Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases
Abstract
1. Introduction
2. Depression and Cardiovascular Disease
3. Obesity
4. Physical Activity
5. Diabetes
6. Inflammation
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 31 March 2023).
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Pope, B.S.; Wood, S.K. Advances in understanding mechanisms and therapeutic targets to treat comorbid depression and cardiovascular disease. Neurosci. Biobehav. Rev. 2020, 116, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Brailean, A.; Curtis, J.; Davis, K.; Dregan, A.; Hotopf, M. Characteristics, comorbidities, and correlates of atypical depression: Evidence from the UK Biobank Mental Health Survey. Psychol. Med. 2020, 50, 1129–1138. [Google Scholar] [CrossRef]
- Frasure-Smith, N.; Lespérance, F. Reflections on Depression as a Cardiac Risk Factor. Psychosom. Med. 2005, 67 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef]
- Möller-Leimkühler, A.M. Gender differences in cardiovascular disease and comorbid depression. Dialog. Clin. Neurosci. 2007, 9, 71–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Ma, L. Depression and cardiovascular disease in elderly: Current understanding. J. Clin. Neurosci. 2018, 47, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Grasso, M.; Santangelo, R.; Lazzarino, G.; Lunte, S.M.; Caraci, F. Inflammation as the Common Biological Link Between Depression and Cardiovascular Diseases: Can Carnosine Exert a Protective Role? Curr. Med. Chem. 2020, 27, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J. Transl. Med. 2015, 13, 13. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 16 September 2022).
- Kones, R.; Rumana, U. Cardiometabolic diseases of civilization: History and maturation of an evolving global threat. An update and call to action. Ann. Med. 2017, 49, 260–274. [Google Scholar] [CrossRef]
- Cui, R. A Systematic Review of Depression. Curr. Neuropharmacol. 2015, 13, 480. [Google Scholar] [CrossRef]
- Available online: https://www.psychiatry.org/psychiatrists/practice/dsm (accessed on 16 September 2022).
- Drevets, W.C.; Price, J.L.; Simpson, J.R., Jr.; Todd, R.D.; Reich, T.; Vannier, M.; Raichle, M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997, 386, 824–827. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Koolschijn, P.C.M.; van Haren, N.E.; Lensvelt-Mulders, G.J.; Pol, H.H.; Kahn, R.S. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009, 30, 3719–3735. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Siemer, M.; Gotlib, I.H. Amygdala volume in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry 2008, 13, 993–1000. [Google Scholar] [CrossRef]
- Ménard, C.; Hodes, G.; Russo, S. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef]
- Lima-Ojeda, J.M.; Rupprecht, R.; Baghai, T.C. Neurobiology of depression: A neurodevelopmental approach. World J. Biol. Psychiatry 2017, 19, 349–359. [Google Scholar] [CrossRef]
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b (accessed on 1 May 2021).
- Forum Przeciw Depresji. Available online: https://forumprzeciwdepresji.pl/depresja/samobojstwo (accessed on 16 September 2022).
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef]
- Shanthi, M.; Pekka, P.; Norrving, B.; World Health Organization; World Heart Federation. World Stroke Organization Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization in Collaboration with the World Heart Federation and the World Stroke Organization: Geneva, Switzerland, 2011; pp. 3–18. [Google Scholar]
- Carney, R.M.; Freedland, K.E.; Veith, R.C. Depression, the Autonomic Nervous System, and Coronary Heart Disease. Psychosom. Med. 2005, 67, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Szyndler, A.; Narkiewicz, K. Świadomość wybranych czynników ryzyka chorób układu sercowo-naczyniowego w populacji młodych osób. Choroby. Serca i Naczyń. 2017, 14, 186–193. [Google Scholar]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a Risk Factor for Poor Prognosis Among Patients with Acute Coronary Syndrome: Systematic Review and Recommendations: A scien-tific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.C.; Nelesen, R.A.; Dimsdale, J.E. Depressive Symptoms Are Associated with Increased Systemic Vascular Resistance to Stress. Psychosom. Med. 2005, 67, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Whang, W.; Kubzansky, L.D.; Kawachi, I.; Rexrode, K.M.; Kroenke, C.H.; Glynn, R.J.; Garan, H.; Albert, C.M. Depression and Risk of Sudden Cardiac Death and Coronary Heart Disease in Women: Results from the Nurses’ Health Study. J. Am. Coll. Cardiol. 2009, 53, 950–958. [Google Scholar] [CrossRef]
- Kimmel, S.E.; Schelleman, H.; Berlin, J.A.; Oslin, D.W.; Weinstein, R.B.; Kinman, J.L.; Sauer, W.H.; Lewis, J.D. The effect of selective serotonin re-uptake inhibitors on the risk of myocardial infarction in a cohort of patients with depression. Br. J. Clin. Pharmacol. 2011, 72, 514–517. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 16 September 2022).
