Abstract
SGLT-2i are the new standard of care for diabetic kidney disease (DKD), but previous studies have not included patients on kidney replacement therapy (KRT). Due to their high risk of cardiovascular, renal complications, and mortality, these patients would benefit the most from this therapy. Residual kidney function (RKF) conveys a survival benefit and cardiovascular health among hemodialysis (HD) patients, especially those on incremental hemodialysis (iHD). We retrospectively describe the safety and efficacy of SGLT2i regarding RKF preservation in seven diabetic patients with different clinical backgrounds who underwent iHD (one or two sessions per week) during a 12-month follow-up. All patients preserved RKF, measured as residual kidney urea clearance (KrU) in 24 h after the introduction of SGLT2i. KrU levels improved significantly from 4.91 ± 1.14 mL/min to 7.28 ± 1.68 mL/min at 12 months (p = 0.028). Pre-hemodialysis blood pressure improved 9.95% in mean systolic blood pressure (SBP) (p = 0.015) and 10.95% in mean diastolic blood pressure (DBP) (p = 0.041); as a result, antihypertensive medication was modified. Improvements in blood uric acid, hemoglobin A1c, urine albumin/creatinine ratio (UACR), and 24 h proteinuria were also significant. Regarding side effects, two patients developed uncomplicated urinary tract infections that were resolved. No other complications were reported. The use of SGLT2i in our sample of DKD patients starting iHD on a 1–2 weekly regimen appears to be safe and effective in preserving RKF.
1. Introduction
Diabetic kidney disease (DKD) is the leading cause of end stage renal disease (ESRD) worldwide and continues to be the major contributor to kidney replacement therapy (KRT) [1,2]. Patients with DKD that develop macroalbuminuria have a greater risk of mortality due to cardiovascular disease (CVD) than they are to progress to ESRD [3]. Despite the significant decline in diabetes-related complications in recent decades, the same trend cannot be observed in chronic kidney disease (CKD) patients due to DKD that requires KRT [4]. Hence, there exists a significant requirement for novel treatment approaches that can enhance glycemic control while minimizing the risk of hypoglycemia, as well as reducing cardiovascular and renal risks within this population. Irrespective of the limitations associated with estimated glomerular filtration rate (eGFR), it is crucial to develop new treatments that can effectively address these concerns [5].
The American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) guidelines 2022 recommend sodium–glucose cotransporter-2 inhibitors (SGLT2i) as first-line treatment in all patients with an eGFR ≥ 20 mL/min/1.73 m2 and metformin in those with an eGFR ≥ 30 mL/min/1.73 m2 [6].
SGLT2i have become the new standard of care for slowing CKD progression in patients with type 2 diabetes mellitus (T2DM) [7,8,9] due to their specific renal and cardiovascular protective effects that are independent of the main metabolic and glucose-lowering effects [10,11]. Most studies on SGLT2i fail to include ESRD patients, particularly those on KRT or kidney transplant (KT) recipients [7,8,9]. These patients, due to their high risk of cardiovascular and renal disease, would benefit the most from these therapies. Literature on SGLT2i use in T2DM patients on KRT is limited. There is emerging evidence in post-hoc analyses and experimental and preclinical trials that support the premise that SGLT2i may be equally effective in preventing cardiovascular and mortality outcomes in patients on KRT, either on hemodialysis (HD), peritoneal dialysis (PD), or even in KT [12,13,14,15,16]. As of 2023, there are four major trials searching for a SGLT2i benefit in this population (NCT05687058, NCT05179668, NCT05141552, and NCT05374291).
In the majority of developed countries, the standard treatment for the vast majority of patients undergoing hemodialysis (HD) involves three sessions per week, each lasting 3–5 h. Regrettably, only a few centers adhere to the 2015 National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines, which permit a reduction in the weekly HD dose for patients with a residual kidney urea clearance (KrU) exceeding 3 mL/min/1.73 m2 [17].
Incremental hemodialysis (iHD) has been proposed as an alternative to conventional HD, whereby dialysis dosage can be individually tailored according to RKF. In recent years, several randomized controlled trials (RCTs) and cohort studies have proved the safety and efficacy of iHD. Most of them developed once or twice-weekly HD regimens that showed no increased risk of mortality, fewer hospitalizations, and less cost when compared with standard HD treatment [18]. In these patients, preservation of residual kidney function (RKF) is associated with lower morbidity and mortality [17,19].
We report a case series on the efficacy and safety of SGLT2i treatment in patients with ESRD due to DKD on incremental hemodialysis with a regimen of 1–2 weekly sessions. In addition, we describe and evaluate the effects of SGLT2i on RKF during 12 months of follow-up.
2. Materials and Methods
Our study was carried out in the hemodialysis unit at Hospital Central de la Defensa Gómez Ulla in Madrid, Spain. We retrospectively collected information on all incident iHD patients from June 2021 until May 2023.
According to our current protocol, for once-a-week iHD, a patient must have a RKF measured as KrU of ≥4 mL/min/1.73 m2 and a urine volume of ≥1 L/day. This is based on the design proposed by Deira et al. in the IHDIP trial [20]. For a twice-weekly iHD, we follow the proposed criteria by Kalantar-Zadeh et al. [21]. Out of 23 patients starting iHD in the period described above, 12 patients (52.17%) had T2DM and 7 of these started treatments with SGLT2i (5 with Dapagliflozin and 2 with Empagliflozin) at a median of 3 months after starting iHD.
This is a retrospective analysis of those cases, and although treatment with SGLT2i has not been specifically approved for use in T2DM patients on HD, the off-label use is based on evidence from clinical trials that confirm that SGLT2i are cardioprotective and nephroprotective regardless of eGFR [7,8,9,10,11,12,22]. All patients signed their informed consent, and the reasons for off-label use of SGLT2i were clearly detailed in their medical records. Data on analytical parameters, RKF and HD, were collected at 0, 3, 6, 9, and 12 months.
