Abstract
Neovascular age-related macular degeneration (nAMD) with choroidal neovascularization (CNV) is a leading cause of blindness in the elderly in developed countries. The disease is currently treated with anti-angiogenic biologics, including aflibercept, against vascular endothelial growth factor (VEGF) but with limited efficacy, treatment resistance and requirement for frequent intravitreal injections. Although anti-VEGF gene therapy may provide sustained therapy that obviates multiple injections, the efficacy and side effects related to VEGF pathway targeting remain, and alternative strategies to block angiogenesis independently of VEGF are needed. We recently reported that secretogranin III (Scg3) induces only pathological angiogenesis through VEGF-independent pathways, and Scg3-neutralizing antibodies selectively inhibit pathological but not physiological angiogenesis in mouse proliferative retinopathy models. Anti-Scg3 antibodies synergize dose-dependently with VEGF inhibitors in a CNV model. Here, we report that an adeno-associated virus-8 (AAV8) vector expressing anti-Scg3 Fab ameliorated CNV with an efficacy similar to that of AAV-aflibercept in a mouse model. This study is the first to test an anti-angiogenic gene therapy protocol that selectively targets pathological angiogenesis via a VEGF-independent mechanism. The findings support further safety/efficacy studies of anti-Scg3 gene therapy as monotherapy or combined with anti-VEGF to treat nAMD.
1. Introduction
Neovascular age-related macular degeneration (nAMD), also known as exudative or wet AMD, is a leading cause of blindness in the elderly populations of developed countries, with the incidence currently estimated to be >1.5 million cases in the US [1]. Wet AMD manifests as choroidal neovascularization (CNV) in the macula and was traditionally treated with thermal laser or photodynamic therapy but with poor efficacy and potential side effects [2]. The advent and approval of anti-angiogenic biological drugs against vascular endothelial growth factor (VEGF), such as ranibizumab and aflibercept, delivered by intravitreal injections represent a major breakthrough for nAMD therapy but with limited efficacy. Clinical trials reported visual acuity improvement (>15 letters) only in 14.6–40.3% of patients treated with anti-VEGF vs. 3.8–5.6% in sham controls [3]. The requirement for frequent intravitreal injections is a major drawback. The half-lives of intravitreally injected ranibizumab and aflibercept are 9 and 11 days, respectively, so monthly injections are required to maintain therapeutic efficacy [4,5]. Frequent intravitreal injections increase the risk of eye complications, including ocular pain, cataract, retinal and vitreous hemorrhage, retinal detachment, endophthalmitis and elevated intraocular pressure [6]. When combined with the physical and psychological stresses associated with repeated ocular injections, the treatment regimens severely impact patient quality of life. Real-world visual outcomes after anti-VEGF therapy often fall short of those in published randomized clinical trials, as a consequence of poor patient compliance, lower injection rates and undertreatment [7,8,9,10].
The possibility for anti-VEGF treatments to be synergized by simultaneously targeting alternative signaling pathways was explored in trials by combining ranibizumab with Fovista or nesvacumab as the antagonist of platelet-derived growth factor (PDGF) or angiopoietin 2 (Ang2), respectively, but failed to achieve endpoints [11,12]. Although safety/efficacy limitations and patient tolerance were cited as reasons for the failures, it seems likely that the common VEGF-dependent signaling pathways of PDGF and Ang2 preclude synergy with anti-VEGF while retaining the limitations of anti-VEGF monotherapy [13,14,15]. Faricimab, a bispecific antibody that simultaneously targets VEGF-A and Ang2, delivered by an intravitreal injection every 12–16 weeks was found to be clinically equivalent to aflibercept for the treatment of nAMD and approved by the FDA on the basis of “no inferiority” [16,17]. The VEGF pathway remains the exclusive target of most ongoing clinical trials [18]. Alternative approaches to replace or synergize with anti-VEGF therapeutics are rarely reported largely because the relevant VEGF-independent regulators of angiogenesis have not been identified.
Gene therapy protocols to deliver aflibercept and ranibizumab are currently in Phase 1/2 clinical trials [19]. The goal is to circumvent the requirement for repeated injections by delivering sustained therapy via a single intraocular injection. The strategy is important but will not address other issues related to limited anti-VEGF efficacy across patient groups. We recently identified secretogranin III (Scg3) as a unique disease-restricted angiogenic factor that drives pathological but not physiological angiogenesis via a VEGF-independent signaling pathway [20]. Scg3-neutralizing antibodies alleviate CNV and dose-dependently synergize with aflibercept in mouse models [21,22,23]. Anti-Scg3 therapeutic antibodies represent potential alternatives or add-ons to anti-VEGF therapy for proliferative ocular diseases but share the same durability obstacles and requirements for repeated injections. The benefits of adapting these therapies to gene therapy are clear and uncontroversial. Here, we investigated the feasibility of anti-Scg3 gene therapy to ameliorate laser-induced CNV in mice and compared efficacies with anti-VEGF gene therapy.
2. Materials and Methods
2.1. Animals and Materials
C57BL/6J mice (6–8 weeks old, strain # 000664) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All animal procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine (Protocol # AN-8362). An anti-Scg3 clone mT4 monoclonal antibody (mAb) was generated by Everglades Biopharma, LLC (Houston, TX, USA), and it was characterized for Scg3-neutralizing activity, as previously described [20,22]. AAV-CAG-mCherry was purchased from Charles River (Rockville, MD, USA).
2.2. Endothelial Cell Proliferation Assay
An endothelial cell proliferation assay was performed to characterize the neutralizing activity of an anti-Scg3 mAb Fab fragment (2.5 μg/mL) in human umbilical vein endothelial cells (HUVECs) in the presence or absence of Scg3 (1 μg/mL), as previously described [23].
2.3. Production of Recombinant AAV Vectors
The cDNA coding sequence of anti-Scg3 mouse Fab was amplified using PCR and cloned into an AAV2-CAG plasmid at EcoRI and XhoI sites (Figure 1A). A furin GT2A cleavage site was inserted between the heavy and light chains, and a signal peptide of the human/murine IgG heavy chain was inserted at the N-termini (Figure 1A) [24,25]. The cDNA for aflibercept with a C-terminal FLAG tag and the same N-terminal signal peptide was synthesized by Synbio Technologies (Monmouth Junction, NJ, USA) and cloned into an AAV2-CAG plasmid at EcoRI and XhoI sites (Figure 1A). All plasmids were verified using DNA sequencing. AAV8 was packaged and purified by SignaGen (Frederick, MD, USA) using CsCl gradient centrifugation, followed by dialysis against phosphate-buffered saline (PBS). The viral genome (vg) was titrated via qPCR using a standard curve and subsequently verified using digital PCR (dPCR) (Applied Biosystems/Thermo Fisher Absolute Q, Waltham, MA, USA) [26].