- Milaneschi, Y.; Simmons, W.K.; Van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Baker, M. Obesity effects on depression: Systematic review of epidemiological studies. Int. J. Obes. 2008, 32, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, G.; Nitka, D.; Schmitz, N. The association between obesity and anxiety disorders in the population: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Jantaratnotai, N.; Mosikanon, K.; Lee, Y.; McIntyre, R.S. The interface of depression and obesity. Obes. Res. Clin. Pract. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puhl, R.M.; Heuer, C.A. Obesity Stigma: Important Considerations for Public Health. Am. J. Public Health 2010, 100, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Steptoe, A.; Oliver, G.; Lipsey, Z. Stress, dietary restraint and food intake. J. Psychosom. Res. 2000, 48, 195–202. [Google Scholar] [CrossRef]
- Hryhorczuk, C.; Sharma, S.; Fulton, S.E. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, L. Obesity and Depression in US Women: Results from the 2005–2006 National Health and Nutritional Examination Survey. Obesity 2010, 18, 347–353. [Google Scholar] [CrossRef]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The Prevalence of Comorbid Depression in Adults with Diabetes. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef]
- Pagoto, S.; Schneider, K.; Appelhans, B.M.; Curtin, C.; Hajduk, A. Psychological Co-morbidities of Obesity. Psychosomatics 2012, 53, 1–72. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Du, F.-M.; Kuang, H.-Y.; Duan, B.-H.; Liu, D.-N.; Yu, X.-Y. Effects of thyroid hormone and depression on common components of central obesity. J. Int. Med. Res. 2019, 47, 3040–3049. [Google Scholar] [CrossRef]
- Fox, C.K.; Gross, A.C.; Rudser, K.D.; Foy, A.M.; Kelly, A.S. Depression, Anxiety, and Severity of Obesity in Adolescents: Is Emotional Eating the Link? Clin. Pediatr. 2016, 55, 1120–1125. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relation-ship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Rogge, B.P.; Cramariuc, D.; Lønnebakken, M.T.; Gohlke-Bärwolf, C.; Chambers, J.B.; Boman, K.; Gerdts, E. Effect of overweight and obesity on cardiovascular events in asymptomatic aortic stenosis: A SEAS substudy (Simvastatin Ezetimibe in Aortic Stenosis). J. Am. Coll. Cardiol. 2013, 62, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Pagidipati, N.J.; Zheng, Y.; Green, J.B.; McGuire, D.K.; Mentz, R.J.; Shah, S.; Aschner, P.; Delibasi, T.; Rodbard, H.W.; Westerhout, C.M.; et al. Association of obesity with cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease: Insights from TECOS. Am. Heart J. 2020, 219, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Daumit, G.L.; Dalcin, A.T.; Dickerson, F.B.; Miller, E.R.; Evins, A.E.; Cather, C.; Jerome, G.J.; Young, D.R.; Charleston, J.B.; Gennusa, J.V.; et al. Effect of a Comprehensive Cardiovascular Risk Reduction Intervention in Persons with Serious Mental Illness: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e207247. [Google Scholar] [CrossRef]
- Faulconbridge, L.F.; Driscoll, C.F.B.; Hopkins, C.M.; Benforado, B.B.; Bishop-Gilyard, C.; Carvajal, R.; Berkowitz, R.I.; DeRubeis, R.; Wadden, T.A. Combined Treatment for Obesity and Depression: A Pilot Study. Obesity 2018, 26, 1144–1152. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Poirier, P.; Alpert, M.A.; Fleisher, L.A.; Thompson, P.D.; Sugerman, H.J.; Burke, L.E.; Marceau, P.; Franklin, B.A. Cardiovascular evaluation and management of severely obese patients undergoing surgery: A science advisory from the American Heart Association. Circulation 2009, 120, 86–95. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/health-topics/physical-activity#tab=tab_1 (accessed on 16 September 2022).
- Villella, M.; Villella, A. Exercise and Cardiovascular Diseases. Kidney Blood Press. Res. 2014, 39, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Fan, H. The associations between screen time-based sedentary behavior and depression: A systematic review and meta-analysis. BMC Public Health 2019, 19, 1524. [Google Scholar] [CrossRef] [PubMed]
- Teychenne, M.; Ball, K.; Salmon, J. Sedentary Behavior and Depression Among Adults: A Review. Int. J. Behav. Med. 2010, 17, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Stubbs, B.; Firth, J.; Van Damme, T.; Koyanagi, A. Sedentary behavior and depressive symptoms among 67,077 adolescents aged 12–15 years from 30 low- and middle-income countries. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 73. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Calcaterra, V.; Vandoni, M.; Rossi, V.; Berardo, C.; Grazi, R.; Cordaro, E.; Tranfaglia, V.; Pellino, V.C.; Cereda, C.; Zuccotti, G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 6908. [Google Scholar] [CrossRef]
- Cattadori, G.; Segurini, C.; Picozzi, A.; Padeletti, L.; Anzà, C. Exercise and heart failure: An update. ESC Heart Fail. 2017, 5, 222–232. [Google Scholar] [CrossRef]
- Lapmanee, S.; Charoenphandhu, J.; Charoenphandhu, N. Beneficial effects of fluoxetine, reboxetine, venlafaxine, and voluntary running exercise in stressed male rats with anxiety- and depression-like behaviors. Behav. Brain Res. 2013, 250, 316–325. [Google Scholar] [CrossRef]
- Danielsson, L.; Papoulias, I.; Petersson, E.-L.; Carlsson, J.; Waern, M. Exercise or basic body awareness therapy as add-on treatment for major depression: A controlled study. J. Affect. Disord. 2014, 168, 98–106. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Soucy, I.; Provencher, M.; Fortier, M.; McFadden, T. Efficacy of guided self-help behavioural activation and physical activity for depression: A randomized controlled trial. Cogn. Behav. Ther. 2017, 46, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Thorp, A.A.; Owen, N.; Neuhaus, M.; Dunstan, D.W. Sedentary Behaviors and Subsequent Health Outcomes in Adults: A Systematic Review of Longitudinal Studies, 1996–2011. Am. J. Prev. Med. 2011, 41, 207–215. [Google Scholar] [CrossRef]
- Reed, J.L.; Terada, T.; Cotie, L.M.; Tulloch, H.E.; Leenen, F.H.; Mistura, M.; Hans, H.; Wang, H.-W.; Vidal-Almela, S.; Reid, R.D.; et al. The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: A randomized controlled trial (CRX study). Prog. Cardiovasc. Dis. 2021, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Svensson, E.; Andersson, G.; Lundgren, J. Trajectories and associations between depression and physical activity in patients with cardiovascular disease during participation in an internet-based cognitive behavioural therapy programme. Eur. J. Cardiovasc. Nurs. 2020, 20, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.C.; Charlson, M.E.; Wells, M.T.; Altemus, M. Depression, Coronary Artery Disease, and Physical Activity: How Much Exercise Is Enough? Clin. Ther. 2014, 36, 1518–1530. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 16 September 2022).