Volemic parameters (bioimpedance spectroscopy, lung ultrasound (LUS), and other parameters for assessing systemic congestion) were collected only at 0, 6, and 12 months. Self-reported adverse events (gastrointestinal symptoms, genital fungal/urinary tract infections, and hypoglycemia events) and SGLT2i discontinuation were also recorded at these intervals.
3. Results
Table 1 presents the patient’s basal characteristics. The median age was 69.71 ± 10.24 years, and 85.7% were males. All were hypertensive, had renin–angiotensin system (RAS) blockade, and maintained furosemide treatment without changes during follow-up. ESRD etiology was DKD for all patients, but there was also secondary focal segmental glomerulosclerosis (FSGS) due to decreased renal mass after radical nephrectomy for renal cancer (Patient 1), right nephroureterectomy for high-grade (Patient 4), crescentic IgA nephropathy (IgAN) (Patient 5), and advanced IgAN (Patient 7).

Table 1.
Basal characteristics before starting SGLT2i.
Five patients started on a once-weekly iHD regimen, with two on a twice-weekly regimen. All patients received post-dilution online hemodiafiltration (OL-HDF) with a dialyzer of asymmetric cellulose triacetate (ATA) membrane and venous administration of low-molecular-weight heparin (LMWH, Enoxaparin) at a dose of 40 or 60 mg in each session.
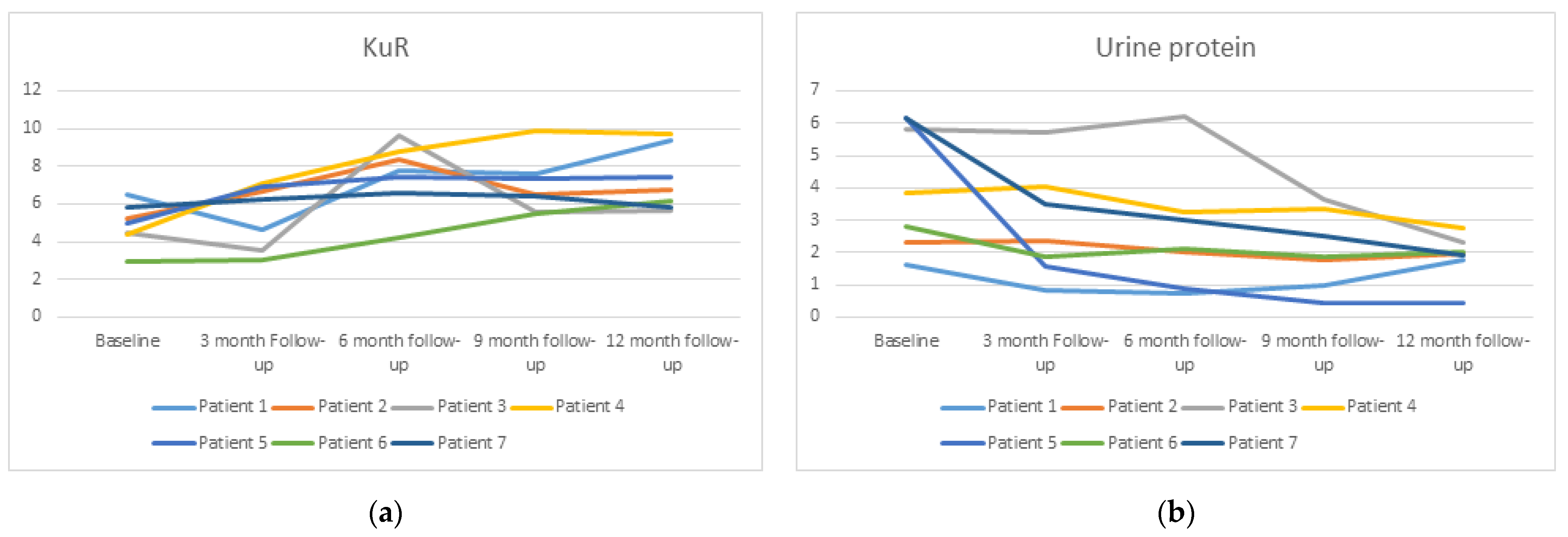
A significant improvement in KrU levels was found. It increased from 4.91 ± 1.14 mL/min to 7.28 ± 1.68 mL/min (p = 0.028) at 12 months (Figure 1a). Urine volume increased from 1742 ± 288 mL/24 h to 2021 ± 532 mL/24 h, and 24 h creatinine clearance also increased from 12.7 ± 3.53 mL/min to 16.35 mL/min ± 6.85 (p = 0.26) but was not significant. Urine albumin/creatinine ratio (UACR) and 24 h proteinuria were also significantly reduced at the end of the study. UACR dropped from 4040 ± 2729 mg/g to 1568 ± 746 mg/g (p = 0.016) and 24 h proteinuria from 4.10 ± 1.95 g/24 h to 1.88 ± 0.71 g/24 h (p = 0.028) (Figure 1b).
Figure 1.
Change in residual kidney urea clearance (KuR) (a) and urine protein (b) over time during the data collection period of 1 year.