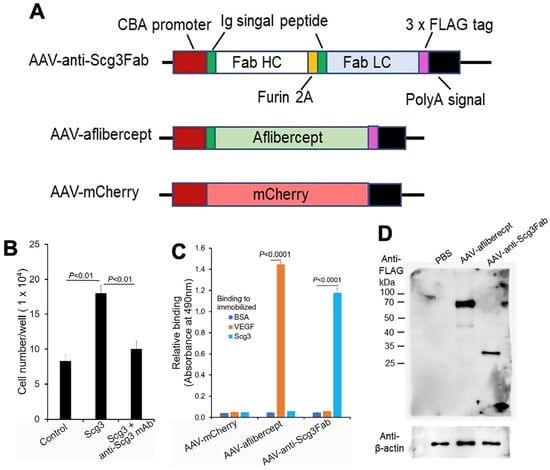
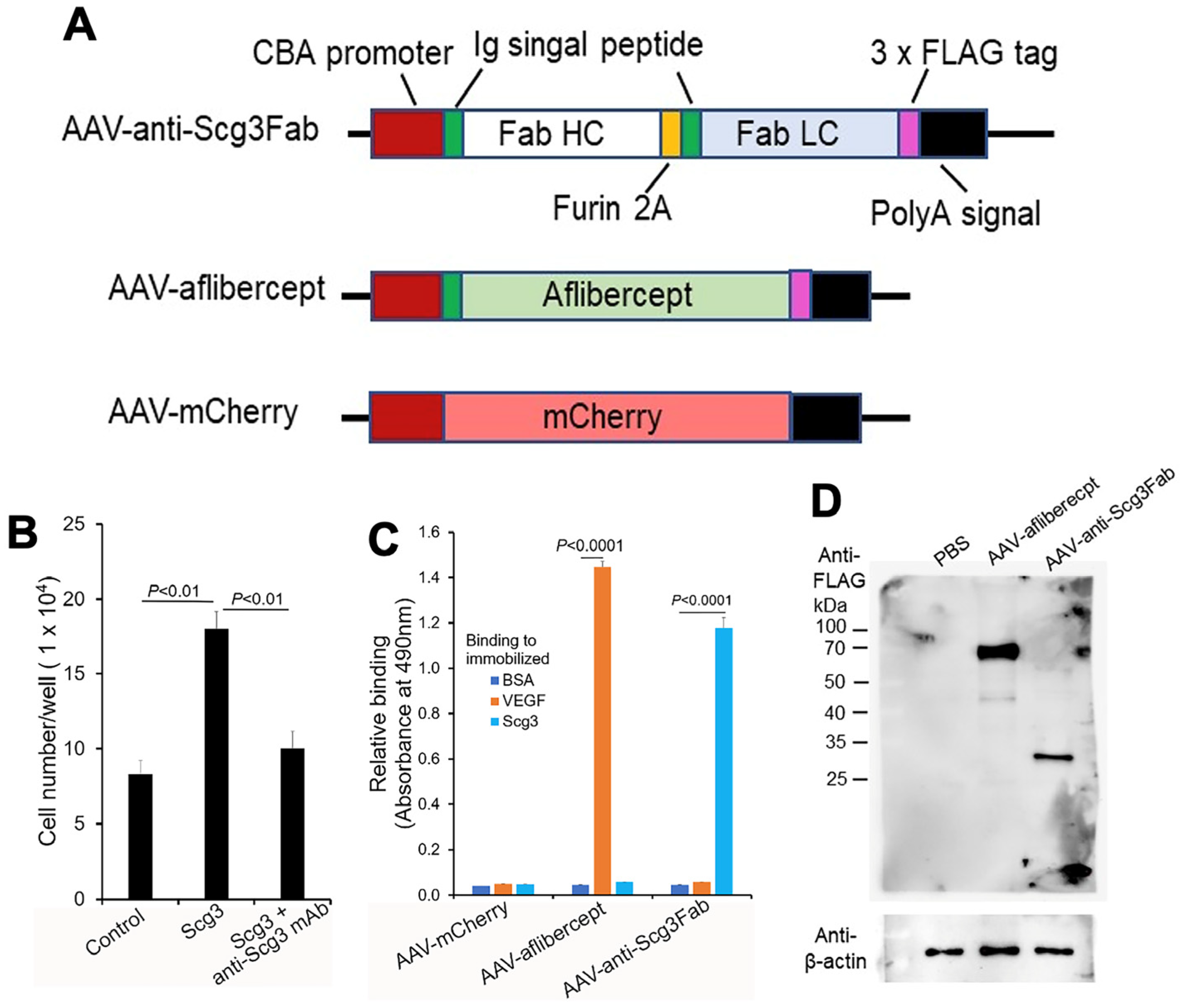
Figure 1.
Construction and characterization of AAVs. (A) Design of AAV-anti-Scg3Fab, AAV-aflibercept and AAV-mCherry. (B) Neutralizing activity of anti-Scg3 mAb to block Scg3-induced proliferation of HUVECs. n = 3 wells/group. (C) Functional validation of AAV-mediated transgene expression. HEK293 cells were transduced by indicated AAVs. Conditioned media were analyzed for binding activity to immobilized Scg3, VEGF or BSA (negative control) using ELISA. n = 3 wells/group. (D) AAV-mediated FLAG-tagged transgene gene expression in mouse retinas. Indicated AAVs were intravitreally injected into mice, and retinas were isolated one month after AAV injection and analyzed using Western blot with anti-FLAG mAb. ± SEM, one-way ANOVA test.
2.4. Characterization of AAV Plasmids and Viral Vectors
Human embryonic kidney 293 cells (HEK293) were seeded on 6-well plates at 2 × 105 cells/well in Dulbecco’s modified Eagle’s minimum essential medium (DMEM) (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), 1x GlutaMAX (Thermo Fisher) and 1% penicillin/streptomycin (Gibco) and incubated at 37 °C overnight in a humidified atmosphere with 5% CO2. The cells were transduced with AAV-anti-Scg3Fab, AAV-aflibercept or AAV-mCherry at 5 × 106 vg/mL. The medium was replaced with a serum-free 293SFM II medium (Thermo Fisher) the following day. Five days post-transduction, the conditioned medium was collected and concentrated using an Amicon® Ultra-4 Centrifugal Filter Unit (UFC801008, Millipore Sigma, St. Louis, MO, USA). An enzyme-linked immunosorbent assay (ELISA) was performed with pre-immobilized Scg3 (5 μg/mL, 100 μL/well, Sino Biological, Wayne, PA, USA), recombinant human VEGF (VEGF, 5 μg/mL, R&D Systems, Minneapolis, MN, USA) or bovine serum albumin (BSA, Sigma). Bound anti-Scg3 mAb and aflibercept were detected with biotin-conjugated anti-FLAG M2 mAb and horseradish peroxidase (HRP)-conjugated streptavidin (Sigma), followed by a colorimetric assay [27].
2.5. AAV Administration
Mice were anesthetized via an intraperitoneal (i.p.) injection of ketamine (40 mg/kg body weight, Covetrus North America, Portland, ME) and xylazine (8 mg/kg, Akom, Lake Forest, IL, USA). AAV-anti-Scg3Fab, AAV-aflibercept or AAV-mCherry (5.0 × 108 vg/1 μL/eye) was blind-coded and intravitreally injected.
2.6. Laser-Induced Choroidal Neovascularization
Mice were subjected to laser photocoagulation to induce CNV at 1 or 4 months after the AAV injection by using the following procedures: For pupil dilation, anesthetized mice received a topical eye drop of 1% tropicamide (Akorn, Lake Forest, IL, USA) and 2.5% phenylephrine (Paragon BiTeck, Portland, ME, USA). A green laser beam (532 nm, 240 mW, 150 ms, 50 µm spot) was applied to the retina around the optic disk (4 spots/retina) using a Micron IV retinal imaging system (Phoenix Research Labs, Pleasanton, CA, USA). Gaseous bubbles formed at laser spots indicated the rupture of Bruch’s membrane. Lesions with retinal hemorrhage on Day 0 and linear or fused lesions on Day 7 were excluded.
2.7. Fluorescein Angiography
Fluorescein angiography was conducted on Day 7 after laser photocoagulation. All fluorescein angiography images were taken 6 min after an injection of fluorescein sodium (0.1 mL/mouse, 2.5%, Akorn) in anesthetized mice with standardized instrument settings using a Spectralis Tracking OCTA system (Heidelberg Engineering, Franklin, MA, USA). Fluorescein angiography images were analyzed using ImageJ software (NIH). The area and intensity of the laser spots were normalized to cognate the entire viewing field of the eye. After fluorescein angiography, the retinal pigment epithelium (RPE)–choroid–sclera eyecups (RPE eyecups) were isolated from the euthanized mice, fixed, stained with Alexa Fluor 488-isolectin B4 (AF488-IB4, 10 μg/mL, Thermo Fisher), flat-mounted, and analyzed using a Keyence BZ-X810 structured illumination microscope (SIM) and Keyence software.
2.8. Immunohistochemistry
The mice with CNV were euthanized with CO2 inhalation after fluorescein angiography, and they were immediately perfused intracardially with PBS, followed by 4% paraformaldehyde (PFA) and eye enucleation. The anterior segments, including the cornea and lens, were removed to yield RPE eyecups that were embedded in the optimal cutting temperature (OCT) compound (Tissue-Tek; Miles Scientific, Napierville, IL, USA) and cryosectioned with a 10 μm thickness. The retinal sections were immunostained with anti-FLAG mouse M2 mAb (Sigma, #F1804, dilution 1:200), followed by Alexa Fluor 594-conjugated anti-mouse IgG F(ab’)2 (Cell Signaling, Danvers, MA, USA; #8889S; dilution 1:1000) and Hoechst staining, and analyzed using SIM microscopy.
2.9. Western Blot
Western blots were performed as previously described [20]. Briefly, the total protein was isolated from the retinas and homogenized in a RIPA buffer (Sigma) supplemented with a protease inhibitor cocktail (Sigma, Cat. #P8340). The total protein was quantified using a BCA protein assay Kit (Thermo Fisher), separated by SDS-PAGE (20 µg/lane), and transferred onto nitrocellulose membranes (Millipore). The membranes were probed with anti-FLAG M2 mAb (1:1000) and a horseradish peroxidase (HRP)-conjugated secondary antibody (Ab) for chemiluminescence signal detection, followed by stripping and reprobing with anti-β-actin mAb.
2.10. Statistical Analysis
Data are expressed as mean ± SEM. A statistical analysis was performed using a one-way ANOVA test. p < 0.05 was considered significant.
