliorates HCV-Induced Portal Hypertension. J. Hepatol. 2016, 65, 692–699. [Google Scholar] [CrossRef]
- Mauro, E.; Crespo, G.; Montironi, C.; Londoño, M.-C.; Hernández-Gea, V.; Ruiz, P.; Sastre, L.; Lombardo, J.; Mariño, Z.; Díaz, A.; et al. Portal Pressure and Liver Stiffness Measurements in the Prediction of Fibrosis Regression after Sustained Virological Response in Recurrent Hepatitis C. Hepatology 2018, 67, 1683–1694. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for Chronic Hepatitis E Virus Infection in Transplant Recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef]
- Goyal, R.; Kumar, A.; Panda, S.K.; Paul, S.B.; Acharya, S.K. Ribavirin Therapy for Hepatitis E Virus-Induced Acute on Chronic Liver Failure: A Preliminary Report. Antivir. Ther. 2012, 17, 1091–1096. [Google Scholar] [CrossRef]
- Pischke, S.; Hardtke, S.; Bode, U.; Birkner, S.; Chatzikyrkou, C.; Kauffmann, W.; Bara, C.L.; Gottlieb, J.; Wenzel, J.; Manns, M.P.; et al. Ribavirin Treatment of Acute and Chronic Hepatitis E: A Single-Centre Experience. Liver Int. 2013, 33, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-Chronic Liver Failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D.; Sengupta, N.; Mellinger, J.; Ratz, D.; Lok, A.S.-F.; Su, G.L. A Risk Score to Predict the Development of Hepatic Encephalopathy in a Population-Based Cohort of Patients with Cirrhosis. Hepatology 2018, 68, 1498–1507. [Google Scholar] [CrossRef]
- Bajaj, J.S.; O’Leary, J.G.; Tandon, P.; Wong, F.; Garcia-Tsao, G.; Kamath, P.S.; Maliakkal, B.; Biggins, S.W.; Thuluvath, P.J.; Fallon, M.B.; et al. Hepatic Encephalopathy Is Associated With Mortality in Patients With Cirrhosis Independent of Other Extrahepatic Organ Failures. Clin. Gastroenterol. Hepatol. 2017, 15, 565–574.e4. [Google Scholar] [CrossRef]
- Piscaglia, A.C.; Arena, V.; Passalacqua, S.; Gasbarrini, A. A Case of Granulocyte-Colony Stimulating Factor/Plasmapheresis-Induced Activation of Granulocyte-Colony Stimulating Factor-Positive Hepatic Progenitors in Acute-on-Chronic Liver Failure. Hepatology 2015, 62, 649–652. [Google Scholar] [CrossRef]
- Gustot, T. Beneficial Role of G-CSF in Acute-on-Chronic Liver Failure: Effects on Liver Regeneration, Inflammation/Immunoparalysis or Both? Liver Int. 2014, 34, 484–486. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Xue, R.; Meng, Q.; Dong, J.; Li, J.; Yao, Q.; Zhu, Y.; Yu, H. Clinical Performance of Stem Cell Therapy in Patients with Acute-on-Chronic Liver Failure: A Systematic Review and Meta-Analysis. J. Transl. Med. 2018, 16, 126. [Google Scholar] [CrossRef]
- Engelmann, C.; Sheikh, M.; Sharma, S.; Kondo, T.; Loeffler-Wirth, H.; Zheng, Y.B.; Novelli, S.; Hall, A.; Kerbert, A.J.C.; Macnaughtan, J.; et al. Toll-like Receptor 4 Is a Therapeutic Target for Prevention and Treatment of Liver Failure. J. Hepatol. 2020, 73, 102–112. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, W.; Tamaki, Z.; Shi, B.; Yeldandi, A.; Tsukimi, Y.; Yamasaki, M.; Varga, J. Pharmacological Inhibition of Toll-Like Receptor-4 Signaling by TAK242 Prevents and Induces Regression of Experimental Organ Fibrosis. Front. Immunol. 2018, 9, 2434. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Klein, J.; Ernst, M.; Christmann, A.; Tropper, M.; Leykauf, T.; Kreis, W.; Munkert, J. Knockout of Arabidopsis Thaliana VEP1, Encoding a PRISE (Progesterone 5β-Reductase/Iridoid Synthase-Like Enzyme), Leads to Metabolic Changes in Response to Exogenous Methyl Vinyl Ketone (MVK). Metabolites 2021, 12, 11. [Google Scholar] [CrossRef]