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldanius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Sartorius, N. Depression and diabetes. Dialog-Clin. Neurosci. 2018, 20, 47–52. [Google Scholar] [CrossRef]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Nouwen, A.; Winkley, K.; Twisk, J.; Lloyd, C.E.; Peyrot, M.; Ismail, K.; Pouwer, F. Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia 2010, 53, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Fisher, L.; Skaff, M.M.; Mullan, J.T.; Arean, P.; Mohr, D.; Masharani, U.; Glasgow, R.; Laurencin, G. Clinical depression versus distress among patients with type 2 diabetes: Not just a question of semantics. Diabetes Care 2007, 30, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Peyrot, M.; McCarl, L.A.; Collins, E.M.; Serpa, L.; Mimiaga, M.J.; Safren, S.A. Depression and Diabetes Treatment Nonadherence: A Meta-Analysis. Diabetes Care 2008, 31, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Gonzalez, J.S.; Polonsky, W.H. The confusing tale of depression and distress in patients with diabetes: A call for greater clarity and precision. Diabet. Med. 2014, 31, 764–772. [Google Scholar] [CrossRef]
- Lustman, P.J.; Clouse, R.E.; Nix, B.D.; Freedland, K.E.; Rubin, E.H.; McGill, J.B.; Williams, M.M.; Gelenberg, A.J.; Ciechanowski, P.S.; Hirsch, I.B. Sertraline for prevention of depression recurrence in diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 2006, 63, 521–529. [Google Scholar] [CrossRef]
- Lustman, P.J.; Anderson, R.J.; Freedland, K.E.; de Groot, M.; Carney, R.M.; Clouse, R.E. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care 2000, 23, 934–942. [Google Scholar] [CrossRef]
- Knol, M.J.; Twisk, J.W.R.; Beekman, A.T.F.; Heine, R.J.; Snoek, F.J.; Pouwer, F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006, 49, 837–845. [Google Scholar] [CrossRef]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Rutter, M.K.; Meigs, J.B.; Sullivan, L.M.; D’agostino, R.B.; Wilson, P.W. Insulin Resistance, the Metabolic Syndrome, and Incident Cardiovascular Events in the Framingham Offspring Study. Diabetes 2005, 54, 3252–3257. [Google Scholar] [CrossRef]
- Laakso, M. Cardiovascular disease in type 2 diabetes: Challenge for treatment and prevention. J. Intern. Med. 2001, 249, 225–235. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Cavallari, I.; Andreotti, F.; Calabrò, P.; Cirillo, P.; Denas, G.; Galli, M.; Golia, E.; Maddaloni, E.; Marcucci, R.; et al. Prevention of atherothrombotic events in patients with diabetes mellitus: From antithrombotic therapies to new-generation glucose-lowering drugs. Nat. Rev. Cardiol. 2018, 16, 113–130. [Google Scholar] [CrossRef]
- Morrish, N.J.; Wang, S.-L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001, 44 (Suppl. 2), S14–S21. [Google Scholar] [CrossRef]
- American Diabetes Association. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S53–S72. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C.; Sowers, J.R. Diabetes and Cardiovascular Disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Harvard Health Publishing. Available online: https://www.health.harvard.edu/heart-disease/ask-the-doctor-what-is-inflammation (accessed on 12 April 2021).
