Multiomics Strategy Reveals the Mechanism of Action and Ameliorating Effect of Deer Velvet Antler Water Extracts on DSS-Induced Colitis
Abstract
:1. Introduction
2. Results
2.1. VAWEs Ameliorated the Symptoms in DSS-Induced Colitis
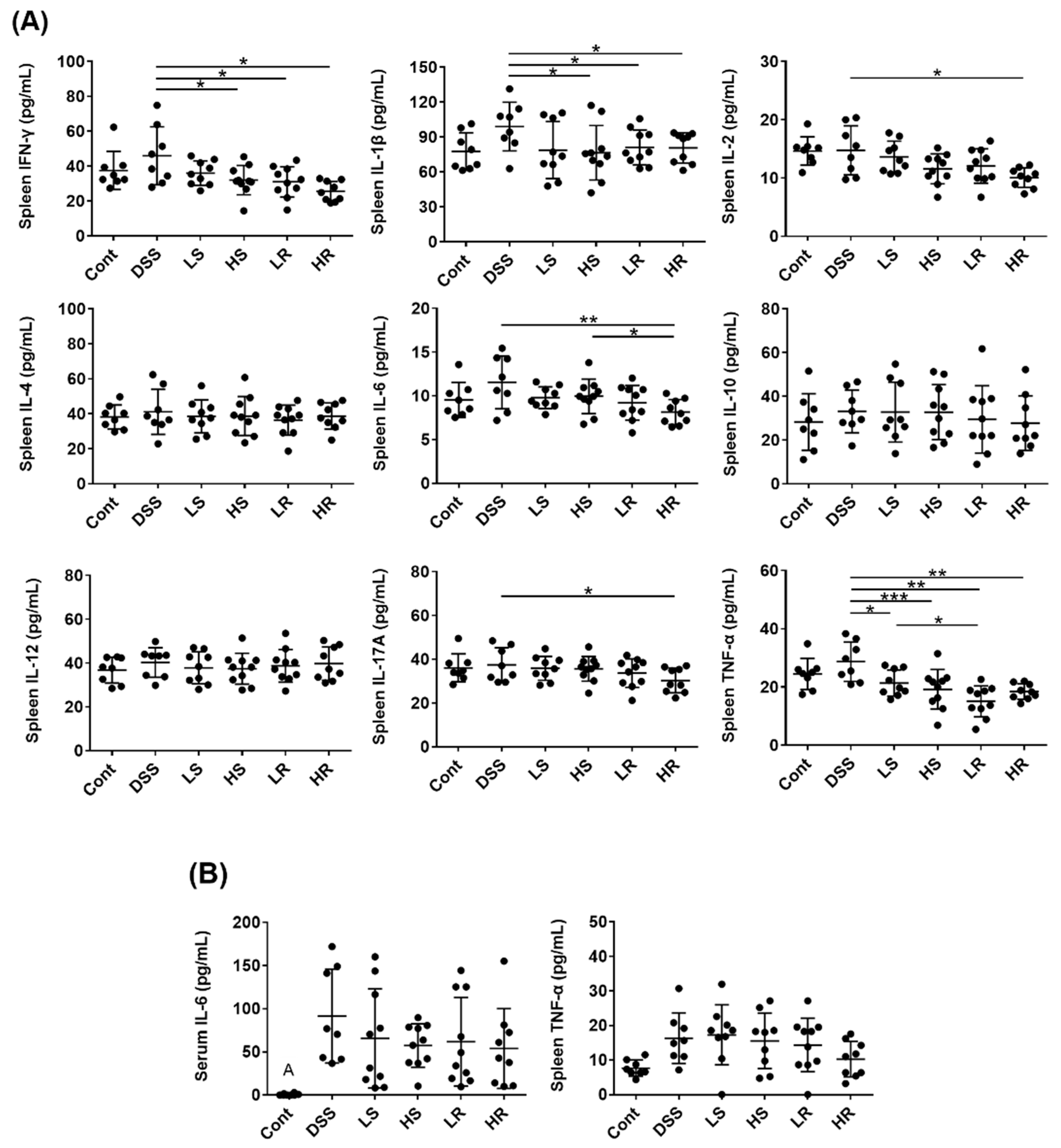
2.2. VAWEs Reduced the Systemic Inflammatory Response in DSS-Induced Injury
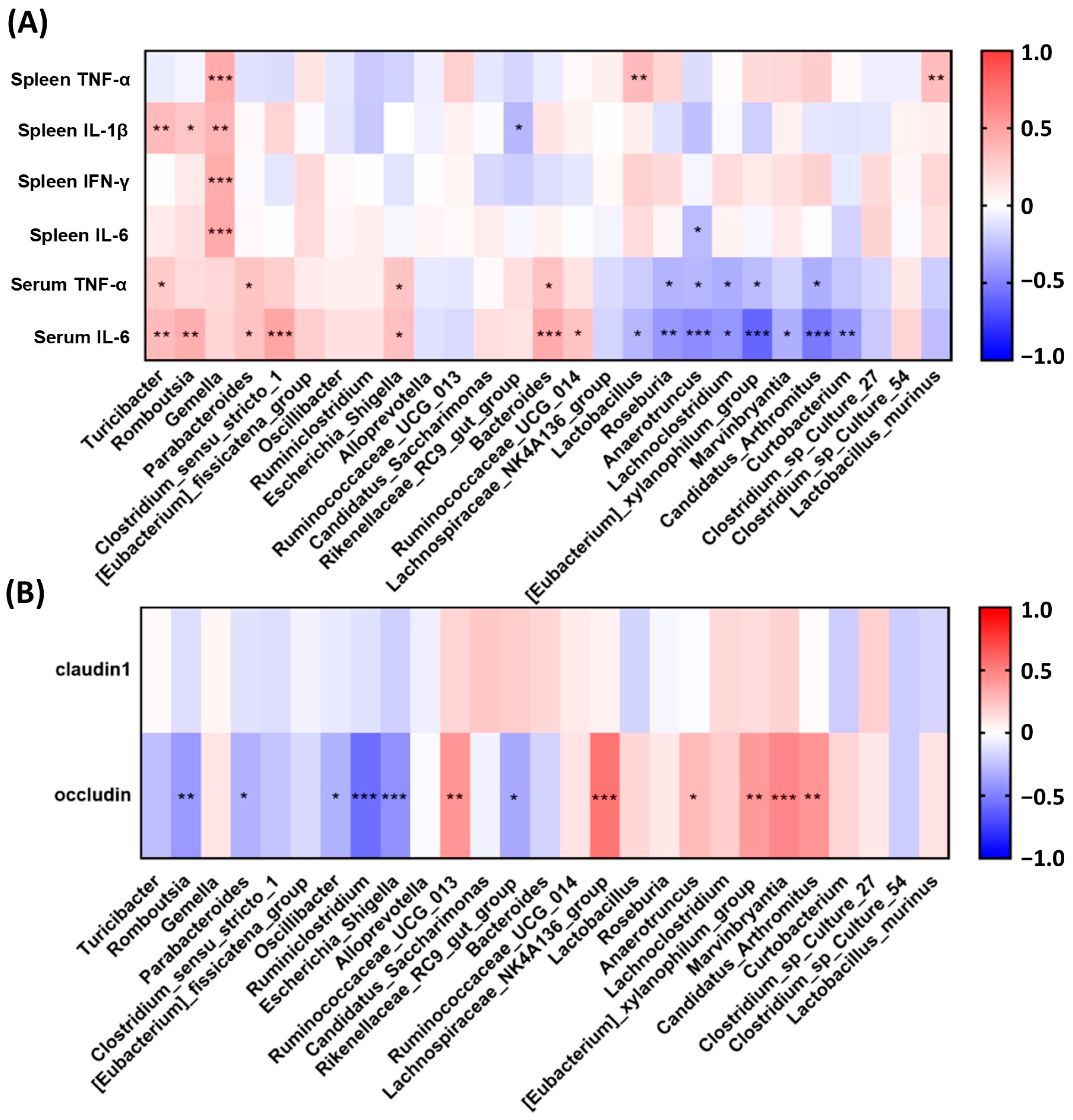
2.3. VAWEs Restored the Tight Junction Associated Proteins against DSS Challenge
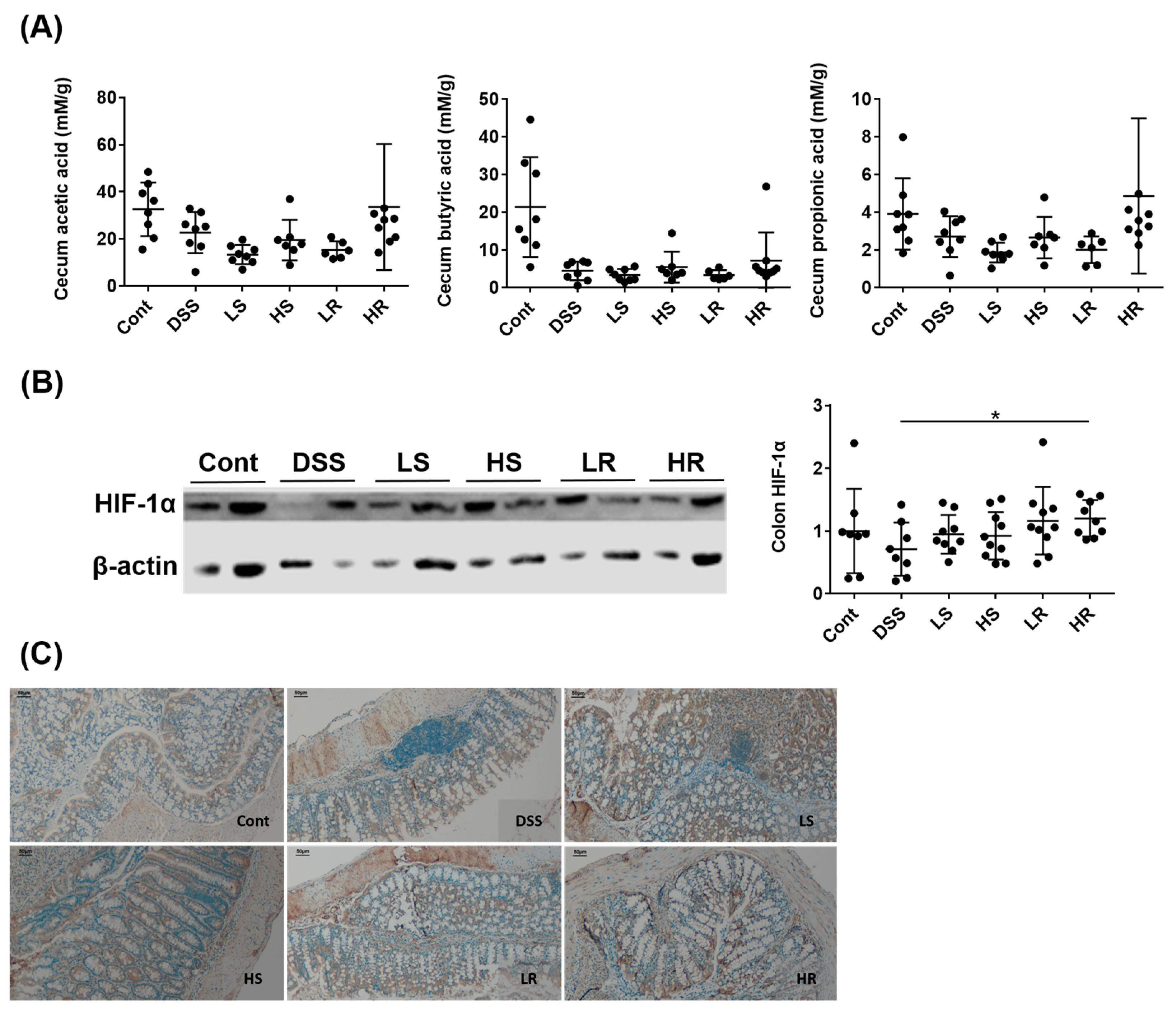
2.4. RVAE Maintained the Secretion of Short Chain Fatty Acids via the HIF-1α Pathway
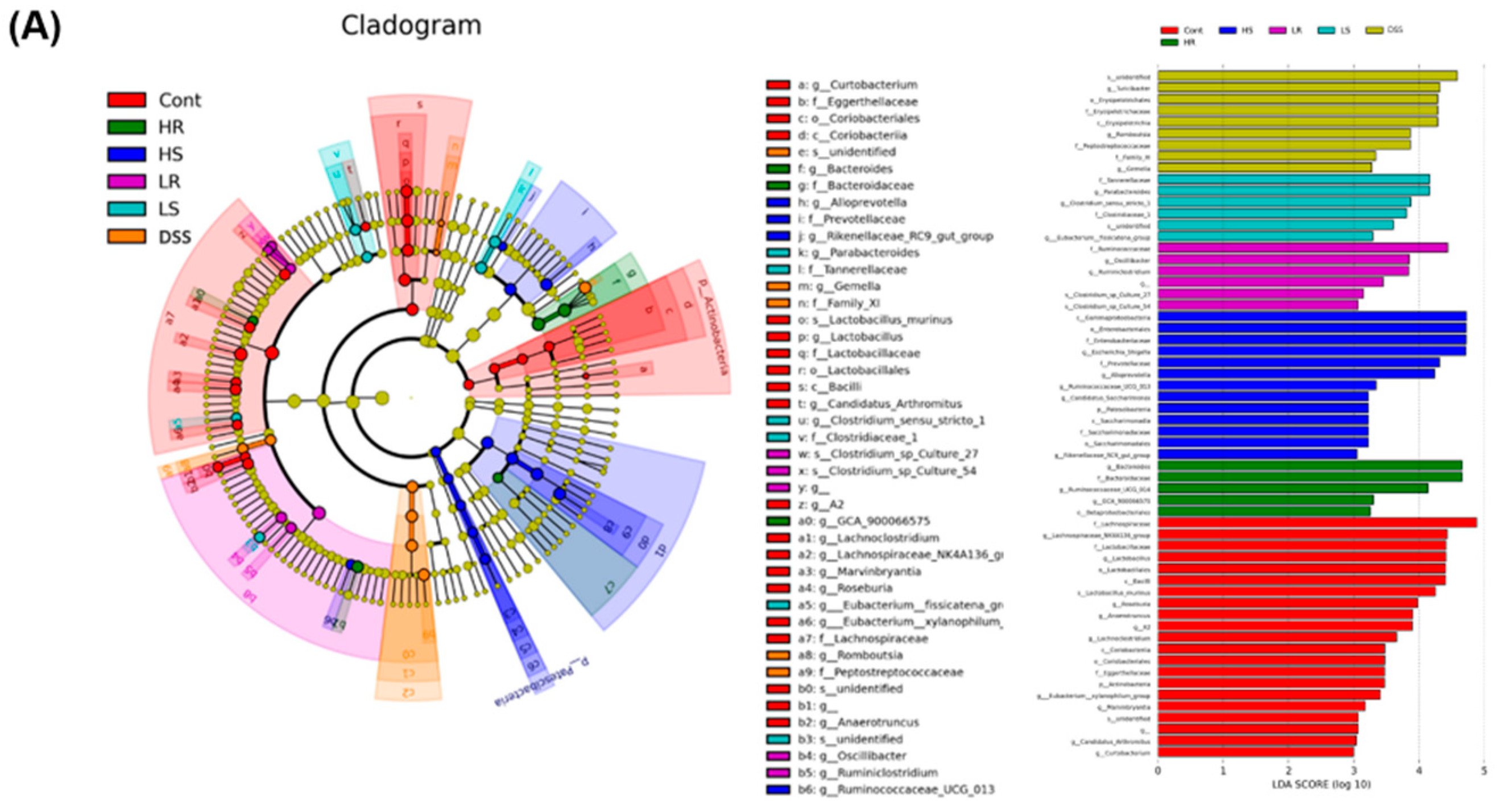
2.5. RVAE Partially Restored the Homeostasis of the Gut Microbiota in DSS-Induced Colitis via Alteration of Enriched Taxa
2.6. The Molecular Weight of Active Components in SVAE and RVAE Was Less than 3 kDa
2.7. Potential Bioactive Components of SVAE and RVAE Were Identified by Untargeted UHPLC-MS/MS and GC-MS/MS
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Velvet Antler Water Extracts
4.3. DSS-Induced Colitis Animal Model
4.4. Magnetic Resonance Imaging (MRI) Colon Monitoring
4.5. Fecal Bleeding Test
4.6. H&E Tissue Staining and Immunohistological Staining
4.7. Cytokine Detection in Serum and Spleen Tissue