- Peters, E.; Masereeuw, R.; Pickkers, P. The Potential of Alkaline Phosphatase as a Treatment for Sepsis-Associated Acute Kidney Injury. Nephron Clin. Pract. 2014, 127, 144–148. [Google Scholar] [CrossRef]
- Gustot, T.; Jalan, R. Acute-on-Chronic Liver Failure in Patients with Alcohol-Related Liver Disease. J. Hepatol. 2019, 70, 319–327. [Google Scholar] [CrossRef]
- Hall, A.R.; Burke, N.; Dongworth, R.K.; Hausenloy, D.J. Mitochondrial Fusion and Fission Proteins: Novel Therapeutic Targets for Combating Cardiovascular Disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef]
- Xue, R.; Yang, J.; Jia, L.; Zhu, X.; Wu, J.; Zhu, Y.; Meng, Q. Mitofusin2, as a Protective Target in the Liver, Controls the Balance of Apoptosis and Autophagy in Acute-on-Chronic Liver Failure. Front. Pharmacol. 2019, 10, 601. [Google Scholar] [CrossRef]
- Bosch, J.; Gracia-Sancho, J.; Abraldes, J.G. Cirrhosis as New Indication for Statins. Gut 2020, 69, 953–962. [Google Scholar] [CrossRef]
- Trebicka, J.; Hennenberg, M.; Laleman, W.; Shelest, N.; Biecker, E.; Schepke, M.; Nevens, F.; Sauerbruch, T.; Heller, J. Atorvastatin Lowers Portal Pressure in Cirrhotic Rats by Inhibition of RhoA/Rho-Kinase and Activation of Endothelial Nitric Oxide Synthase. Hepatology 2007, 46, 242–253. [Google Scholar] [CrossRef]
- Marrone, G.; Maeso-Díaz, R.; García-Cardena, G.; Abraldes, J.G.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. KLF2 Exerts Antifibrotic and Vasoprotective Effects in Cirrhotic Rat Livers: Behind the Molecular Mechanisms of Statins. Gut 2015, 64, 1434–1443. [Google Scholar] [CrossRef]
- Pollo-Flores, P.; Soldan, M.; Santos, U.C.; Kunz, D.G.; Mattos, D.E.; da Silva, A.C.; Marchiori, R.C.; Rezende, G.F. da M. Three Months of Simvastatin Therapy vs. Placebo for Severe Portal Hypertension in Cirrhosis: A Randomized Controlled Trial. Dig. Liver Dis. 2015, 47, 957–963. [Google Scholar] [CrossRef]
- Millea, P.J. N-Acetylcysteine: Multiple Clinical Applications. Am. Fam. Physician 2009, 80, 265–269. [Google Scholar]
- Otrubová, O.; Turecký, L.; Uličná, O.; Janega, P.; Luha, J.; Muchová, J. Therapeutic Effects of N-Acetyl-L-Cysteine on Liver Damage Induced by Long-Term CCl4 Administration. Gen. Physiol. Biophys. 2018, 37, 23–31. [Google Scholar] [CrossRef]
- Wang, M.-L.; Yin, X.-J.; Li, X.-L.; Wang, F.-D.; Zhou, J.; Tao, Y.-C.; Wang, Y.-H.; Wu, D.-B.; Chen, E.-Q. Retrospective Analysis of the Clinical Efficacy of N-Acetylcysteine in the Treatment of Hepatitis B Virus Related Acute-on-Chronic Liver Failure. Front. Med. 2021, 8, 724224. [Google Scholar] [CrossRef]
- Khanam, A.; Kottilil, S. Acute-on-Chronic Liver Failure: Pathophysiological Mechanisms and Management. Front. Med. 2021, 8, 752875. [Google Scholar] [CrossRef]
- Chan, A.C.Y.; Fan, S.T. Criteria for Liver Transplantation in ACLF and Outcome. Hepatol. Int. 2015, 9, 355–359. [Google Scholar] [CrossRef]
- Sundaram, V.; Jalan, R.; Wu, T.; Volk, M.L.; Asrani, S.K.; Klein, A.S.; Wong, R.J. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology 2019, 156, 1381–1391.e3. [Google Scholar] [CrossRef]