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Scott, L., Jr.; Li, N.; Dobrev, D. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 2019, 287, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Adibfar, A.; Herrmann, N.; Gallagher, D.; Lanctôt, K.L. Evidence for Inflammation-Associated Depression. Curr. Top. Behav. Neurosci. 2017, 31, 3–30. [Google Scholar] [CrossRef]
- Gałecki, P. Teoria zapalna depresji—Podstawowe fakty. Psychiatria 2012, 9, 68–75. [Google Scholar]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Tabaei, A.S. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Bailey, M.L.; Andridge, R.; Peng, J.; Jaremka, L.M.; Fagundes, C.P.; Malarkey, W.B.; Laskowski, B.; Belury, M.A. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98, 52–60. [Google Scholar] [CrossRef]
- Vaccarino, V.; Johnson, B.D.; Sheps, D.S.; Reis, S.E.; Kelsey, S.F.; Bittner, V.; Rutledge, T.; Shaw, L.J.; Sopko, G.; Merz, C.N.B. National Heart, Lung, and Blood Institute. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: The National Heart, Lung, and Blood Institute-sponsored WISE study. J. Am. Coll. Cardiol. 2007, 50, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Smykiewicz, P.; Segiet, A.; Keag, M.; Żera, T. Proinflammatory cytokines and ageing of the cardiovascular-renal system. Mech. Ageing Dev. 2018, 175, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, D.D.-H.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. PROMINENT, REDUCE-IT, and STRENGTH Investigators. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef]
- Koenig, W.; Sund, M.; Fröhlich, M.; Fischer, H.-G.; Löwel, H.; Döring, A.; Hutchinson, W.L.; Pepys, M.B. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999, 99, 237–242. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Garcia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef]
- Sandoo, A.; Kitas, G.D.; Carroll, D.; van Zanten, J.J.V. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: A cross-sectional and longitudinal study. Arthritis. Res. Ther. 2012, 14, R117. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Gałecki, P.; Gałecka, E.; Maes, M.; Chamielec, M.; Orzechowska, A.; Bobińska, K.; Lewiński, A.; Szemraj, J. The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. J. Affect. Disord. 2012, 138, 360–366. [Google Scholar] [CrossRef]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Khoury, M.K.; Yang, H.; Liu, B. Macrophage Biology in Cardiovascular Diseases. Arter. Thromb. Vasc. Biol. 2021, 41, e77–e81. [Google Scholar] [CrossRef]
- Hellmann-Regen, J.; Clemens, V.; Grözinger, M.; Kornhuber, J.; Reif, A.; Prvulovic, D.; Goya-Maldonado, R.; Wiltfang, J.; Gruber, O.; Schüle, C.; et al. Effect of Minocycline on Depressive Symptoms in Patients with Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2230367. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, K.; Mohebbi, M.; Gray, L.; Walker, A.J.; Bortolasci, C.C.; Turner, A.; Berk, M.; Walder, K.; Maes, M.; Kanchanatawan, B.; et al. Exploring interleukin-6, lipopolysaccharide-binding protein and brain-derived neurotrophic factor following 12 weeks of adjunctive minocycline treatment for depression. Acta Neuropsychiatr. 2021, 34, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Bliźniewska-Kowalska, K.; Szewczyk, B.; Gałecka, M.; Su, K.-P.; Maes, M.; Szemraj, J.; Gałecki, P. Is Interleukin 17 (IL-17) Expression A Common Point in the Pathogenesis of Depression and Obesity? J. Clin. Med. 2020, 9, 4018. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Ministrini, S.; Carbone, F.; Camici, G.G.; Montecucco, F. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic Res. Cardiol. 2021, 116, 23. [Google Scholar] [CrossRef]
- Pan, W.; Stone, K.P.; Hsuchou, H.; Manda, V.K.; Zhang, Y.; Kastin, A.J. Cytokine Signaling Modulates Blood-Brain Barrier Function. Curr. Pharm. Des. 2011, 17, 3729–3740. [Google Scholar] [CrossRef]
- Gałecka, M.; Bliźniewska-Kowalska, K.; Maes, M.; Su, K.-P.; Gałecki, P. Update on the neurodevelopmental theory of depression: Is there any ‘unconscious code’? Pharmacol. Rep. 2020, 73, 346–356. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef]
- Kruse, J.L.; Vasavada, M.M.; Olmstead, R.; Hellemann, G.; Wade, B.; Breen, E.C.; Brooks, J.O.; Congdon, E.; Espinoza, R.; Narr, K.L.; et al. Depression treatment response to ketamine: Sex-specific role of interleukin-8, but not other inflammatory markers. Transl. Psychiatry 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Z.; Sun, D.; Xu, Z.; Zhang, X.; Li, L. The serum interleukin-18 is a potential marker for development of post-stroke depression. Neurol. Res. 2010, 32, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-M.; Yin, M.X.C.; Chan, J.S.M.; Chan, C.H.Y.; Fong, T.C.T.; Li, A.; So, K.-F.; Yuen, L.-P.; Chen, J.-P.; Chung, K.-F.; et al. Impact of mind–body intervention on proinflammatory cytokines interleukin 6 and 1β: A three-arm randomized controlled trial for persons with sleep disturbance and depression. Brain Behav. Immun. 2021, 99, 166–176. [Google Scholar] [CrossRef]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Gałecki, P.; Leonard, B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef]