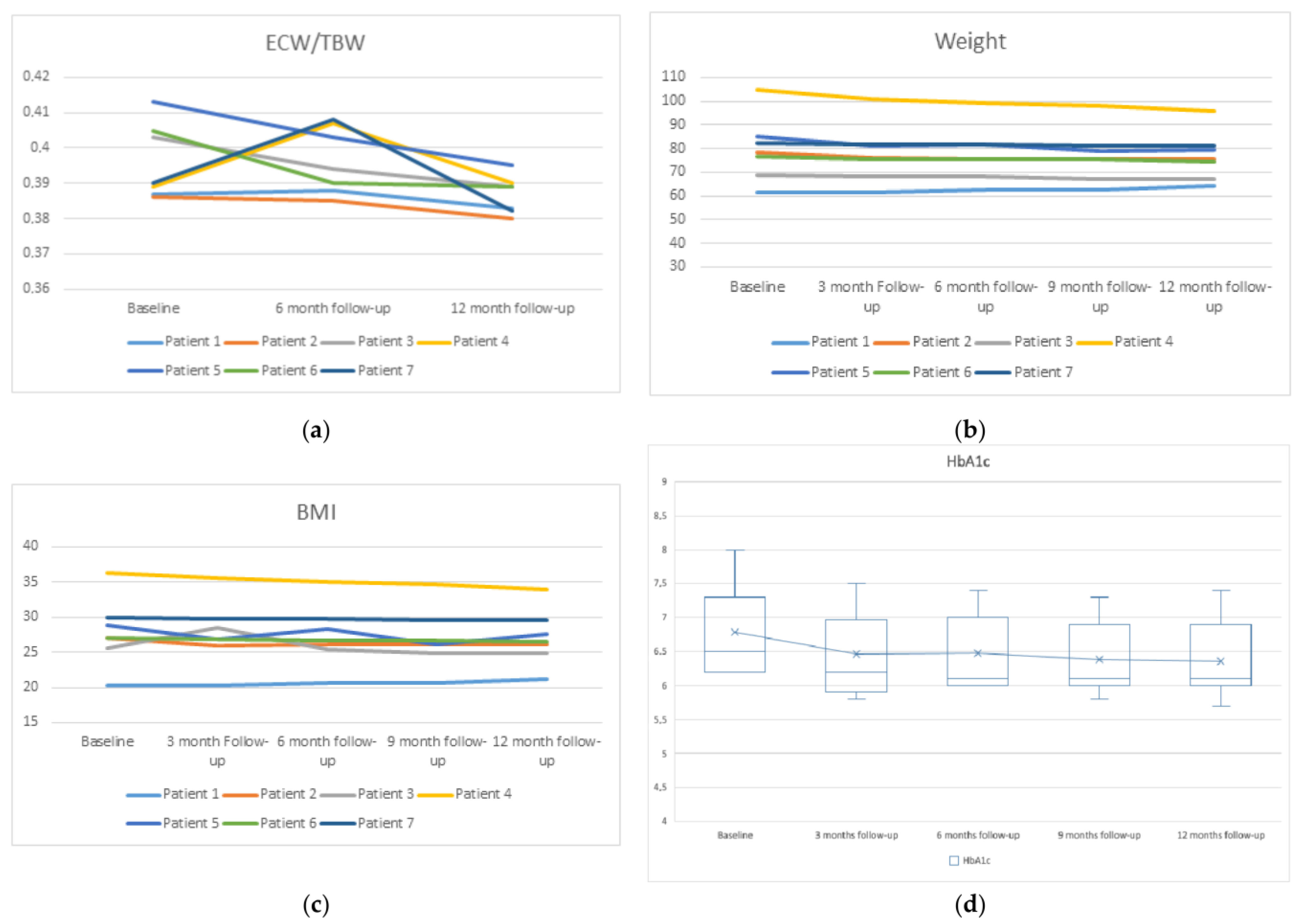
The extracellular water to total body water ratio (ECW/TBW), by bioimpedance measurement, was significantly reduced at 12 months (Figure 2a). Weight, body mass index (BMI), and fat mass reduction were non-significant, as was the increase in lean mass (Figure 2b,c).
Figure 2.
Changes in ECW/TBW (a), weight (b), BMI (c), and glycated hemoglobin A1c (HbA1c) (d) with respect to their baseline.
As shown in Table 2, a significant drop was observed in pre-hemodialysis blood pressure (both systolic and diastolic) at the end of the study. As a result, antihypertensive medication was modified. Calcium channel blocker (CCB) was discontinued in Patients 6 and 7, the same as angiotensin-converting enzyme inhibitors (ACEi) in Patients 3 and 5. Beta-blocker (BB) dose was reduced in Patients 5 and 7.

Table 2.
Evolution of dialysis parameters, residual renal function, bioimpedance, volemic parameters, and laboratory characteristics.
There was also a significant improvement in blood uric acid (from 7.67 ± 1.95 mg/dL to 5.96 ± 0.52 mg/dL, p = 0.018), serum potassium levels (from 5.45 ± 0.43 mmol/L to 4.62 ± 0.39 mmol/L, p = 0.001), serum phosphorus levels (from 5.7 ± 0.39 mg/dL to 4.68 ± 0.39, p = 0.001), 25-Hydroxyvitamin D (25OHD) serum levels (from 18.94 ± 4.85 mg/dL to 27.08 ± 2.02 mg/dL, p = 0.007), and hemoglobin A1c (HbA1c) levels (6.79 ± 0.68 g/dL to 6.36 ± 0.59 g/dL, p = 0.018) (Figure 2d). We observed no significant difference in calcium levels or parathyroid hormone (PTH) (p = 0.42 and 0.122, respectively). Of note, at the third month of starting treatment with dapagliflozin, Patients 4 and 7, who were treated with semaglutide and insulin, were able to discontinue (patient 4) and reduce their usual insulin doses by 20% (Patient 7).
Regarding side effects, two patients developed urinary tract infections (UTIs). Patient 3 developed a UTI after 3 weeks of treatment initiation with dapagliflozin. Patient 6 developed it after 8 weeks with empagliflozin. Both episodes were treated and resolved. SGLT2i discontinued in none of the patients.
No vascular access complications, change in mean KT or infusion volume in post-dilutional OL-HDF, or problems with blood flow rate (QB), arterial pressure flow (APF), or venous pressure flow (VPF) developed during the 12-month follow-up (Table 2).
4. Discussion
In this report, we describe the safety and efficacy of SGLT2i regarding RKF preservation in seven diabetic patients with different clinical backgrounds who underwent iHD (one or two sessions per week) during a 12-month follow-up.
SGLT2i, originally developed as oral hypoglycemic drugs, have shown renal-specific and cardioprotective effects to prevent the progression of CKD in numerous landmark cardiovascular outcome (CVO) trials [7,8,9]. Evidence is not clear whether these benefits can be extrapolated to patients with more advanced CKD or on dialysis, particularly those maintaining RKF on PD or iHD.