- Abbas, N.; Rajoriya, N.; Elsharkawy, A.M.; Chauhan, A. Acute-on-Chronic Liver Failure (ACLF) in 2022: Have Novel Treatment Paradigms Already Arrived? Expert Rev. Gastroenterol. Hepatol. 2022, 16, 639–652. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Bajaj, J.S. Acute-on-Chronic Liver Failure and Liver Transplantation: Putting the Cart Before the Horse in Data Analyses and Advocating for Model for End-Stage Liver Disease Exceptions. Liver Transpl. 2022, 28, 535–538. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Waleed, M.; Bell, M.G.; Nelson, M.; Wong, R.; Sundaram, V.; Singal, A.K. Systematic Review with Meta-Analysis: Liver Transplant Provides Survival Benefit in Patients with Acute on Chronic Liver Failure. Aliment. Pharmacol. Ther. 2020, 52, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Subramanian, R.M. Current Evidence for Extracorporeal Liver Support Systems in Acute Liver Failure and Acute-on-Chronic Liver Failure. Crit. Care Clin. 2016, 32, 439–451. [Google Scholar] [CrossRef]
- Kribben, A.; Gerken, G.; Haag, S.; Herget-Rosenthal, S.; Treichel, U.; Betz, C.; Sarrazin, C.; Hoste, E.; Van Vlierberghe, H.; Escorsell, A.; et al. Effects of Fractionated Plasma Separation and Adsorption on Survival in Patients with Acute-on-Chronic Liver Failure. Gastroenterology 2012, 142, 782–789.e3. [Google Scholar] [CrossRef]
- Bañares, R.; Nevens, F.; Larsen, F.S.; Jalan, R.; Albillos, A.; Dollinger, M.; Saliba, F.; Sauerbruch, T.; Klammt, S.; Ockenga, J.; et al. Extracorporeal Albumin Dialysis with the Molecular Adsorbent Recirculating System in Acute-on-Chronic Liver Failure: The RELIEF Trial. Hepatology 2013, 57, 1153–1162. [Google Scholar] [CrossRef]
- Yue-Meng, W.; Yang, L.-H.; Yang, J.-H.; Xu, Y.; Yang, J.; Song, G.-B. The Effect of Plasma Exchange on Entecavir-Treated Chronic Hepatitis B Patients with Hepatic de-Compensation and Acute-on-Chronic Liver Failure. Hepatol. Int. 2016, 10, 462–469. [Google Scholar] [CrossRef]
- Jalan, R.; Pavesi, M.; Saliba, F.; Amorós, A.; Fernandez, J.; Holland-Fischer, P.; Sawhney, R.; Mookerjee, R.; Caraceni, P.; Moreau, R.; et al. The CLIF Consortium Acute Decompensation Score (CLIF-C ADs) for Prognosis of Hospitalised Cirrhotic Patients without Acute-on-Chronic Liver Failure. J. Hepatol. 2015, 62, 831–840. [Google Scholar] [CrossRef]
- Thanapirom, K.; Teerasarntipan, T.; Treeprasertsuk, S.; Choudhury, A.; Sahu, M.K.; Maiwall, R.; Pamecha, V.; Moreau, R.; Al Mahtab, M.; Chawla, Y.K.; et al. Impact of Compensated Cirrhosis on Survival in Patients with Acute-on-Chronic Liver Failure. Hepatol. Int. 2022, 16, 171–182. [Google Scholar] [CrossRef]
- Shalimar; Kumar, D.; Vadiraja, P.K.; Nayak, B.; Thakur, B.; Das, P.; Gupta, S.D.; Panda, S.K.; Acharya, S.K. Acute on Chronic Liver Failure Because of Acute Hepatic Insults: Etiologies, Course, Extrahepatic Organ Failure and Predictors of Mortality. J. Gastroenterol. Hepatol. 2016, 31, 856–864. [Google Scholar] [CrossRef]
- Shin, J.; Yu, J.H.; Jin, Y.-J.; Yim, H.J.; Jung, Y.K.; Yang, J.M.; Song, D.S.; Kim, Y.S.; Kim, S.G.; Kim, D.J.; et al. Acute-on-Chronic Liver Failure as a Major Predictive Factor for Mortality in Patients with Variceal Bleeding. Clin. Mol. Hepatol. 2020, 26, 540–553. [Google Scholar] [CrossRef]