- Koo, J.W.; Duman, R.S. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr. Opin. Investig. Drugs 2009, 10, 664–671. [Google Scholar] [PubMed]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2020, 18, 58–68. [Google Scholar] [CrossRef]
- Ting, E.Y.-C.; Yang, A.C.; Tsai, S.-J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef]
- Kim, C.W.; Oh, E.; Park, H.J. A strategy to prevent atherosclerosis via TNF receptor regulation. FASEB J. 2021, 35, e21391. [Google Scholar] [CrossRef]
- Uzzan, S.; Azab, A.N. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Crother, T.R.; Arditi, M. Emerging Role of IL-17 in Atherosclerosis. J. Innate Immun. 2010, 2, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 Cells in Atherosclerosis: Subtle and contextual roles. Arter. Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef]
- Beurel, E.; Lowell, J.A. Th17 cells in depression. Brain, Behav. Immun. 2018, 69, 28–34. [Google Scholar] [CrossRef]
- Nicholson, A.; Kuper, H.; Hemingway, H.; Adrie, C.; Cariou, A.; Mourvillier, B.; Laurent, I.; Dabbane, H.; Hantala, F.; Rhaoui, A.; et al. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J. 2006, 27, 2763–2774. [Google Scholar] [CrossRef]
- van Melle, J.P.; de Jonge, P.; Spijkerman, T.A.; Tijssen, J.G.P.; Ormel, J.; van Veldhuisen, D.J.; van den Brink, R.H.S.; van den Berg, M.P. Prognostic Association of Depression Following Myocardial Infarction with Mortality and Cardiovascular Events: A Meta-analysis. Psychosom. Med. 2004, 66, 814–822. [Google Scholar] [CrossRef]
- Thombs, B.D.; de Jonge, P.; Coyne, J.C.; Whooley, M.A.; Frasure-Smith, N.; Mitchell, A.J.; Zuidersma, M.; Eze-Nliam, C.; Lima, B.B.; Smith, C.G.; et al. Depression Screening and Patient Outcomes in Cardiovascular Care. JAMA 2008, 300, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Yu, C.; Liu, N.; He, M.; Lv, J.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Zhang, X.; et al. Association of Depression with All-Cause and Cardiovascular Disease Mortality Among Adults in China. JAMA Netw. Open 2020, 3, e1921043. [Google Scholar] [CrossRef]
- Lett, H.S.; Blumenthal, J.A.; Babyak, M.A.; Sherwood, A.; Strauman, T.; Robins, C.; Newman, M.F. Depression as a Risk Factor for Coronary Artery Disease: Evidence, Mechanisms, and Treatment. Psychosom. Med. 2004, 66, 305–315. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E.; Miller, G.E.; Jaffe, A.S. Depression as a risk factor for cardiac mortality and morbidity. J. Psychosom. Res. 2002, 53, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Berkman, L.F.; Blumenthal, J.; Burg, M.; Carney, R.M.; Catellier, D.; Cowan, M.J.; Czajkowski, S.M.; DeBusk, R.; Hosking, J.; Jaffe, A.; et al. Effects of Treating Depression and Low Perceived Social Support on Clinical Events After Myocardial Infarction: The Enhancing Re-covery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003, 289, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Schumacher, M.; Herrmann-Lingen, C. Depression as a Risk Factor for Mortality in Patients with Coronary Heart Disease: A Meta-analysis. Psychosom. Med. 2004, 66, 802–813. [Google Scholar] [CrossRef]
- Whooley, M.A.; de Jonge, P.; Vittinghoff, E.; Otte, C.; Moos, R.; Carney, R.M.; Ali, S.; Dowray, S.; Na, B.; Feldman, M.D.; et al. Depressive Symptoms, Health Behaviors, and Risk of Cardiovascular Events in Patients with Coronary Heart Disease. JAMA 2008, 300, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.J.; Lin, E.H.; Von Korff, M.; Ciechanowski, P.; Ludman, E.J.; Young, B.; Peterson, D.; Rutter, C.M.; McGregor, M.; McCulloch, D. Collaborative Care for Patients with Depression and Chronic Illnesses. N. Engl. J. Med. 2010, 363, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and cardiovascular disease: The deep blue sea of women’s heart. Trends Cardiovasc. Med. 2020, 30, 170–176. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Gender differences in depression. Int. Rev. Psychiatry 2010, 22, 429–436. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Connelly, P.J.; Azizi, Z.; Alipour, P.; Delles, C.; Pilote, L.; Raparelli, V. The Importance of Gender to Understand Sex Differences in Cardiovascular Disease. Can. J. Cardiol. 2021, 37, 699–710. [Google Scholar] [CrossRef]