3. Results
3.1. Construction and In Vitro Characterization of AAVs
We chose anti-Scg3 mAb over the related humanized antibody (hAb) [20,23] for this project to minimize potential mouse anti-human IgG Ab that may attenuate the efficacy of anti-Scg3 gene therapy. We constructed a mouse anti-Scg3 mAb Fab fragment in an AAV2 vector with a CAG promoter [28]. The heavy and light chains contained the same human/murine IgG heavy chain signal peptide, separated by a furin GT2A cleavage site (Figure 1A) [24,25]. A FLAG tag was attached to the C-terminus of the light chain. AAV-aflibercept was constructed in a similar fashion. Anti-Scg3 mAb Fab was verified for its neutralizing activity to block Scg3-induced endothelial proliferation of HUVECs in a culture before AAV construction (Figure 1B). After packaging into AAV8, purified AAVs were used to transduce HEK293 cells. Conditioned media were collected and analyzed for binding activity to immobilized VEGF or Scg3 using ELISA. The results show that the HEK293 cells transduced with AAV-anti-Scg3Fab and AAV-aflibercept secreted functionally active anti-Scg3 Fab and aflibercept with corresponding binding activity to Scg3 and VEGF, respectively (Figure 1C). The results suggest that the heavy and light chains of anti-Scg3 Fab are appropriately processed by the furin protease cleavage and assembled into a functionally active Fab fragment. By contrast, a similar construct of anti-Scg3 Fab without the second signal peptide produced non-functional Fab without Scg3-binding activity.
3.2. Transgene Expression In Vivo
To characterize gene expression in mouse retinas, we intravitreally injected AAV-anti-Scg3Fab, AAV-aflibercept and AAV-mCherry into mice and analyzed gene expression one month post-injection. Western blot detected the expressions of the FLAG-tagged anti-Scg3 Fab light chain and aflibercept at approximately 27 kDa and 67 kDa, respectively, under reduced and denaturing conditions (Figure 1D). The predicted molecular weight was 26.7 kDa for anti-Scg3 light chain-FLAG and 51.4 kDa for aflibercept-FLAG without glycosylation. The size of the anti-Scg3 light chain also confirmed the cleavage by the furin protease. According to FDA drug information, the aflibercept dimer produced from CHO cells is 97 kDa (monomer 48.5 kDa) but migrates as 115 kDa, with the additional 15% of the total molecular weight attributed to glycosylation [29]. Therefore, aflibercept expressed in the mouse retina by AAV-aflibercept was glycosylated with a ~30% increase in the molecular weight.
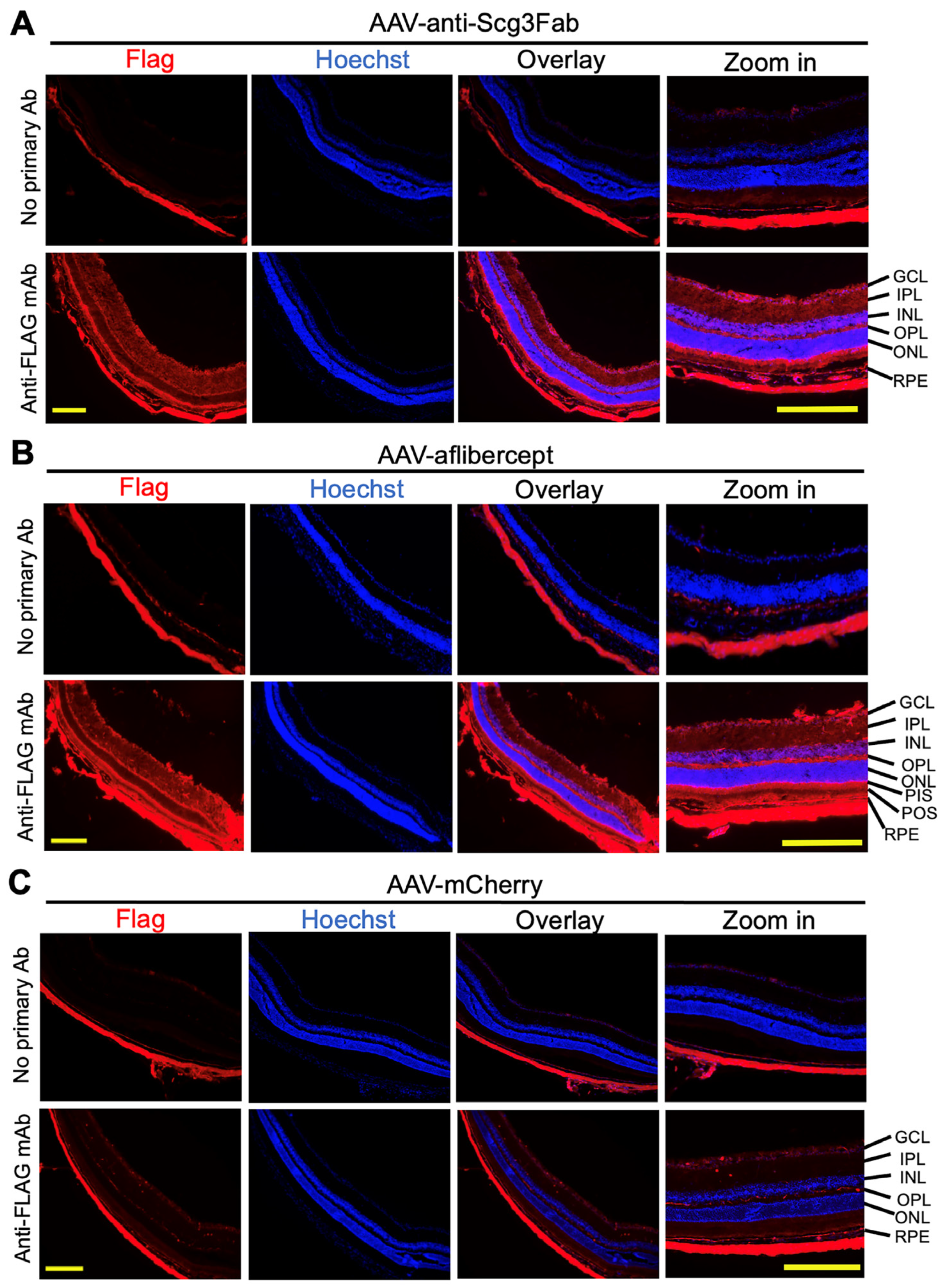
To independently validate the transgene expression pattern in the mouse retinas, we performed immunohistochemistry one month after the AAV injection using anti-FLAG mAb. The assay detected the expressions of FLAG-tagged anti-Scg3 Fab and aflibercept in the retinal ganglion cell (RGC) layer, inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), photoreceptor inner segments (PISs) and RPE. A reduced expression was also detected in the outer nuclear layer (ONL) and photoreceptor outer segments (POSs) (Figure 2). These expression patterns suggest that the transgenes are expressed throughout the entire retina. No or a minimal FLAG signal was detected for AAV-mCherry or retinal sections without the primary Ab, supporting the signal specificity.
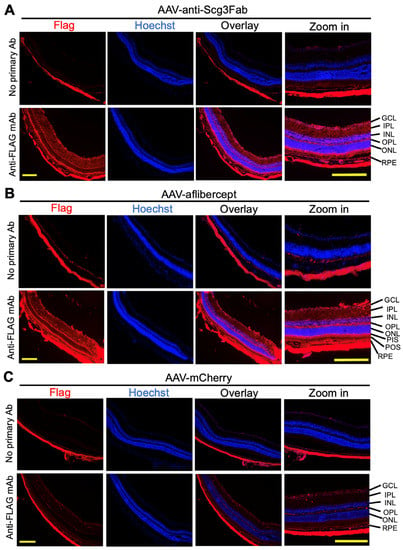
Figure 2.
Immunohistochemistry to detect AAV-mediated gene expression in mouse retinas. AAV-anti-Scg3Fab (A), AAV-aflibercept (B) and AAV-mCherry (C) were intravitreally injected into mice, and eyes were isolated from euthanized mice 1 month after AAV injection. Immunohistochemistry was performed using anti-FLAG mAb. Yellow scale bar = 200 µm. GCL, retinal ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PIS, photoreceptor inner segment; POS, photoreceptor outer segment; RPE, retinal pigment epithelium.