A post hoc analysis of The CREDENCE trial [7] described the efficacy and safety of canagliflozin in participants with eGFR below 30 mL/min/1.73 m2. In total, 174 individuals (4%) out of the total 4401 participants randomized for the trial had an eGFR below 30 mL/min/1.73 m2 at the time of randomization, with an average eGFR of 26 mL/min/1.73 m2. This analysis revealed that canagliflozin effectively slowed down the progression of kidney disease in advanced DKD patients without increasing the risk of acute kidney injury (AKI) [22]. Additionally, Chertow et al. [12] showed the beneficial effects of dapagliflozin on the reduction of renal and cardiovascular events, as well as the delay in the progression of eGFR decline in patients with an eGFR below 30 mL/min/1.73 m2, independently of the presence of type 2 diabetes (T2DM).
The recently completed EMPA-KIDNEY trial included patients with CKD (T2DM and without T2DM) who had an eGFR between 20 mL/min/1.73 m2 and 90 mL/min/1.73 m2 with UACR ≥ 200 mg/g. Patients were randomly assigned to receive empagliflozin (10 mg once daily) or a matching placebo. The primary outcome was a composite of the progression of kidney disease or death from cardiovascular causes. Of the 6609 patients that underwent randomization, 1131 (34%) in the control group and 1151 (35%) in the placebo group had an eGFR < 30 mL/min/1.73 m2. This trial demonstrated consistent benefits of empagliflozin treatment in reducing the risk of kidney disease progression or death from cardiovascular causes compared to the placebo, even in the population with lower eGFR [9].
Ongoing randomized clinical trials (RCT) should provide further evidence to support the usefulness of SGLT2i in HD. We look forward with optimism to the results of the following studies currently underway: (1) SGLT2i in hemodialysis (DAPA-HD). Examine the effect of dapagliflozin for left ventricular mass indexed to body surface area (LVMi) reduction as measured by cardiac magnetic resonance imaging at baseline and after 6 months of treatment in comparison with a placebo in patients undergoing replacement therapy with hemodialysis (NCT05179668). (2) Safety, tolerability, and feasibility of empagliflozin therapy in dialysis-dependent ESKD (EM-PA-HD). The aim is to include 75 diabetic and non-diabetic patients on dialysis for >3 months. They are randomized into three treatment arms: empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The primary variable is the proportion of patients still on treatment at each dose at the end of 12 weeks of treatment. In the secondary variables, the trial will look at the adherence, safety, and pharmacokinetics of empagliflozin in these dialyzed patients (NCT05687058). (3) The RENAL LIFECYCLE trial is a pragmatic, randomized, controlled clinical trial with a basket design. It plans to enrol 1500 participants (including 450 to 525 patients with CKD stages G4/5, 400 to 475 patients on dialysis, and 550 to 650 patients with a KT), consisting of a screening period and a double-blind treatment period with two arms. The primary outcome measure is the combined endpoint of all-cause mortality, kidney failure, and hospitalization for heart failure in the overall study population. It should be noted that when pre-dialysis patients start dialysis or dialysis patients are transplanted, they will continue in the trial, and this will not be a reason to discontinue the study. Of note, the inclusion criteria for the dialysis patients specify that they must maintain a residual diuresis >500 mL/24 h at least 3 months after the start of dialysis (NCT05374291). In addition, the included dialysis patients should have a residual diuresis >500 mL/24 h at least 3 months after starting dialysis (NCT05374291). (4) The safety of dapagliflozin in hemodialysis patients with heart failure (SDHF) is an open, randomized, controlled study. The aim is to recruit 20 hemodialysis patients with heart failure. Among these participants, 10 individuals will receive a daily dose of dapagliflozin at 10mg for a duration of 12 weeks. The primary outcome measure focuses on determining the number of patients who experience hypoglycemia or urinary infection. The secondary outcome involves assessing the changes in NT-proBNP levels (NCT05141552). These and still developing trials support the premise that, even in the context of minimal diuresis and low SGLT2 receptor disposition, as expected in incident iHD patients who still maintain significant RKF, SGLT2i may exert favorable direct and indirect effects in preventing cardiovascular and mortality outcomes and other benefits such as the preservation of the RKF [13].
RKF conveys a survival benefit and cardiovascular health among HD patients. Clearance of protein-bound and middle molecules, reduction of inflammation, and improved fluid management are among the proposed mechanisms [23]. Therefore, preservation of RKF is associated with better patient outcomes, including survival and better quality of life [24,25]. There is no uniform definition for RKF, it can be estimated and measured, but an optimal method for it has not been established. The NKF-KDOQI guidelines advocate measuring RKF by calculating the residual kidney urea clearance (KrU) in 24 h urine and expressing it in mL/min/1.73 m2 in HD patients.
Preservation of RKF in iHD patients requires not only adequate BP, proteinuria, diuretics, and volume control but also avoiding intradialytic hypotension and continuous adjustment of hemodialysis prescription by measurement and monitorization of RKF [23].
In our center, the intensity of the iHD regimen is tailored based on residual kidney function (RKF). Patients may begin with a once-weekly regimen if their KrU is in the range of 4 to 5 mL/min/1.73 m2. If the KrU decreases to a range of 2 to 4 mL/min/1.73 m2, the regimen progresses to twice weekly. Finally, if the KrU falls below 2 mL/min/1.73 m2, the regimen is adjusted thrice weekly. This approach allows for individualized treatment based on the specific level of residual kidney function.
The seven patients preserved creatinine clearance (CrCl) and KrU 24 h after the introduction of SGLT2i, and two of them (Patient 1 and 5) even improved their eGFR, making it possible to discontinue hemodialysis. In all cases except for Patient 7, KrU and CrCl increased with an average of 2.7 mL/min and 3.65 mL/min, respectively. Although CrCl decreased in this patient, urinary creatinine and urea excretion normalized to kilogram weight were maintained. A possible cause of the decrease in CrCl is the significant increase in lean mass and serum Cr.
Currently, there are no trials analyzing the role of SGLT2i in the preservation of RKF in patients with advanced DKD undergoing iHD. The KrU findings from our case series may suggest some additional beneficial effects of SGLT2i on RKF. Nevertheless, the importance of regularly monitoring RKF in all KRT modalities and adjusting dialysis prescriptions cannot be stressed enough.