- Saeed, A.; Kampangkaew, J.; Nambi, V. Prevention of Cardiovascular Disease in Women. Methodist DeBakey Cardiovasc. J. 2017, 13, 185–192. [Google Scholar] [CrossRef]
- Dudek, K.A.; Dion-Albert, L.; Kaufmann, F.N.; Tuck, E.; Lebel, M.; Menard, C. Neurobiology of resilience in depression: Immune and vascular insights from human and animal studies. Eur. J. Neurosci. 2021, 53, 183–221. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Nava, M.; Ortega, Á.; Rojas, M.; Núñez, V.; Salazar, J.; Bermúdez, V.; Rojas-Quintero, J. Depression as an Immunometabolic Disorder: Exploring Shared Pharmacotherapeutics with Cardiovascular Disease. Curr. Neuropharmacol. 2020, 18, 1138–1153. [Google Scholar] [CrossRef]
- Heissel, A.; Heinen, D.; Brokmeier, L.L.; Skarabis, N.; Kangas, M.; Vancampfort, D.; Stubbs, B.; Firth, J.; Ward, P.B.; Rosenbaum, S.; et al. Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression. Br. J. Sports Med. 2023. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Akbaraly, T.N.; Brunner, E.J.; Ferrie, J.E.; Marmot, M.G.; Kivimaki, M.; Singh-Manoux, A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry 2009, 195, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Czernichow, S.; Kivimaki, M.; Okereke, O.I.; Lucas, M.; Manson, J.E.; Ascherio, A.; Hu, F.B. Bidirectional association between depression and obesity in middle-aged and older women. Int. J. Obes. 2011, 36, 595–602. [Google Scholar] [CrossRef]
- Lavie, C.J.; McAuley, P.A.; Church, T.S.; Milani, R.V.; Blair, S.N. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J. Am. Coll. Cardiol. 2014, 63, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M. Risk Factors for Development of Depression and Psychosis. Ann. N. Y. Acad. Sci. 2009, 1179, 144–152. [Google Scholar] [CrossRef]
- Rozanski, A.; Blumenthal, J.A.; Kaplan, J. Impact of Psychological Factors on the Pathogenesis of Cardiovascular Disease and Implications for Therapy. Circulation 1999, 99, 2192–2217. [Google Scholar] [CrossRef] [PubMed]
- Joynt, K.E.; Whellan, D.J.; O’Connor, C.M. Depression and cardiovascular disease: Mechanisms of interaction. Biol. Psychiatry 2003, 54, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H. Depression and cardiovascular comorbidity. Dialog. Clin. Neurosci. 2007, 9, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr. Top. Behav. Neurosci. 2017, 31, 45–70. [Google Scholar] [CrossRef]
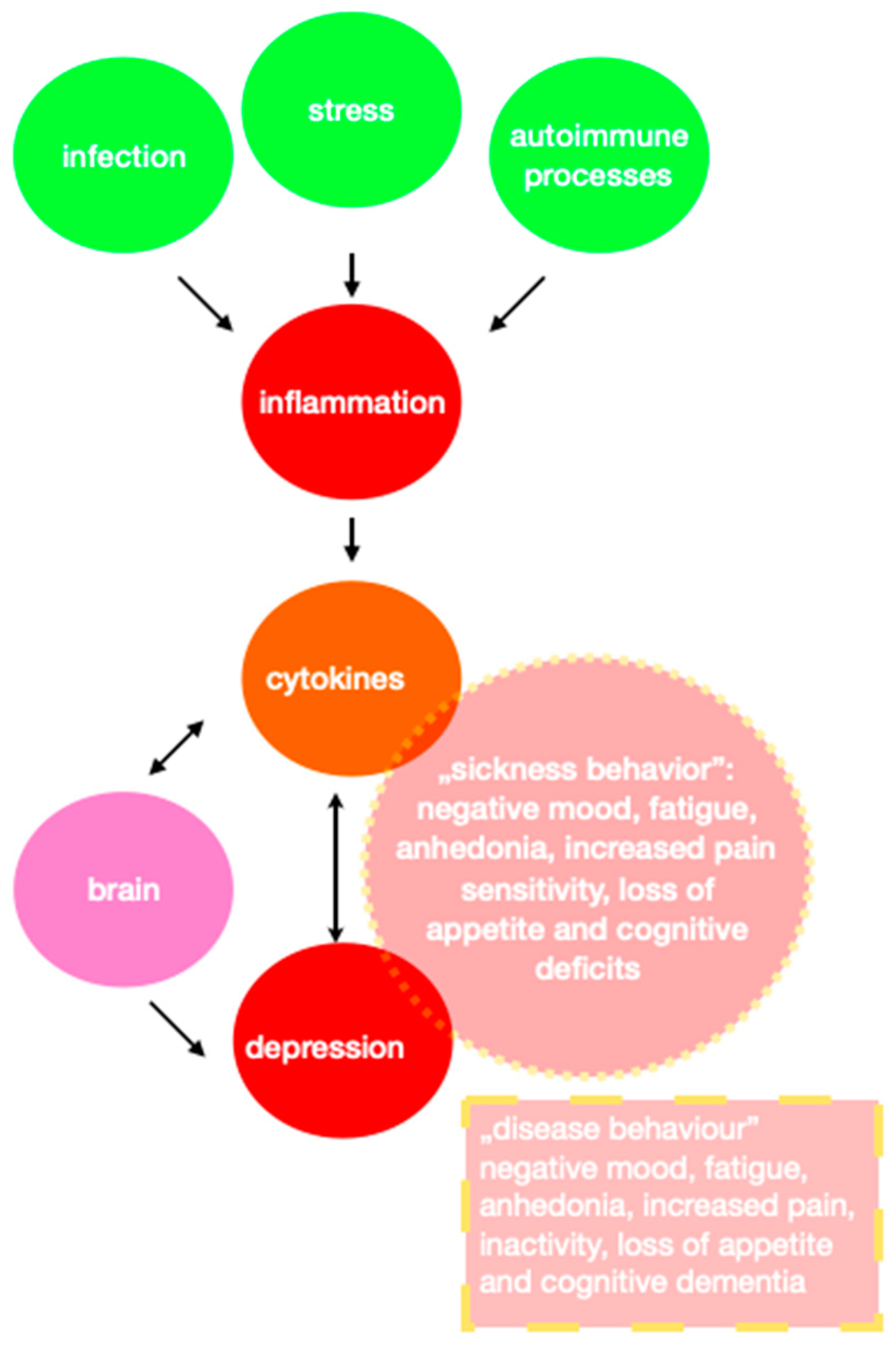

| Depressed mood, or loss of interest or pleasure. |
| Depressed most of the day, almost every day, as indicated by subjective feelings (e.g., feeling sad, empty, hopeless) or observations of others (e.g., crying). |
| Markedly decreased interest or pleasure in all or nearly all activities most of the day, almost every day (according to subjective report or observation). |
| Significant weight loss when not dieting or gaining weight (e.g., change of more than 5% of body weight in a month) or a decrease or increase in appetite almost every day. |
| Insomnia or hypersomnia almost daily. Psychomotor agitation or retardation almost daily (observed by others, not just a subjective feeling of restlessness or slowness). |
| Fatigue or loss of energy almost every day. |
| Feelings of worthlessness or excessive or inappropriate guilt (which may be delusional) almost daily (not just remorse or guilt about illness). |
| Decreased ability to think or concentrate, or indecisiveness almost every day (according to subjective accounts or observed by others). |
| Recurrent thoughts of death (not just fear of death), recurrent suicidal thoughts without a specific plan, or suicide attempts or a specific plan to commit suicide. Symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| The questions: |
| Do depression and CVD have common risk factors? |
| Are depression and CVD related to diabetes? |
| Are depression and CVD related to physical activity? |
| Are depression and CVD related to obesity? |
| Is it worth conducting CVD prophylaxis in patients with depression? |
| Is it worth conducting depression prevention in patients with CVD? |
| Is it advisable and necessary to conduct further research in this field? |
| Cytokine | Behavior |
|---|---|
| IL-1 | Has a pivotal role in the occurrence of fatigue as assessed by a decreased resistance to forced exercise on a treadmill [129,130] |
| IL-1β i TNF-α | Flatten the diurnal rhythm of activity by decreasing the expression of steady-state mRNAs for clock genes that control the amplitude but not the period of activity rhythms [126] |
| IL-10 and insulin-like growth factor I (IGF-I) | Growth factor that behaves like an anti-inflammatory cytokine in the brain, attenuates behavioral signs of sickness induced by centrally injected LPS [126] |