3.3. Anti-Angiogenic Gene Therapy to Inhibit CNV
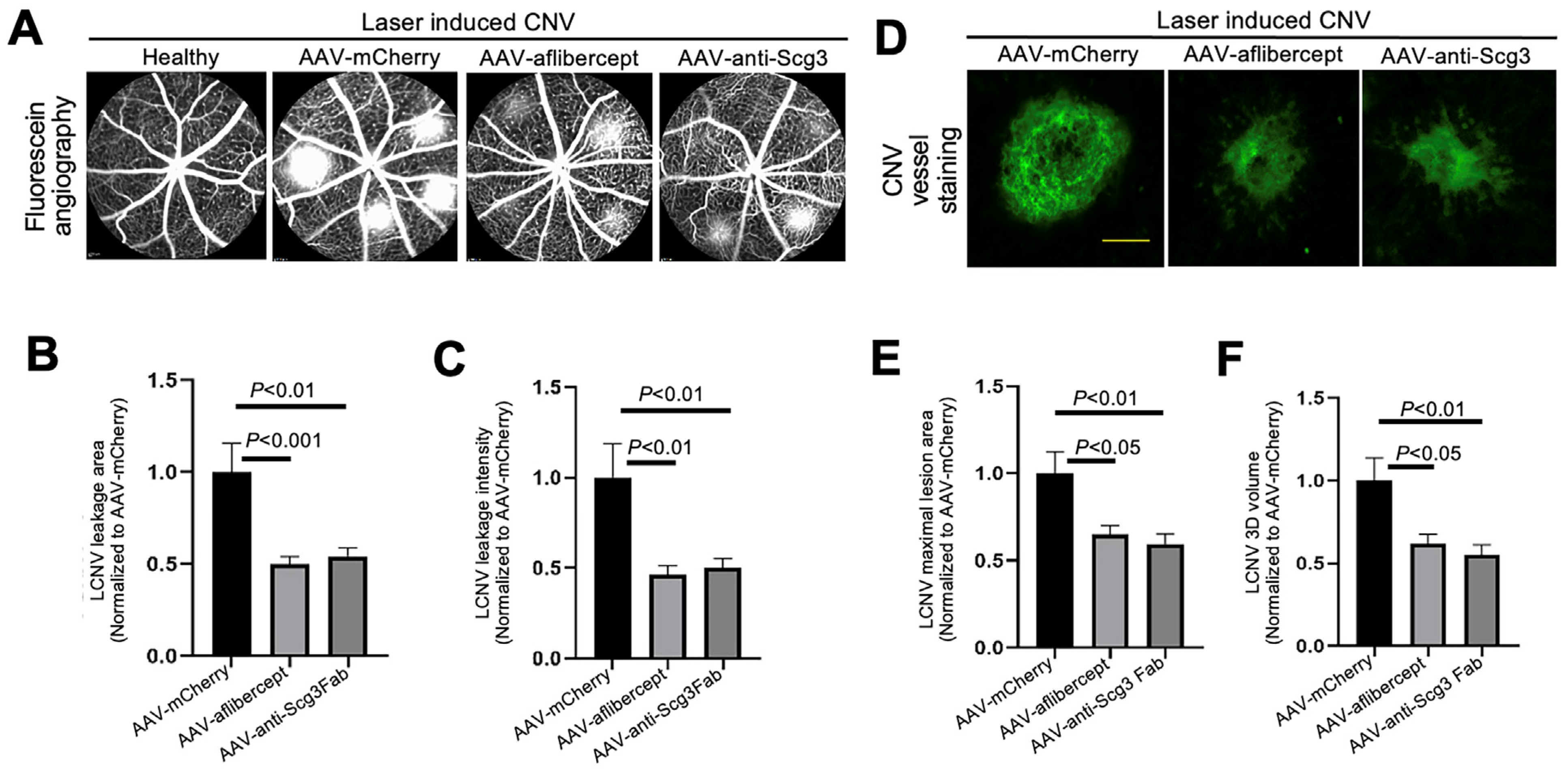
To compare efficacies, we intravitreally injected AAV-anti-Scg3Fab, AAV-aflibercept and AAV-mCherry into mice, followed by CNV induction one month after the AAV injection. We quantified the CNV leakage and related therapeutic efficacy in anesthetized mice using fluorescein angiography 7 days after CNV induction. The results indicate that AAV-anti-Scg3Fab significantly ameliorated CNV leakage in terms of the leakage area and intensity (Figure 3A–C). AAV-aflibercept as a positive control reduced the CNV leakage area and intensity by the same degree as the vehicle control vector AAV-mCherry.
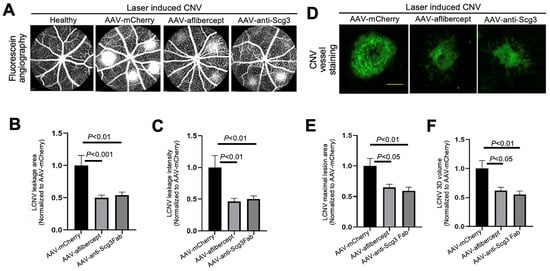
Figure 3.
Short-term therapeutic efficacy of AAV-anti-Scg3 and AAV-aflibercept in alleviating CNV. Indicated AAVs (blind-coded) were injected intravitreally into mice. After one month, mice were treated with laser photocoagulation to induce CNV. (A) Representative images of fluorescein angiography performed in anesthetized mice 7 days post-laser. (B) Quantification of CNV leakage area in (A). (C) Quantification of CNV leakage intensity in (A). n = 27 laser spots in 9 eyes (AAV-mCherry), 28 laser spots/8 eyes (AAV-aflibercept) and 32/10 (AAV-anti-Scg3Fab). (D) Representative images of eyecups isolated from mice 7 days post-laser and immunostained with Alexa Fluor 488-isolection B4 (AF488-IB4). (E) Quantification of CNV maximal lesion area in (D). (F) Quantification of CNV 3D volume in (D). n = 29 laser spots/9 eyes (AAV-mCherry), 21/8 (AAV-aflibercept) and 25/10 (AAV-anti-Scg3Fab). ± SEM; one-way ANOVA test. Scale bar = 100 µm.
To quantify CNV lesions using histopathology, we euthanized the mice after fluorescein angiography, and we isolated and stained the RPE–choroid–sclera eyecups with AF488-IB4 to label CNV vessels. An SIM microscopy analysis confirmed that AAV-anti-Scg3Fab and AAV-aflibercept significantly reduced CNV lesion size and 3D volume with similar efficacy (Figure 3D–F).