KDOQI guidelines [17] recommend ACEi or ARB-II to control BP in patients with significant RKF either on PD or HD because this treatment was associated with an improvement in residual glomerular filtration rate (GFR) and urine volume on HD [26]. We know now that renin–angiotensin system (RAS) inhibitors continuation in non-dialysis advanced CKD patients is not associated with a significant loss of RKF in the long term [27]. Improvements in BP control are associated with hemodynamic stability during dialysis and with the maintenance of RKF and fewer cardiovascular events [28].
It is well known that the risk of hyperkalemia in patients with HD is high and multifactorial. It could be associated with long interdialytic intervals, acidosis, food intake, or medications such as ACEi/ARB-II or mineralocorticoid receptor antagonists (MRA). In our series, we observed a significant drop in the levels of potassium at the end of the follow-up. Although the exact mechanism is not clear, there is a hypothesis from a recent meta-analysis that SGLT2i may increase distal sodium and water delivery, enhancing the electronegative charge in the tubular lumen that regulates potassium excretion in the distal nephron caused on some occasions by the combined regimen including ACEi or MRA [29].
To reconcile available evidence, controversial results exist that the use of SGLT2 may have some deleterious effects on bone health in specific subgroups of patients at high risk for bone fracture [30]. This is associated with the combination of decreased 1,25-dihydroxyvitamin D (1,25(OH)2D) and increased PTH and fibroblast growth factor-23 (FGF-23) serum levels, which combined possibly contribute to the increased fracture risk associated with SGLT2i, but such results were not present in our series.
In our study, we observed a significant reduction of SBP after 12 months of SGLT2i treatment. Similar data were obtained in a cohort of 50 patients with T2DM and advanced CKD on automated peritoneal dialysis (APD) who had SGLT2i (dapagliflozin) added to their insulin therapy. Compared to SBP at the start of treatment versus 6 months after, the authors observed a significant decrease from 148 ± 5.2 mmHg to 134 ± 6.5 mmHg (p = 0.0431) [31]. Although modest, to our knowledge, these effects on BP may contribute to the potential reno-protective and RKF-preserving actions of SGLT2i. Studies in animal models demonstrate that SGLT2 inhibition causes sympathetic inhibition of renal nerve function, which could explain the glucose-independent effect of SGLT2 inhibition on BP [32]. The denervation hypothesis is consistent with the glucose-independent renal and cardiovascular benefits observed in the subgroup of patients with eGFR below 30 mL/min/1.73 m2 treated with canagliflozin [22], as well as in the DAPA-HD study with dapagliflozin [33]. Of note, there are contradictory data on the benefits of ACEi and ARB-II in reducing mortality and preserving RFK in patients on hemodialysis, independent of attained blood pressure. Xydakis et al. [26] conducted a study in 42 hypertensive patients on HD treated with enalapril, showing the association of increased preservation of RRF at 1-year follow-up. In contrast, Kjaergaard et al. [34] did not find the same benefit with irbesartan in HD patients. Patients in our series were already on these drugs before starting hemodialysis. Therefore, we hypothesized that SGLT2i has additional benefits when used concomitantly with RAS blockade, not only for the reduction in BP but also for its antiproteinuric effect despite extended glomerular damage in ESRD [35]. T2DM is a leading cause of proteinuria in ESRD patients, which is related to cardiovascular events [36]. However, persistent proteinuria is associated with a decline in RKF, independent of ESRD etiology [37]. RAS blockade and SGLT2i reduce albuminuria by decreasing intraglomerular pressure (SGLT2i by afferent arteriolar vasoconstriction, RAAS blockers by efferent arteriolar vasodilation). It is well known that one of the most relevant factors for the preservation of RKF is the reduction in urinary albumin excretion. Therefore, SGLT2i should have beneficial effects on RKF in patients with iHD and PD. In our case series, we observed significant albuminuria reduction, consistent with the findings of clinical trials performed in non-hemodialysis T2DM CKD patients [7,8,9].
During the 2022 KDIGO controversies conference on blood pressure and volume management in dialysis, reference was made to the fact that the balance between correcting chronic hypervolemia and preventing acute intravascular volume depletion remains a critical challenge in the care of HD patients. Various strategies, such as diuretics and volume control, have been proposed for volume management in HD patients, and this will depend primarily on the availability of accurate and objective methods to assess volume status. Clinical examination is currently the mainstay of volume assessment; however, this approach is inaccurate and unreliable. Other tools such as biomarkers, LUS, and bioimpedance designed to objectively aid in the assessment of ECW are currently being tested for efficacy and safety. In our hemodialysis unit, we use bioimpedance, an 8-zone LUS protocol and assessment of the inferior vena cava (IVC), portal, and hepatic vein to avoid high ultrafiltration rates and the risk of intradialytic cardiac stress, intradialytic hypotension (IDH), organ damage, and loss of RKF. Our results show that these tools are very useful in preserving RKF; however, more studies are needed to validate the efficacy and safety of these interventions in hemodialysis patients.
In addition, the positive effects of diuretic use in preventing excessive volume overload and preservation of RKF in HD patients are known. The Dialysis Outcomes and Practice Pattern Study (DOPPS) [38] showed the benefits of loop diuretics on better control of hyperkalemia, lower interdialytic weight gain, greater likelihood of preservation of RKF, and lower cardiac mortality below 14%. At the moment, it is unclear whether the diuretic effect of SGLT2i is attributable to osmotic diuresis or natriuresis, or both. SGLT2i can reduce intravascular volume status and thus preserve RKF. The records of our patients showed an increase in diuresis after starting the medication; these data agree with the results obtained in the study performed by Alhwiesh et al. [31] in T2DM patients with RKF in APD treated with SGLT2i.