| IL-6 | Chronic mild stress showed anhedonia and increased levels of circulating pro-inflammatory cytokines, including IL-6 [131,132] |
| Cytokine | Atherosclerosis | Depression |
|---|---|---|
| IL-1 | Changes the functions of cardiac myocytes and cells in the blood vessel wall, impairing systolic function, and may intensify ischemia-reperfusion injury and expansive cardiac remodeling [138] | Can cause mood disorders, a key mediator in various behavioral effects of stress [139] |
| IL-6 | Impaired function of vascular mitochondria accelerates the development of atherosclerosis. In mouse studies, it caused dysfunction associated with increased levels of the inflammatory cytokine IL-6 in the aorta. Human and mouse studies—aging leads to the deterioration of vascular mitochondrial function and the impairment of mitophagy. The aging of blood vessels and bone marrow cells is associated with IL-6 signaling [140] | was tested on animals and in clinical trials. Increased IL-6 activity is a factor contributing to the development of depression by activating the hypothalamic-pituitary-adrenal axis or by affecting the metabolism of neurotransmitters [141] |
| TNF-α | The development of atherosclerosis is closely related to the activation of TNF-α, and promotes various inflammatory reactions associated with atherosclerosis, induces vascular adhesion molecules and the recruitment and proliferation of monocytes/macrophages, participates in lipid metabolism, inhibits the activity of 7α-hydroxylase and lipoprotein lipase, and enhances the production of triglycerides in the liver [142] | Elevated plasma concentrations of tumor necrosis factor (TNF)-α in patients with mood disorders, disturbances in TNF-α levels and mental deterioration, including suicidal thoughts and response to treatment, collide [143] |
| IL-17 | T helper-17 lymphocytes produce interleukin-17, which are important in the defense of the host mucosa against pathogenic microorganisms and fungi, constituting the anti-atherosclerotic effect of IL-17, but also the pro-atherogenic effect of IL-17 [144,145] | In depression, the number of Th17 cells increase. Th17 cells produce interleukin-17A (IL-17A), through which they promote inflammation of the nerves and activation of microglia and astrocytes. In this mechanism, they can contribute to neuronal damage, which is secondary to depression [146] |
| Increased risk of cardiovascular disease | Depression is associated with an increased risk of developing cardiovascular disease. Many studies show that people with depression are more likely to develop cardiovascular conditions, such as coronary artery disease, heart attacks, heart failure, and strokes. |
| Common Risk Factors | Depression and CVD share several common risk factors, such as a sedentary lifestyle, poor eating habits, smoking, excessive alcohol consumption, and obesity. These risk factors can contribute to both depression and cardiovascular problems. |
| Biological mechanisms | There are a wide range of biological mechanisms that may help explain the relationship between depression and CVD. Chronic stress, which is often associated with depression, can lead to increased inflammation and oxidative stress within the human body. These processes may contribute to the development and progression of cardiovascular diseases. |
| Behavioral factors | Depression can also affect an individual’s behavior and lifestyle choices in ways that increase the risk of developing CVD. For example, people with depression may engage in less physical activity, have difficulty complying with medical advice, or use unhealthy coping mechanisms, such as overeating or substance abuse. |
| Poor adherence to treatment | People with depression may have poor adherence to treatment required for cardiovascular disease. This lack of commitmernt can lead to inadequate treatment of risk factors, exacerbation of CVD symptoms, and increased complications. |
| Depression | Cardiovascular Disease | Depression and Cardiovascular Disease | |
|---|---|---|---|
| Obesity | The prevalence of depression was 17.83% in patients with central obesity and 12.6% in non-obese patients [39] | That reducing BMI reduces the risk of CVD, at least in overweight or moderately obese patients [31] | Behavioral weight control resulted in short-term improvements in weight, mood, and CVD risk, comparable to a combination treatment of cognitive-behavioral therapy for depression [45] |
| That depression and anxiety were associated with more severe obesity among adolescents seeking treatment [40] | |||
| Physical activity | A strong antidepressant effect of exercise [52] | The impact of physical fitness and physical activity on the risk, management, and prognosis of heart failure [49] | The online cognitive-behavioral therapy group, a significant correlation was found between changes in depressive symptoms and changes in physical activity [54] |