3.4. Long-Term Efficacy in Alleviating CNV
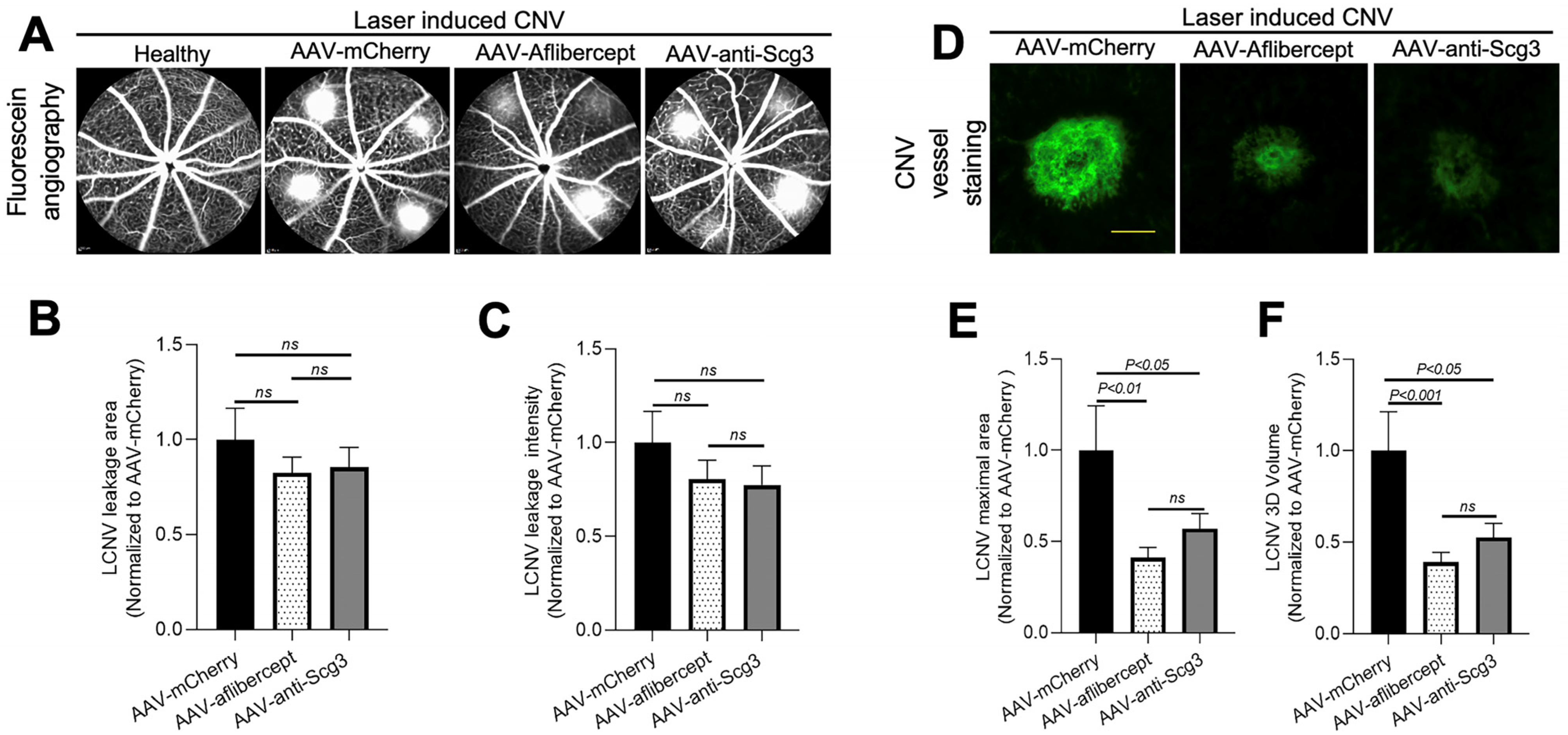
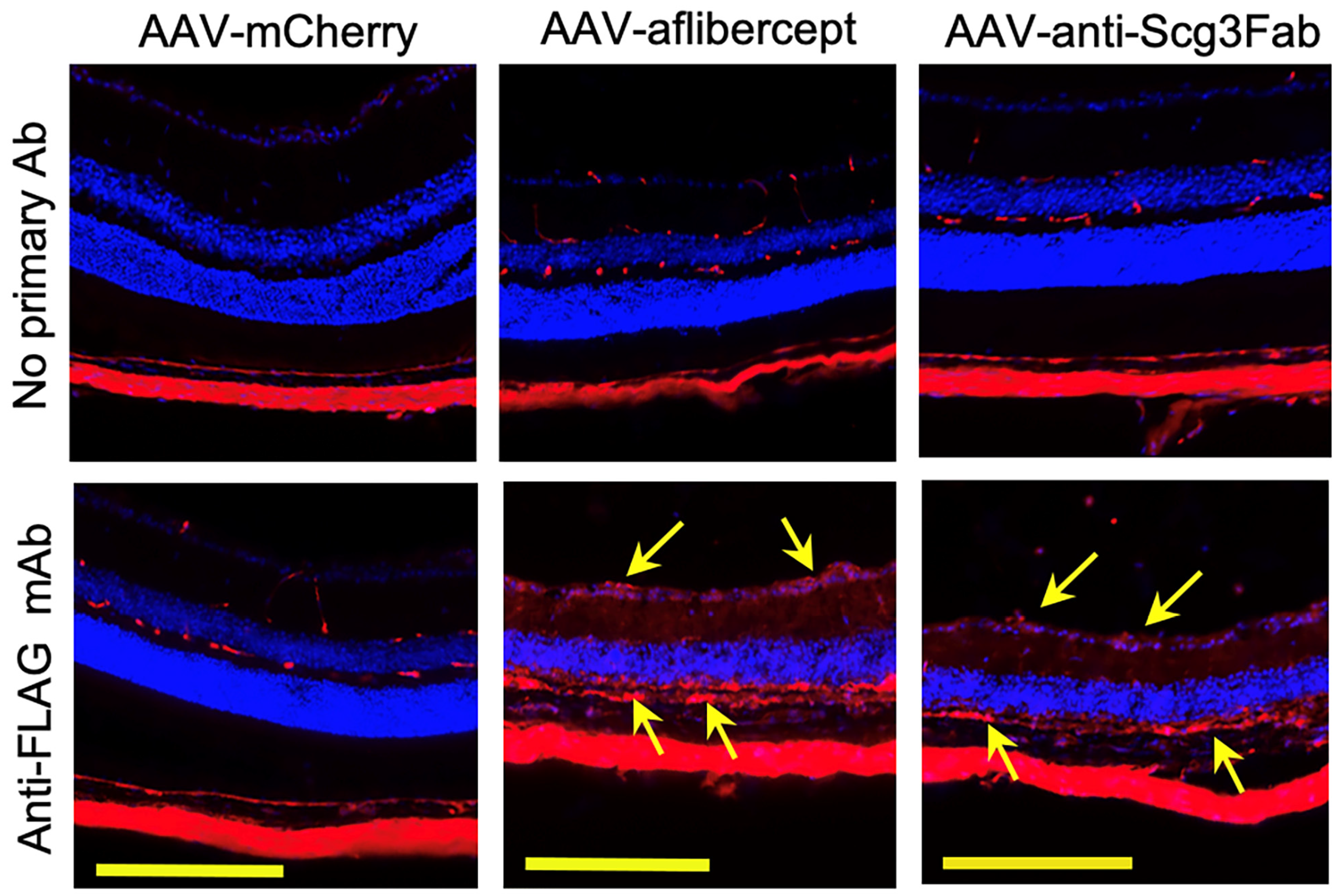
To investigate whether gene therapy in this model provides long-term therapeutic benefits, we induced CNV in mice 4 months after AAV transduction and analyzed therapeutic efficacy via fluorescein angiography and immunostaining with AF488-IB4 7 days after the CNV induction. The results show that both AAV-anti-Scg3Fab and AAV-aflibercept significantly and similarly reduced CNV lesion size and 3D volume (Figure 4D–F). However, unlike the 1-month protocol, the CNV leakage size and intensity were not significantly reduced by either treatment at 4 months (Figure 4A–C). Compared to transgene expression at one month after the AAV injection, immunohistochemistry revealed that the transgene expression at 4 months was markedly reduced (Figure 2 vs. Figure 5).
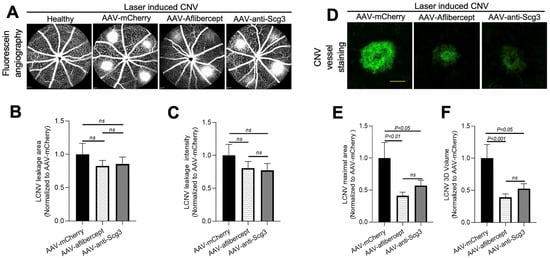
Figure 4.
Long-term therapeutic efficacy of AAV-anti-Scg3 and AAV-aflibercept in alleviating CNV. Indicated AAVs (blind-coded) were injected intravitreally into mice. After 4 months, mice were treated with laser photocoagulation to induce CNV and analyzed, as described in Figure 3. (A) Representative images of fluorescein angiography. (B) Quantification of CNV leakage area in (A). (C) Quantification of CNV leakage intensity in (A). n = 19 laser spots in 6 eyes (AAV-mCherry), 30 laser spots/9 eyes (AAV-aflibercept) and 26/8 (AAV-anti-Scg3Fab). (D) Representative images of eyecups isolated from mice 7 days post-laser and immunostained with AF488-IB4. (E) Quantification of CNV maximal lesion area in (D). (F) Quantification of CNV 3D volume in (D). n = 14/6 (AAV-mCherry), 25/9 (AAV-aflibercept) and 24/8 (AAV-anti-Scg3Fab). ± SEM; one-way ANOVA test. Scale bar = 100 µm.
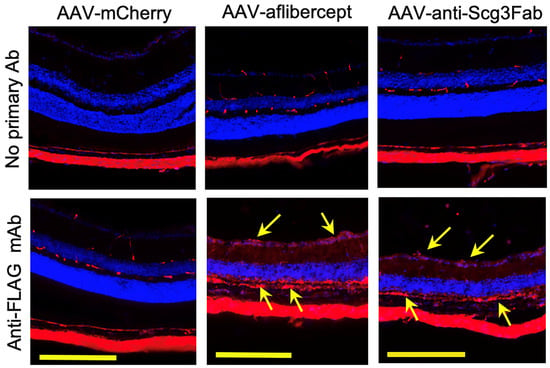
Figure 5.
Immunohistochemistry to detect long-term AAV-mediated gene expression in mouse retinas. AAV-anti-Scg3Fab, AAV-aflibercept and AAV-mCherry were intravitreally injected into mice, and eyes were isolated from euthanized mice 4 months after AAV injection. Immunohistochemistry was performed using anti-FLAG mAb. Yellow arrows indicate FLAG+ transgene signals. Yellow scale bar = 200 µm.