Another relevant observation is that our patients did not require hospital admissions for heart failure; only one patient (Patient 4) required three extra hemodialysis sessions for volume adjustment. This low incidence of volemic complications could also be attributed to the strict control that we performed during the follow-up based on the point of care ultrasound (POCUS) and bioimpedance, as well as the constant periodical assessment by the nursing staff. The weight and ECW/TBW ratio of our patients decreased by a mean of 2.8 kg (p = 0.128) and 0.009 (p = 0.028), respectively. All these findings are similar to those found in patients with non-hemodialysis CKD.
High ultrafiltration rates (UFR) in HD patients are a risk factor for RKF loss [39]. There are a large number of observational studies demonstrating the association of higher UFR with all-cause and cardiovascular mortality. The findings of an observational study indicate that patients who experience a higher proportion of HD sessions with UFR exceeding 13 mL/h/kg during the initial three months of HD have an elevated risk of mortality. This increased risk persists even when the average UFR during that period remains below 13 mL/h/kg [40]. In our case series, interdialytic weight gain decreased by a mean of 0.39 L, with a consequent low UFR. We believe that SGLT2i use in incident iHD patients could help reduce interdialytic weight gain, avoiding high UFR rates, and preserving RKF.
Another important point is the effects of SGLT2i on body composition in patients with T2DM. Bioimpedance spectroscopy also measures other body composition parameters such as BMI, fat mass, and lean mass. In our case series, patients had a mean reduction in fat mass of 3 kg, with a mean increase in lean mass of 2 kg after starting SGLT2i, measured by bioimpedance. These findings are inconsistent with recent evidence pointing to an increased risk of sarcopenia by loss of skeletal muscle mass in diabetic patients treated with SGLT2i [41,42,43,44,45,46,47,48]. A recent meta-analysis in type 2 diabetic patients treated with SGLT2i showed a significant reduction in skeletal muscle mass compared to other antihyperglycemic agents [49]. The hypothesis put forward in this regard is that SGLT2i induces skeletal muscle loss to increase the release of amino acids into the systemic circulation as a catabolic response to renal glucose loss, preventing hypoglycemia [50,51]. Quiroga et al. [52], in a recent editorial, mention this issue, the effect of SGLT2i on skeletal muscle mass makes renal endpoints based on serum creatinine questionable (due to overestimation of eGFR), especially in cardiorenal patients, who per se present with muscle mass loss and sarcopenia. These aspects could influence the interpretation of the true renal and CV beneficial effects of SGLT2i. The authors recommend considering the use of muscle mass-independent estimates of eGFR, such as the calculation of eGFR based on cystatin C measurement.
The inhibitor effects of the coupled reabsorption of sodium and glucose in the proximal tubule of the kidney from SGLT2i lead to natriuresis and glycosuria. Therefore, SGLT2i promote the renal excretion of glucose and modestly lower elevated blood glucose levels. In patients with T2DM and moderate renal impairment, it was assumed that the HbA1c-lowering efficacy and micro-macrovascular preventive action of SGLT2i are attenuated or absent due to a reduction in the number of functional nephrons in proximal tubules containing SGLT2 receptors [53]. Nevertheless, despite this reduction in the capacity for tubular glucose reabsorption, SGLT-2i have been shown to be safe in diabetic patients with mild to moderate CKD [54]. The reduced glucosuric effect associated with low eGFR was observed in our cases series, but despite this, we observed a significant reduction in HbA1c and increased glycosuria. We assume that this improvement is due to two of the seven patients receiving GLP-1-RA treatment and not because of the direct effect of SGLT2i since the magnitude of serum glucose reduction is known to be dependent on glycosuria. We observed a beneficial effect of SGLT2i regarding serum uric acid with a mean reduction of 1.71 mg/dL, which is in agreement with the study by Alhwiesh et al. [31]. A meta-analysis revealed a significant reduction in uric acid levels after treatment with any of the SGLT2i [55]. The possible mechanisms underlying the hypouricemic effect of SGLT2i have not yet been established.
Regarding side effects, SGLT2i treatment in our case series was safe. Two patients developed a single episode of UTI. The medication was not discontinued in any of them. No cases of euglycemic ketoacidosis, bone fractures, or amputations were reported, but it must be addressed that our sample was small, and the follow-up period may have been too short.
The main limitation of our study is the small sample size. The absence of a control group and the fact that all data were retrospectively collected should also be of note. However, our study, as far as we are concerned, is the first report describing the possible beneficial effects of SGLT2i on RKF preservation in T2DM patients on iHD.
5. Conclusions
The use of SGLT2i in our small sample of patients with ESRD and DKD starting iHD on a 1–2 weekly regimen appears to be safe and effective in lowering HbA1c, improving BP control, reducing proteinuria, serum uric acid levels, and interdialytic weight gain, and most importantly in preserving RKF.
Clinical data supporting the cardio and nephroprotective effects of SGLT2i are currently limited to patients with eGFR >20 mL/min/1.73 m2, but emerging evidence is expanding its use and indication. The necessity of more studies on the effects of SGLT2i on patients with advanced CKD, kidney transplants, and dialysis patients cannot be stressed enough. The findings in this case series need to be confirmed in a large prospective study with a longer follow-up period.
Author Contributions
Conceptualization, J.C.D.L.F. and D.V.; methodology, J.C.D.L.F., R.Z., M.R., D.V., L.C., J.A., F.V., A.M., M.C. and J.D.; software, A.M., J.A. and M.C.; validation, J.C.D.L.F., D.V., J.A. and A.M.; formal analysis, J.C.D.L.F., D.V. and A.M.; investigation, J.C.D.L.F., R.Z., M.R., D.V., L.C., F.V., M.C. and J.D.; resources, J.C.D.L.F.; data curation, A.M.; writing—original draft preparation, J.C.D.L.F., D.V., L.C., J.A., M.C. and F.V.; writing—review and editing, J.C.D.L.F., D.V., A.M., R.Z., M.R., M.C., F.V. and J.D.; visualization, M.C. and J.D.; supervision, J.D., M.R. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not required for this study, according to our Institutional Review Board (Approval code: 29/23), due to the nature of our research as a case series in routine clinical practice.
Informed Consent Statement
Informed written consent was obtained from the patients for the publication of this article (including the publication of images).