| Behavioral activation (BA) and physical activity (PA) can reduce the severity of depressive symptoms in adults [52] | Physical activity of ≥336 kcal/week had significantly lower rates of cardiovascular morbidity and mortality at 12 months [55] | ||
| Physical exercise in a physiotherapeutic setting seems to have an effect on depressive severity and performance in major depression. Their findings suggest that physical therapy may be a viable clinical strategy that inspires and guides people with major depression to exercise [50] | Patients with coronary heart disease, exercise programs were well attended, safe, and beneficial in terms of improving physical and mental health [53] | ||
| Diabetes | The dysregulation of the hypothalamic-pituitary-adrenal HPA axis is a critical link in the high incidence of depression and comorbid diabetes [48] |
| Pros | Limitations |
|---|---|
| Improved understanding: Conducting a study can help researchers gain a better understanding of the relationship between depression and cardiovascular disease. It can shed light on the underlying mechanisms, contributing factors, and potential interactions between these two conditions. | Methodological challenges: Conducting a study on depression and cardiovascular disease can present methodological challenges. Designing appropriate research protocols, selecting appropriate study participants, and accurately measuring variables related to depression and cardiovascular health can be complex and require careful consideration. |
| Early detection and prevention: Identifying the link between depression and cardiovascular disease can lead to early detection and prevention strategies. This knowledge can help healthcare professionals identify individuals at risk and implement appropriate interventions to reduce the incidence of cardiovascular disease among those with depression. | Confounding factors: The relationship between depression and cardiovascular disease is multifaceted and influenced by various confounding factors. It can be challenging to isolate the effects of depression on cardiovascular health, as other factors such as lifestyle habits, socioeconomic status, and genetic predisposition may also contribute to the observed outcomes. |
| Treatment strategies: A study can inform the development of targeted treatment strategies. Understanding the relationship between depression and cardiovascular disease can help clinicians tailor interventions that address both conditions simultaneously, leading to better overall outcomes for patients. | Causality and directionality: Establishing a causal relationship between depression and cardiovascular disease can be difficult. It is unclear whether depression leads to cardiovascular disease, cardiovascular disease contributes to the development of depression, or if there are shared underlying factors that contribute to both conditions. |
| Public health implications: Findings from a study on depression and cardiovascular disease can have significant public health implications. It can inform public health policies, healthcare guidelines, and educational campaigns aimed at raising awareness about the relationship between these conditions and promoting preventive measures. | Ethical considerations: Studying depression and cardiovascular disease may involve ethical considerations. Researchers must prioritize participant welfare, ensure informed consent, and address potential psychological distress that may arise during the study. Safeguarding participant confidentiality and privacy is also crucial. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewska-Nowak, J.; Wachowska, K.; Nowak, A.; Orzechowska, A.; Szulc, A.; Płaza, O.; Gałecki, P. Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines 2023, 11, 1903. https://doi.org/10.3390/biomedicines11071903
Sobolewska-Nowak J, Wachowska K, Nowak A, Orzechowska A, Szulc A, Płaza O, Gałecki P. Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines. 2023; 11(7):1903. https://doi.org/10.3390/biomedicines11071903
Chicago/Turabian StyleSobolewska-Nowak, Justyna, Katarzyna Wachowska, Artur Nowak, Agata Orzechowska, Agata Szulc, Olga Płaza, and Piotr Gałecki. 2023. "Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases" Biomedicines 11, no. 7: 1903. https://doi.org/10.3390/biomedicines11071903
APA StyleSobolewska-Nowak, J., Wachowska, K., Nowak, A., Orzechowska, A., Szulc, A., Płaza, O., & Gałecki, P. (2023). Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines, 11(7), 1903. https://doi.org/10.3390/biomedicines11071903