4. Discussion
We provide the first evidence that an Scg3 antagonist with a signaling pathway distinct from that of VEGF reduced CNV lesion size and leakage in a gene therapy protocol that is quantitatively equivalent to AAV-aflibercept administered according to the same regimen and gene dosing. Both genes were delivered by AAV8, a serotype appropriate for ocular indications [25]. The vectors delivered equivalent levels of expression at one month after intravitreal injections in mice with 5.0 × 108 vg/eye and generated secreted gene products that displayed the expected selective binding to immobilized VEGF and Scg3 ligands. Immunohistochemistry revealed common distributions of the respective AAV-aflibercept and AAV-anti-Scg3Fab gene products in all retinal layers (Figure 2). AAV-anti-Scg3Fab and AAV-aflibercept reduced the CNV 3D volume and maximal lesion area by the same degrees, indicating equivalent efficacy at both short and extended time intervals after AAV transduction. However, protection against CNV leakage was significantly effective only in the 1-month group and not at 4 months after transduction. We attribute the diminished efficacy to the relatively low vector dose, resulting in a gradual reduction in transgene expression over time (Figure 2 vs. Figure 5). The reduced transgene expression over time may be due to epigenetic regulations after AAV integration. Although the sustained expression of AAV in ocular tissues is well established [25,30,31,32], Liu et al. reported a strict dose-dependent expression and therapeutic efficacy of AAV8 anti-VEGF Fab at 1 month in a mouse CNV model with a markedly reduced efficacy at doses below ~109 vg/eye [25]. This is also consistent with the high dose of 2 × 1012 vg/eye used to express AAV-aflibercept in African green monkeys, equivalent to ~2 × 109 vg/eye in mice assuming a factor of ~660× for primate versus mouse vitreous volume [30,31,32,33]. Such a low-dose instability may account for the variable results seen in some gene therapy trials. In a Phase I trial of intravitreal AAV2-sFLT01, gene expressions in the aqueous humor of patients injected with <2 × 1010 vg/eye were below the limits of detection [34]. An insufficient transgene expression of 1 × 1011 vg/eye AAV2 via subretinal delivery in patients is suspected for the low efficacy and failure of sFLT01 clinical trials [35,36]. The results suggest a narrow window or threshold of the AAV vector dose to achieve sustained therapeutic gene expression [25]. In future studies, we will characterize the dose–response curves of AAV-anti-Scg3Fab and AAV-aflibercept in parallel to determine the dose requirement for persistent anti-Scg3 gene therapy. Additionally, we will investigate whether AAV2 and AAV2.7m8 further improve therapeutic duration [34,37].
Aflibercept contains the Ig domains of VEGFR1 and VEGFR2 fused to the Fc region of human IgG and functions as a soluble VEGF decoy receptor that binds and neutralizes VEGF-A, VEGF-B and placental growth factor (PIGF) [38]. Aflibercept is currently a first-line therapy for nAMD, macular edema following retinal vein occlusion, DR and diabetic macular edema [38]. The related and competing first-line VEGF-neutralizing reagents that are also FDA-approved for the same or similar ocular indications include ranibizumab; bevacizumab (used off-label for nAMD); brolucizumab; and more recently faricimab, a bispecific anti-VEGF and anti-Ang2 Ab [39]. To achieve stable efficacy for nAMD, all the approved drugs of this category require frequent intraocular injections, a requirement that adversely affects patient quality of life and treatment compliance [9,10]. Multiple studies have confirmed the requirement for rigid adherence to injection regimens and the loss or reversal of therapy caused by non-compliance [7,8]. Consequently, intense efforts are underway to circumvent monthly injections by devising sustained-release technologies and/or gene therapy that promises persistent therapy via a single intraocular injection [37]. After the failure of early efforts to translate endogenous angiogenesis inhibitors, such as angiostatin/endostatin and pigment epithelial-derived factor, into gene therapy applications, the field focused on adapting the current FDA-approved repertoire of VEGF pathway blockers [40]. After promising preclinical results [37], ADVM-022, an optimized AAV2 vector encoding aflibercept, is currently in a Phase 2 trial to treat nAMD [19,41]. In a second ongoing Phase I/IIa trial, RGX-314, an AAV8 vector expressing a mAb fragment similar to ranibizumab, delivered by a subretinal injection is being evaluated in patients with nAMD [36]. The safety/efficacy results at 1.5 years reported an improved or stabilized best-corrected visual acuity (BCVA), a reduced central retinal thickness (CRT) and markedly decreased requirements for supplemental anti-VEGF injections [42].
The progression of anti-angiogenesis protocols to gene therapy clinical trials promises to relieve the constraints of frequent intravitreal injections for patients with nAMD but fails to address the confounding issues of suboptimal therapy and other adverse side effects associated with current anti-VEGF therapy. Recent studies reported that VEGF inhibitors are effective in alleviating CNV in young but not aged animal models [43,44], implying that the promised efficacy of anti-VEGF in young animals may not be translated quantitatively to aged patients with nAMD. The alternating use and/or combination of these reagents with inhibitors of VEGF-dependent accessory factors, such as PDGF, Ang2 and semaphorin 6A [45], are unlikely to significantly improve efficacy as next-generation pharmacology or gene therapy for CNV indications [11,12]. Central to this dilemma is that the independence of VEGF signaling is a likely prerequisite for synergistic combinations with current anti-VEGF protocols, and, except for anti-Scg3, no inhibitors of pathological angiogenesis that work independently of VEGF signaling have been described.
Scg3 was discovered by our group using a novel comparative ligandomics technology to screen for disease-restricted ligands in mouse models of DR and CNV [20,22]. Scg3 is a disease-restricted angiogenic factor that selectively binds to diseased but not healthy vessels. Scg3-neutralizing Abs alleviated CNV, DR and retinopathy of prematurity (ROP) in mouse models with an efficacy equivalent to that of aflibercept [20,21,22,23,46,47,48]. The inhibition of angiogenesis by anti-Scg3 Abs in all cases was independent of VEGF, consistent with separate angiogenic signaling pathways and synergy between anti-Scg3 hAb and aflibercept [23]. In Scg3, we appear to have uncovered a hitherto invisible but long-sought-after disease-restricted proangiogenic pathway that operates in parallel but independent of VEGF in pathological states [20]. Such a property that restricts Scg3 actions to pathological angiogenesis is consistent with our findings of safety and wide therapeutic windows of anti-Scg3 vs. anti-VEGF [46,47]. Because, like anti-VEGF biologics, intraocular anti-Scg3 hAb is expected to have a short therapeutic duration, and clinical applications will also require repeated injections. Therefore, translation from protein pharmacology to gene therapy is an important next step to relieve these constraints.
Safety concerns have overshadowed the progression of anti-VEGF gene therapy since its inception. In addition to indiscriminate binding to diseased and healthy vessels, VEGF possesses neurotrophic and neuroprotective properties that promote neuronal growth and survival [49,50,51]. We and others have shown that intravitreal aflibercept induces abnormalities in electroretinography (ERG) and retinal structure in animal models [47,48,52]. Similar adverse effects on retinal function and structure were also reported in patients treated with VEGF inhibitors in some clinical studies [53,54,55,56]. Clinical studies also revealed that intravitreal anti-VEGF agents can interfere with the function of the central nervous system [57,58,59]. Long-term anti-VEGF therapy for nAMD may increase the risk of geographic atrophy [60]. A recent study reported trends of b-wave suppression by AAV-aflibercept in adult non-human primates 19 months after gene therapy [37]. Aged neurons in patients with AMD may be more susceptible to low-ambience VEGF ligands caused by anti-VEGF therapy, thereby contributing to a limited improvement in visual acuity [3]. Indeed, the possible neurotoxicity of anti-VEGF was implied in clinical trials of nAMD and diabetic macular edema (DME), in which high-dose ranibizumab inversely reduced long-term visual acuity despite improving vascular symptoms [61,62]. It is unclear whether neurotoxicity is caused by the direct effects of VEGF blockade on neurons or indirectly through the suppression of healthy vessels. It seems possible that patients with nAMD are more sensitive to manipulations of VEGF homeostasis due to age and comorbidities. Safety concerns related to chronic VEGF suppression prompted the testing of multiple strategies to regulate anti-VEGF gene therapy [63,64,65]. Because our studies indicate that Scg3 regulates only pathological angiogenesis, with no effect on healthy vessels or neurons [24,26], such safety issues may not apply, and long-term constitutive gene therapy with anti-Scg3 hAb is expected to be safe. Our findings warrant further investigation to compare the efficacy and safety of optimized AAV-anti-Scg3 and AAV-anti-VEGF for monotherapy or combination therapy in large animal models.