Data Availability Statement
No new data were created or analyzed in this study. The data used to support the findings of this study are available from the corresponding author on request (Contact J.C.D.L.F., josedelaflor81@yahoo.com or jflomer@mde.es). I confirm that all the figures and tables are the original work of this manuscript’s authors. All have been performed by the authors of this manuscript, have not been adapted from other authors, and do not present an online link.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stel, V.S.; Awadhpersad, R.; Pippias, M.; Ferrer-Alamar, M.; Finne, P.; Fraser, S.D.; Heaf, J.G.; Hemmelder, M.H.; Martínez-Castelao, A.; De Meester, J.; et al. International comparison of trends in patients commencing renal replacement therapy by primary renal disease. Nephrology 2019, 24, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Tamura, M.K.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; De Boer, I.H. Kidney Disease and Increased Mortality Risk in Type 2 Diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evi-dence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- De la Flor, J.C.; Lorenzo, J.D.; Marschall, A.; Valga, F.; Vázquez, T.M.; Cícero, E.R. Efficacy and Safety of Semaglutide, a Glucagon-Like Peptide-1 Receptor Agonist in Real-Life: A Case Series of Patients in Maintenance Incremental Hemodialysis. Case Rep. Nephrol. Dial. 2022, 12, 238–247. [Google Scholar] [CrossRef]
- De Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.M.P.H.; Agarwal, R.; Bakris, G. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Maegawa, H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J. Diabetes Investig. 2017, 8, 416–427. [Google Scholar] [CrossRef]
- Yau, K.; Dharia, A.; Alrowiyti, I.; Cherney, D.Z.I. Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney Int. Rep. 2022, 7, 1463–1476. [Google Scholar] [CrossRef]
- Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Gorriz, J.L.; Hou, F.F.; McMurray, J.J.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Borkum, M.; Jamal, A.; Singh, R.S.; Levin, A. The rationale for the need to study sodium-glucose co-transport 2 inhibitor usage in peritoneal dialysis patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2023, 43, 139–144. [Google Scholar] [CrossRef] [PubMed]
- AlKindi, F.; Al-Omary, H.L.; Hussain, Q.; Al Hakim, M.; Chaaban, A.; Boobes, Y. Outcomes of SGLT2 Inhibitors Use in Diabetic Renal Transplant Patients. Transplant. Proc. 2020, 52, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S.; Al-Hadhrami, Z.; Moore, S.; Awad, S.; Shamseddin, M.K. Use of Sodium-Glucose Cotransporter-2 Inhibitors in Renal Transplant Patients with Diabetes: A Brief Review of the Current Literature. Can. J. Diabetes 2022, 46, 207–212. [Google Scholar] [CrossRef]
- Lim, J.-H.M.; Kwon, S.M.; Jeon, Y.M.; Kim, Y.H.M.; Kwon, H.M.; Kim, Y.S.M.; Lee, H.M.; Kim, Y.-L.M.; Kim, C.-D.M.; Park, S.-H.M.; et al. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation 2022, 106, e404–e412. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef] [PubMed]
- Caton, E.; Sharma, S.; Vilar, E.; Farrington, K. Impact of incremental initiation of haemodialysis on mortality: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2023, 38, 435–446. [Google Scholar] [CrossRef]
- Mathew, A.T.; Fishbane, S.; Obi, Y.; Kalantar-Zadeh, K. Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int. 2016, 90, 262–271. [Google Scholar] [CrossRef]
- Deira, J.; Suárez, M.A.; López, F.; García-Cabrera, E.; Gascón, A.; Torregrosa, E.; García, G.E.; Huertas, J.; De La Flor, J.C.; Puello, S.; et al. IHDIP: A controlled randomized trial to assess the security and effectiveness of the incremental hemodialysis in incident patients. BMC Nephrol. 2019, 20, 8. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Unruh, M.; Zager, P.G.; Kovesdy, C.P.; Bargman, J.M.; Chen, J.; Sankarasubbaiyan, S.; Shah, G.; Golper, T.; Sherman, R.A.; et al. Twice-Weekly and Incremental Hemodialysis Treatment for Initiation of Kidney Replacement Therapy. Am. J. Kidney Dis. 2014, 64, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.; Oshima, M.; Mahaffey, K.W.; Agarwal, R.; Cannon, C.P.; Capuano, G.; Charytan, D.M.; De Zeeuw, D.; Edwards, R.; Greene, T.; et al. Effects of Canagliflozin in Patients with Baseline eGFR <30 mL/min per 1.73 m2. Clin. J. Am. Soc. Nephrol. 2020, 15, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wilcox, C.S.; Lipkowitz, M.S.; Gordon-Cappitelli, J.; Dragoi, S. Rationale and Strategies for Preserving Residual Kidney Function in Dialysis Patients. Am. J. Nephrol. 2019, 50, 411–421. [Google Scholar] [CrossRef]
- Chandna, S.M.; Farrington, K. Reviews: Residual Renal Function: Considerations on Its Importance and Preservation in Dialysis Patients. Semin. Dial. 2004, 17, 196–201. [Google Scholar] [CrossRef]
- Vilar, E.; Farrington, K. Emerging Importance of Residual Renal Function in End-Stage Renal Failure. Semin. Dial. 2011, 24, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Xydakis, D.; Papadogiannakis, A.; Sfakianaki, M.; Kostakis, K.; Stylianou, K.; Petrakis, I.; Ergini, A.; Voskarides, K.; Dafnis, E. Residual Renal Function in Hemodialysis Patients: The Role of Angiotensin-Converting Enzyme Inhibitor in Its Preservation. ISRN Nephrol. 2013, 2013, 184527. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. Renin–Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef]