- Shalimar; Saraswat, V.; Singh, S.P.; Duseja, A.; Shukla, A.; Eapen, C.E.; Kumar, D.; Pandey, G.; Venkataraman, J.; Puri, P.; et al. Acute-on-Chronic Liver Failure in India: The Indian National Association for Study of the Liver Consortium Experience. J. Gastroenterol. Hepatol. 2016, 31, 1742–1749. [Google Scholar] [CrossRef]
- Karvellas, C.J.; Bagshaw, S.M. Advances in Management and Prognostication in Critically Ill Cirrhotic Patients. Curr. Opin. Crit. Care 2014, 20, 210–217. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Milic, S.; Radic, M.; Orlic, L.; Bagic, Z.; Stimac, D. Clinical Profile, Natural History, and Predictors of Mortality in Patients with Acute-on-Chronic Liver Failure (ACLF). Wien. Klin. Wochenschr. 2015, 127, 283–289. [Google Scholar] [CrossRef]
- Garg, H.; Kumar, A.; Garg, V.; Sharma, P.; Sharma, B.C.; Sarin, S.K. Clinical Profile and Predictors of Mortality in Patients of Acute-on-Chronic Liver Failure. Dig. Liver Dis. 2012, 44, 166–171. [Google Scholar] [CrossRef]

| Precipitating Factor | Prevalence |
|---|---|
| Alcohol | 48.08% |
| Sepsis | 16.35% |
| Gastrointestinal bleeding | 19.13% |
| HBV | 8.2% |
| HEV | 7.21% |
| Tuberculostatic treatment | 6.25% |
| Autoimmune hepatitis | 0.96% |
| Unknown | 3.85% |
| Etiology | Total Patients | ACLF |
|---|---|---|
| Hepatitis C | 13,959 | 809 (5.79%) |
| Hepatitis B | 1967 | 145 (7.37%) |
| Alcohol | 23,484 | 1798 (7.65%) |
| Hepatitis C + Alcohol | 22,343 | 1791 (8.01%) |
| NAFLD | 15,893 | 929 (5.84%) |
| Other | 2737 | 181 (6.61%) |
| ACLF Grade | Characteristics | Mortality Rate (28 Days) |
|---|---|---|
| 1 | →kidney failure OR →liver failure, coagulation disorder, or pulmonary impairment + serum creatinine level 1.5–1.9 mg/dL OR →hepatic encephalopathy of grade 1 or 2 ± serum creatinine level 1.5–1.9 mg/dL | 22.1% |
| 2 | →presence of at least two combined organ failures | 32.0% |
| 3 | →three or more organ failures are implied | 76.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marginean, C.M.; Pirscoveanu, D.; Popescu, M.; Vasile, C.M.; Docea, A.O.; Mitruț, R.; Mărginean, I.C.; Iacob, G.A.; Firu, D.M.; Mitruț, P. Challenges in Diagnosis and Therapeutic Approach of Acute on Chronic Liver Failure—A Review of Current Evidence. Biomedicines 2023, 11, 1840. https://doi.org/10.3390/biomedicines11071840
Marginean CM, Pirscoveanu D, Popescu M, Vasile CM, Docea AO, Mitruț R, Mărginean IC, Iacob GA, Firu DM, Mitruț P. Challenges in Diagnosis and Therapeutic Approach of Acute on Chronic Liver Failure—A Review of Current Evidence. Biomedicines. 2023; 11(7):1840. https://doi.org/10.3390/biomedicines11071840
Chicago/Turabian StyleMarginean, Cristina Maria, Denisa Pirscoveanu, Mihaela Popescu, Corina Maria Vasile, Anca Oana Docea, Radu Mitruț, Iulia Cristina Mărginean, George Alexandru Iacob, Dan Mihai Firu, and Paul Mitruț. 2023. "Challenges in Diagnosis and Therapeutic Approach of Acute on Chronic Liver Failure—A Review of Current Evidence" Biomedicines 11, no. 7: 1840. https://doi.org/10.3390/biomedicines11071840
APA StyleMarginean, C. M., Pirscoveanu, D., Popescu, M., Vasile, C. M., Docea, A. O., Mitruț, R., Mărginean, I. C., Iacob, G. A., Firu, D. M., & Mitruț, P. (2023). Challenges in Diagnosis and Therapeutic Approach of Acute on Chronic Liver Failure—A Review of Current Evidence. Biomedicines, 11(7), 1840. https://doi.org/10.3390/biomedicines11071840