Author Contributions
Conceptualization, W.L., K.A.W. and H.T.; methodology, validation, formal analysis, investigation and data curation, C.H., L.J., A.K. and P.W.; resources, H.T.; writing—original draft preparation, C.H. and W.L.; writing—review and editing, W.L., K.A.W. and H.T.; funding acquisition, H.T., K.A.W. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by NIH R43EY031238 (H.T., K.A.W. and W.L.), R01EY027749 (W.L.), R24EY028764 (W.L. and K.A.W.), R24EY028764-01A1S1 (W.L. and K.A.W.), R43EY032827 (H.T. and W.L.), R41EY027665 (W.L. and H.T.), NIH P30EY002520, Knights Templar Eye Foundation Endowment in Ophthalmology (W.L.) and unrestricted institutional grants from Research to Prevent Blindness (RPB) to Department of Ophthalmology, Baylor College of Medicine.
Institutional Review Board Statement
All animal procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine (Protocol #AN-8362).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this study are reported herein.
Acknowledgments
The authors thank Yingbin Fu for scientific discussion.
Conflicts of Interest
H.T. and W.L. are shareholders of Everglades Biopharma, LLC, and LigandomicsRx, LLC. W.L. is an inventor of issued and pending patents. The remaining authors declare no competing financial interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- ElSheikh, R.H.; Chauhan, M.Z.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Neovascular Age-Related Macular Degeneration. Biomolecules 2022, 12, 1629. [Google Scholar] [CrossRef] [PubMed]
- Dedania, V.S.; Bakri, S.J. Current perspectives on ranibizumab. Clin. Ophthalmol. 2015, 9, 533–542. [Google Scholar] [PubMed]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Rhoades, W.; Nguyen, Q.D. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina 2020, 40, 643–647. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Lasave, A.F.; Wu, L.; Acón, D.; Berrocal, M.H.; Diaz-Llopis, M.; Gallego-Pinazo, R.; Serrano, M.; Alezzandrini, A.A.; Rojas, S.; et al. Intravitreal Bevacizumab for Choroidal Neovascularization in Age-Related Macular Degeneration: 5-Year Results of The Pan-American Collaborative Retina Study Group. Retina 2016, 36, 859–867. [Google Scholar] [CrossRef]
- Holz, F.G.; Tadayoni, R.; Beatty, S.; Berger, A.; Cereda, M.G.; Cortez, R.; Hoyng, C.B.; Hykin, P.; Staurenghi, G.; Heldner, S.; et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br. J. Ophthalmol. 2015, 99, 220–226. [Google Scholar] [CrossRef]
- Monés, J.; Singh, R.P.; Bandello, F.; Souied, E.; Liu, X.; Gale, R. Undertreatment of Neovascular Age-Related Macular Degeneration after 10 Years of Anti-Vascular Endothelial Growth Factor Therapy in the Real World: The Need for A Change of Mindset. Ophthalmologica 2020, 243, 1–8. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Mimoun, G.; Oubraham, H.; Zourdani, A.; Malbrel, C.; Quere, S.; Schneider, V.; LUMIERE Study Group. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: The LUMIERE study. Retina 2013, 33, 474–481. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Dunn, E.N.; Hariprasad, S.M.; Sheth, V.S. An Overview of the Fovista and Rinucumab Trials and the Fate of Anti-PDGF Medications. Ophthalmic Surg Lasers Imaging Retin. 2017, 48, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, H.J.; Kazlauskas, A.; Cavenee, W.K. Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res. 1999, 59, 1464–1472. [Google Scholar] [PubMed]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, H.; Hui, R. Single chain Fv antibody against angiopoietin-2 inhibits VEGF-induced endothelial cell proliferation and migration in vitro. Biochem. Biophys. Res. Commun. 2003, 309, 946–951. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef]
- Khachigian, L.M.; Liew, G.; Teo, K.Y.C.; Wong, T.Y.; Mitchell, P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J. Transl. Med. 2023, 21, 133. [Google Scholar] [CrossRef]
- Khanani, A.M.; Thomas, M.J.; Aziz, A.A.; Weng, C.Y.; Danzig, C.J.; Yiu, G.; Kiss, S.; Waheed, N.K.; Kaiser, P.K. Review of gene therapies for age-related macular degeneration. Eye 2022, 36, 303–311. [Google Scholar] [CrossRef]
- LeBlanc, M.E.; Wang, W.; Chen, X.; Caberoy, N.B.; Guo, F.; Shen, C.; Ji, Y.; Tian, H.; Wang, H.; Chen, R.; et al. Secretogranin III as a disease-associated ligand for antiangiogenic therapy of diabetic retinopathy. J. Exp. Med. 2017, 214, 1029–1047. [Google Scholar] [CrossRef]
- LeBlanc, M.E.; Wang, W.; Ji, Y.; Tian, H.; Liu, D.; Zhang, X.; Li, W. Secretogranin III as a novel target for the therapy of choroidal neovascularization. Exp. Eye Res. 2019, 181, 120–126. [Google Scholar] [CrossRef]
- Ji, L.; Waduge, P.; Wan, W.; Tian, H.; Li, J.; Zhang, J.; Li, W. Comparative ligandomics implicates secretogranin III as a disease-restricted angiogenic factor in laser-induced choroidal neovascularization. FEBS J. 2022, 289, 3521–3534. [Google Scholar] [CrossRef]
- Ji, L.; Waduge, P.; Hao, L.; Kaur, A.; Wan, W.; Wu, Y.; Tian, H.; Zhang, J.; Webster, K.A.; Li, W. Selectively targeting disease-restricted secretogranin III to alleviate choroidal neovascularization. FASEB J. 2022, 36, e22106. [Google Scholar] [CrossRef]
- Chng, J.; Wang, T.; Nian, R.; Lau, A.; Hoi, K.M.; Ho, S.C.; Gagnon, P.; Bi, X.; Yang, Y. Cleavage efficient 2A peptides for high level monoclonal antibody expression in CHO cells. MAbs 2015, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fortmann, S.D.; Shen, J.; Wielechowski, E.; Tretiakova, A.; Yoo, S.; Kozarsky, K.; Wang, J.; Wilson, J.M.; Campochiaro, P.A. AAV8-antiVEGFfab Ocular Gene Transfer for Neovascular Age-Related Macular Degeneration. Mol. Ther. 2018, 26, 542–549. [Google Scholar] [CrossRef]
- Sanmiguel, J.; Gao, G.; Vandenberghe, L.H. Quantitative and Digital Droplet-Based AAV Genome Titration. Methods Mol. Biol. 2019, 1950, 51–83. [Google Scholar] [PubMed]
- Caberoy, N.B.; Zhou, Y.; Jiang, X.; Alvarado, G.; Li, W. Efficient identification of tubby-binding proteins by an improved system of T7 phage display. J. Mol. Recognit. 2010, 23, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, A.N.; Couchman, J.R.; Whiteford, J.R. The CMV early enhancer/chicken beta actin (CAG) promoter can be used to drive transgene expression during the differentiation of murine embryonic stem cells into vascular progenitors. BMC Cell Biol. 2008, 9, 1–11. [Google Scholar] [CrossRef]
- FDA Drug Information for Eylea. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf (accessed on 3 July 2023).