- Dasgupta, I.; Thomas, G.N.; Clarke, J.; Sitch, A.; Martin, J.; Bieber, B.; Hecking, M.; Karaboyas, A.; Pisoni, R.; Port, F.; et al. Associations between Hemodialysis Facility Practices to Manage Fluid Volume and Intradialytic Hypotension and Patient Outcomes. Clin. J. Am. Soc. Nephrol. 2019, 14, 385–393. [Google Scholar] [CrossRef]
- Luo, X.; Xu, J.; Zhou, S.; Xue, C.; Chen, Z.; Mao, Z. Influence of SGLT2i and RAASi and Their Combination on Risk of Hyperkalemia in DKD: A Network Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2023. [Google Scholar] [CrossRef]
- Edwards, A.; Bonny, O. A model of calcium transport and regulation in the proximal tubule. Am. J. Physiol. Physiol. 2018, 315, F942–F953. [Google Scholar] [CrossRef]
- Alhwiesh, A.K.; Sarah Al-Wa, I.; Abdul-Rahman, S.; Ahmad Nasreldin, M.; Moaz Mohammed, A.; Al-Oudah, S.; Al Awal, A.; Alsenpisi, Z.; Abdulrahman, A.; Al-Warthan, S. The Use of SGLT2 Inhibitors in Peritoneal Dialysis Patients: A Shade of Light on Dapagliflozin. Arch. Nephrol. Urol. 2022, 5, 1–8. Available online: https://www.fortunejournals.com/articles/the-use-of-sglt2-inhibitors-in-peritoneal-dialysis-patients-a-shade-of-light-on-dapagliflozin.html (accessed on 13 May 2023). [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Kjaergaard, K.D.; Peters, C.D.; Jespersen, B.; Tietze, I.N.; Madsen, J.K.; Pedersen, B.B.; Novosel, M.K.; Laursen, K.S.; Bibby, B.M.; Strandhave, C.; et al. Angiotensin Blockade and Progressive Loss of Kidney Function in Hemodialysis Patients: A Randomized Controlled Trial. Am. J. Kidney Dis. 2014, 64, 892–901. [Google Scholar] [CrossRef] [PubMed]
- De La Flor Merino, J.C.; Apaza Chávez, J.; Valga Amado, F.; Díaz Crespo, F.; Justo Avila, P.; Marschall, A. Remission of Pro-teinuria in a Patient Affected by Crescentic IgA Nephropathy with Rapidly Progressive Glomerulonephritis Treated by So-dium-Glucose Cotransporter-2 Inhibitors: Casual or Causal Relationship? Kidney Dial. 2022, 2, 545–552. [Google Scholar] [CrossRef]
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.; Chalmers, J.; Heerspink, H.J.L.; Lee, B.J.; Perkins, R.M.; Rossing, P.; Sairenchi, T.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef]
- Szeto, C.-C.; Kwan, B.C.-H.; Chow, K.-M.; Chung, S.; Yu, V.; Cheng, P.M.-S.; Leung, C.-B.; Law, M.-C.; Li, P.K.-T. Predictors of Residual Renal Function Decline in Patients Undergoing Continuous Ambulatory Peritoneal Dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2015, 35, 180–188. [Google Scholar] [CrossRef]
- Bragg-Gresham, J.L.; Fissell, R.B.; Mason, N.A.; Bailie, G.R.; Gillespie, B.W.; Wizemann, V.; Cruz, J.M.; Akiba, T.; Kurokawa, K.; Ramirez, S.; et al. Diuretic Use, Residual Renal Function, and Mortality Among Hemodialysis Patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS). Am. J. Kidney Dis. 2007, 49, 426–431. [Google Scholar] [CrossRef]
- Jansen, M.A.M.; Hart, A.A.M.; Korevaar, J.C.; Dekker, F.W.; Boeschoten, E.W.; Krediet, R.T. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2022, 62, 1046–1053. [Google Scholar] [CrossRef]
- Navarrete, J.E.; Rajabalan, A.; Cobb, J.; Lea, J.P. Proportion of Hemodialysis Treatments with High Ultrafiltration Rate and the Association with Mortality. Kidney360 2022, 3, 1359–1366. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017, 47, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Otsuka, Y.; Ashida, K.; Nagayama, A.; Hasuzawa, N.; Iwata, S.; Hara, K.; Tsuruta, M.; Wada, N.; Motomura, S.; et al. Improvement of skeletal muscle insulin sensitivity by 1 week of SGLT2 inhibitor use. Endocr. Connect. 2020, 9, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Miyoshi, H.; Ono, K.; Sugawara, H.; Kameda, R.; Ichiyama, M.; Yamamoto, K.; Nomoto, H.; Nakamura, A.; Atsumi, T. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr. J. 2016, 63, 589–596. [Google Scholar] [CrossRef]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H. Luseogliflozin reduces epicardial fat accumu-lation in patients with type 2 diabetes: A pilot study. Cardiovasc. Diabetol. 2017, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and sim-ultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japa-nese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Tobita, H.; Sato, S.; Miyake, T.; Ishihara, S.; Kinoshita, Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr. Ther. Res. 2017, 87, 13–19. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef]
- Miyake, T.; Yoshida, S.; Furukawa, S.; Sakai, T.; Tada, F.; Senba, H.; Yamamoto, S.; Koizumi, Y.; Yoshida, O.; Hirooka, M.; et al. Ipragliflozin ameliorates liver damage in non-alcoholic fatty liver disease. Open Med. 2018, 13, 402–409. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Post, A.; Groothof, D.; Eisenga, M.F.; Bakker, S.J.L. Sodium-Glucose Cotransporter 2 Inhibitors and Kidney Outcomes: True Reno-protection, Loss of Muscle Mass or Both? J. Clin. Med. 2020, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, B.; Díez, J. Estimation of glomerular filtration rate in cardiorenal patients: A step forward. Clin. Kidney J. 2023, 16, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Veltkamp, S.A.; Smulders, R.A.; Kadokura, T. Renal glucose handling: Impact of chronic kidney disease and sodi-um-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013, 36, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Delanaye, P. SGLT2 inhibitors in patients with chronic kidney disease: From clinical trials to guidelines and new prospects for clinical practice. Rev. Med. Liege 2021, 76, 186–194. [Google Scholar]
- Zhao, Y.; Xu, L.; Tian, D.; Xia, P.; Zheng, H.; Wang, L.; Chen, L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2018, 20, 458–462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).