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef]
- Glogowski, S.; Ward, K.W.; Lawrence, M.S.; Goody, R.J.; Proksch, J.W. The use of the African green monkey as a preclinical model for ocular pharmacokinetic studies. J. Ocul. Pharmacol. Ther. 2012, 28, 290–298. [Google Scholar] [CrossRef]
- Gelfman, C.M.; Grishanin, R.; Bender, K.O.; Nguyen, A.; Greengard, J.; Sharma, P.; Nieves, J.; Kiss, S.; Gasmi, M. Comprehensive Preclinical Assessment of ADVM-022, an Intravitreal Anti-VEGF Gene Therapy for the Treatment of Neovascular AMD and Diabetic Macular Edema. J. Ocul. Pharmacol. Ther. 2021, 37, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.J.; Chiang, C.W.; Chen, J.; Song, S.K. Vitreous Volume of the Mouse Measured by Quantitative High-Resolution MRI. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4414. [Google Scholar]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef]
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-related Macular Degeneration. EBioMedicine 2016, 14, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Magno, A.L.; Lai, C.M.; Pierce, C.M.; Degli-Esposti, M.A.; Blumenkranz, M.S.; Constable, I.J. Three-Year Follow-Up of Phase 1 and 2a rAAV.sFLT-1 Subretinal Gene Therapy Trials for Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 204, 113–123. [Google Scholar] [CrossRef]
- Kiss, S.; Bender, K.O.; Grishanin, R.N.; Hanna, K.M.; Nieves, J.D.; Sharma, P.; Nguyen, A.T.; Rosario, R.J.; Greengard, J.S.; Gelfman, C.M.; et al. Long-Term Safety Evaluation of Continuous Intraocular Delivery of Aflibercept by the Intravitreal Gene Therapy Candidate ADVM-022 in Nonhuman Primates. Transl. Vis. Sci. Technol. 2021, 10, 34. [Google Scholar] [CrossRef]
- Sarwar, S.; Clearfield, E.; Soliman, M.K.; Sadiq, M.A.; Baldwin, A.J.; Hanout, M.; Agarwal, A.; Sepah, Y.J.; Do, D.V.; Nguyen, Q.D. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016, 2, CD011346. [Google Scholar] [CrossRef]
- Parravano, M.; Costanzo, E.; Scondotto, G.; Trifirò, G.; Virgili, G. Anti-VEGF and Other Novel Therapies for Neovascular Age-Related Macular Degeneration: An Update. BioDrugs 2021, 35, 673–692. [Google Scholar] [CrossRef]
- Lin, F.L.; Wang, P.Y.; Chuang, Y.F.; Wang, J.H.; Wong, V.H.Y.; Bui, B.V.; Liu, G.S. Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update. Mol. Ther. 2020, 28, 2120–2138. [Google Scholar] [CrossRef] [PubMed]
- BBusbee, B.; Boyer, D.S.; Khanani, K.M.; Wykoff, C.C.; Pieramici, D.J.; Regillo, C.; Danzig, C.J.; Joondeph, B.C.; Major, J.; Hoang, C.; et al. Phase 1 Study of Intravitreal Gene Therapy with ADVM-022 for neovascular AMD (OPTIC Trial). Investig. Ophthalmol. Vis. Sci. 2021, 62, 352. [Google Scholar]
- REGENXBIO. REGENXBIO Announces Additional Positive Interim Phase I/IIa and Long-Term Follow-Up Data of RGX-314 for the Treatment of Wet AMD [Internet]. Available online: https://www.prnewswire.com/news-releases/regenxbio-announces-additional-positive-interim-phase-iiia-and-long-term-follow-up-data-of-rgx-314-for-the-treatment-of-wet-amd-301228344.html (accessed on 3 July 2023).
- Zhu, L.; Parker, M.; Enemchukwu, N.; Shen, M.; Zhang, G.; Yan, Q.; Handa, J.T.; Fang, L.; Fu, Y. Combination of apolipoprotein-A-I/apolipoprotein-A-I binding protein and anti-VEGF treatment overcomes anti-VEGF resistance in choroidal neovascularization in mice. Commun. Biol. 2020, 3, 386. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Henry, J.; Zhe, J.; Kieu, Q.; Qian, W.; Fu, Y.; Wang, X.; Paulus, Y.M. Age differential response to bevacizumab therapy in choroidal neovascularization in rabbits. Exp. Eye Res. 2022, 223, 109215. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Ohnuki, H.; Maric, D.; Salvucci, O.; Hou, X.; Kumar, A.; Li, X.; Tosato, G. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood 2012, 120, 4104–4115. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; LeBlanc, M.E.; Wang, W.; Liang, D.; Chen, P.; Chou, T.-H.; Tian, H.; Li, W. Anti-secretogranin III therapy of oxygen-induced retinopathy with optimal safety. Angiogenesis 2019, 22, 369–382. [Google Scholar] [CrossRef]
- Dai, C.; Waduge, P.; Ji, L.; Huang, C.; He, Y.; Tian, H.; Zuniga-Sanchez, E.; Bhatt, A.; Pang, I.; Su, G.; et al. Secretogranin III stringently regulates pathological but not physiological angiogenesis in oxygen-induced retinopathy. Cell Mol. Life Sci. 2022, 79, 63. [Google Scholar] [CrossRef]
- He, Y.; Tian, H.; Dai, C.; Wen, R.; Li, X.; Webster, K.A.; Li, W. Optimal Efficacy and Safety of Humanized Anti-Scg3 Antibody to Alleviate Oxygen-Induced Retinopathy. Int. J. Mol. Sci. 2021, 23, 350. [Google Scholar] [CrossRef]
- Sondell, M.; Sundler, F.; Kanje, M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000, 12, 4243–4254. [Google Scholar] [CrossRef]
- Nishijima, K.; Ng, Y.-S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P.; et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67. [Google Scholar] [CrossRef]
- Romano, M.R.; Biagioni, F.; Besozzi, G.; Carrizzo, A.; Vecchione, C.; Fornai, F.; Lograno, M.D. Effects of bevacizumab on neuronal viability of retinal ganglion cells in rats. Brain Res. 2012, 1478, 55–63. [Google Scholar] [CrossRef]
- Tokunaga, C.C.; Mitton, K.; Dailey, W.; Massoll, C.; Roumayah, K.; Guzman, E.; Tarabishy, N.; Cheng, M.; Drenser, K.A. Effects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1884–1892. [Google Scholar] [CrossRef]
- Miyata, R.; Kondo, M.; Kato, K.; Sugimoto, M.; Matsubara, H.; Ikesugi, K.; Ueno, S.; Yasuda, S.; Terasaki, H. Supernormal Flicker ERGs in Eyes With Central Retinal Vein Occlusion: Clinical Characteristics, Prognosis, and Effects of Anti-VEGF Agent. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5854–5861. [Google Scholar] [CrossRef]
- Lepore, D.; Quinn, G.E.; Molle, F.; Baldascino, A.; Orazi, L.; Sammartino, M.; Purcaro, V.; Giannantonio, C.; Papacci, P.; Romagnoli, C. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: Report on fluorescein angiographic findings. Ophthalmology 2014, 121, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Lepore, D.; Quinn, G.E.; Molle, F.; Orazi, L.; Baldascino, A.; Ji, M.H.; Sammartino, M.; Sbaraglia, F.; Ricci, D.; Mercuri, E. Follow-up to Age 4 Years of Treatment of Type 1 Retinopathy of Prematurity Intravitreal Bevacizumab Injection versus Laser: Fluorescein Angiographic Findings. Ophthalmology 2018, 125, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.N.; Strampe, M.; Fagbemi, O.E.; Visotcky, A.; Tarima, S.; Carroll, J.; Costakos, D.M. Foveal Development in Infants Treated with Bevacizumab or Laser Photocoagulation for Retinopathy of Prematurity. Ophthalmology 2018, 125, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Scondotto, G.; Cutroneo, P.M.; Morgante, F.; Trifirò, G. Intravitreal Anti-VEGF Drugs and Signals of Dementia and Parkinson-Like Events: Analysis of the VigiBase Database of Spontaneous Reports. Front. Pharmacol. 2020, 11, 315. [Google Scholar] [CrossRef]
- Arima, M.; Akiyama, M.; Fujiwara, K.; Mori, Y.; Inoue, H.; Seki, E.; Nakama, T.; Tsukamoto, S.; Ochiai, M.; Ohga, S.; et al. Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS ONE 2020, 15, e0230678. [Google Scholar] [CrossRef]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.-N.; Shah, V.; Shah, P.S.; Kelly, E.N. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Pistilli, M.; Daniel, E.; Ying, G.-S.; Pan, W.; Jaffe, G.J.; Toth, C.A.; Hagstrom, S.A.; Maguire, M.G.; Martin, D.F. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2017, 124, 97–104. [Google Scholar] [CrossRef]
- Sepah, Y.J.; Sadiq, M.A.; Boyer, D.; Callanan, D.; Gallemore, R.; Bennett, M.; Marcus, D.; Halperin, L.; Hassan, M.; Campochiaro, P.A.; et al. Twenty-four-Month Outcomes of the Ranibizumab for Edema of the Macula in Diabetes–Protocol 3 with High Dose (READ-3) Study. Ophthalmology 2016, 123, 2581–2587. [Google Scholar] [CrossRef]
- Ho, A.C.; Busbee, B.G.; Regillo, C.D.; Wieland, M.R.; Van Everen, S.A.; Li, Z.; Rubio, R.G.; Lai, P. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014, 121, 2181–2192. [Google Scholar] [CrossRef]
- Reid, C.A.; Nettesheim, E.R.; Connor, T.B.; Lipinski, D.M. Development of an inducible anti-VEGF rAAV gene therapy strategy for the treatment of wet AMD. Sci. Rep. 2018, 8, 11763. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, C.J.; Smith, G.W.; Dorey, C.K.; Prentice, H.M.; Webster, K.A.; Blanks, J.C. Robust hypoxia-selective regulation of a retinal pigment epithelium-specific adeno-associated virus vector. Mol. Vis. 2008, 14, 471–480. [Google Scholar] [PubMed]
- Chen, J.; Lin, F.-L.; Leung, J.Y.K.; Tu, L.; Wang, J.-H.; Chuang, Y.-F.; Li, F.; Shen, H.-H.; Dusting, G.J.; Wong, V.H.Y.; et al. A drug-tunable Flt23k gene therapy for controlled intervention in retinal neovascularization. Angiogenesis 2021, 24, 97–110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).