4.8. Western Blot Analysis
4.9. Short-Chain Fatty Acid Analysis
4.10. DNA Extraction and Next-Generation Sequencing of Gut Microbiota
4.11. Caco-2 Cell Culture
4.12. Preparation of Different Molecular Fragments of VAWEs
4.13. Measurement of CCL-20 Production
4.14. Untargeted Metabolomics Analysis of Ultra-High Performance Liquid Chromatography-Mass/Mass (UHPLC-MS/MS) and Gas Chromatography-Mass/Mass (GC-MS/MS)
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Munkholm, P. The epidemiology of inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 942–951. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.J.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Seow, C.H.; Steinhart, A.H.; Griffiths, A.M. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane. Database Syst. Rev. 2008, 2, CD006792. [Google Scholar] [CrossRef]
- Dorrington, A.M.; Selinger, C.P.; Parkes, G.C.; Smith, M.; Pollok, R.C.; Raine, T. The historical role and contemporary use of corticosteroids in inflammatory bowel disease. J. Crohns Colitis 2020, 14, 1316–1329. [Google Scholar] [CrossRef]
- Theochari, N.A.; Stefanopoulos, A.; Mylonas, K.S.; Economopoulos, K.P. Antibiotics exposure and risk of inflammatory bowel disease: A systematic review. Scand. J. Gastroenterol. 2018, 53, 1–7. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Peretz, A.; Saliba, W. Role of antibiotics for treatment of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, Z.; Shen, M.J.; Twardus, S.W.; Beuttler, M.M.; Chen, L.A.; Ailson, B.-H. Fecal microbiota transplantation: Uses, questions, and ethics. Med. Microecol. 2020, 6, 100027. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, H.; Jin, L.; Li, X.; Ma, Y.; You, J.; Li, S.; Xu, Y. Deer antler base as a traditional Chinese medicine: A review of its traditional uses, chemistry and pharmacology. J. Ethnopharmacol. 2013, 145, 403–415. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, L.; Huo, Y.; Zhang, Y. Bioactive components of velvet antlers and their pharmacological properies. J. Pharm. Biomed. Anal. 2014, 87, 229–240. [Google Scholar] [CrossRef]
- Suh, S.J.; Kim, K.S.; Lee, A.R.; Ha, K.T.; Kim, J.K.; Kim, D.S.; Lee, Y.C.; Kim, M.S.; Kwon, D.Y.; Kim, C.H. Prevention of collagen-induced arthritis in mice by Cervus korean TEMMINCK var. mantchuricus Swinhoe. Environ. Toxicol. Pharmacol. 2007, 23, 147–153. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Wang, T.; Dai, T.Y.; Wang, C.H.; Chen, K.N.; Chen, Y.P.; Chen, M.J. Effect of thevelvet antler of Formosan sambar deer (Cervus unicolor swinhoei) on the prevention of an allergic airway response in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 481318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhuang, Z.; Sun, Y.; Ma, S.; Yang, W.; Lei, H.; Zuo, J.; Ouyang, J.; Wang, Y. Velvet antler polypeptide is able to induce differentiation of neural stem cells towards neurons in vitro. J. Tradit. Chin. Med. 2017, 37, 308–310. [Google Scholar]
- Xiao, X.; Xu, S.; Li, L.; Mao, M.; Wang, J.; Li, Y.; Wang, Z.; Ye, F.; Huang, L. The effect of velvet antler proteins on cardiac microvascular endothelial cells challenged with ischemia-hypoxia. Front Pharmacol. 2017, 8, 601. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.Y.; Wang, C.H.; Chen, K.N.; Huang, I.N.; Hong, W.S.; Wang, S.Y.; Chen, Y.P.; Kuo, C.Y.; Chen, M.J. The anti-infective effects of velvet antler of Formosan sambar deer (Cervus unicolor swinhoei) on Staphylococcus aureus-infected mice. Evid. Based Complement. Alternat. 2011, 2011, 534069. [Google Scholar]
- Hung, Y.K.; Ho, S.T.; Kuo, C.Y.; Chen, M.J. In vitro effects of velvet antler water extracts from Formosan Sambar deer and red deer on barrier integrity in Caco-2 cell. Int. J. Med. Sc. 2021, 18, 1782–1783. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Cheng, Y.T.; Ho, S.T.; Yu, C.C.; Chen, M.J. Comparison of anti-inflammatory effect and protein profile between the water extracts from Formosan sambar deer and red deer. J. Food Drug Anal. 2018, 26, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Mudter, J.; Finotto, S.; Müllberg, J.; Jostock, T.; Wirtz, S.; Schütz, M.; Bartsch, B.; Holtmann, M.; Becker, C.; et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000, 6, 583. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrin-Biroulet, L. Anti-TNF therapy in inflammatory bowel diseases: A huge review. Minerva. Gastroenterol. Dietol. 2010, 56, 233–243. [Google Scholar]
- Yamamoto, M.; Yoshizaki, K.; Kishimoto, T.; Ito, H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Imminol. 2000, 164, 4878–4882. [Google Scholar] [CrossRef] [Green Version]
- Parkes, M.; Satsangi, J.; Jewell, D. Contribution of IL-2 and IL-10 genes to inflammatory bowel disease (IBD) susceptibility. Clin. Exp. Immunol. 1998, 113, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; He, S.H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 620–625. [Google Scholar] [CrossRef]
- Kotlarz, D.; Beier, R.; Murugan, D.; Diestelhorst, J.; Jensen, O.; Boztug, K.; Pfeifer, D.; Kreipe, H.; Pfister, E.; Baumann, U.; et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: Implications for diagnosis and therapy. Gastroenterology 2012, 143, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Jayme, T.S.; Leung, G.; Wang, A.; Workentine, M.L.; Rajeev, S.; Shute, A.; Callejas, B.E.; Mancini, N.; Beck, P.L.; Panaccione, R.; et al. Human interleukin-4-treated regulatory macrophages promote epithelial wound healing and reduce colitis in a mouse model. Sci. Adv. 2020, 6, eaba4376. [Google Scholar] [CrossRef]
- Xiong, J.; Lin, Y.H.; Bi, L.H.; Wang, J.D.; Bai, Y.; Liu, S.D. Effects of interleukin-4 or interleukin-10 gene therapy on trinitrobenzenesulfonic acid-induced murine colitis. BMC Gastroenterol. 2013, 13, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel. Dis. 2010, 17, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Nalle, S.C.; Shen, L.; Turner, E.S.; Singh, G.; Breskin, L.A.; Khramtsova, E.A.; Khramtsova, G.; Tsai, P.Y.; Fu, Y.X.; et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013, 145, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Scharl, M.; Paul, G.; Barrett, K.E.; McCole, D.F. AMP-activated protein kinase mediates the interferon-γ-induced decrease in intestinal epithelial barrier function. Agric. Biol. Chem. 2009, 284, 27952–27963. [Google Scholar] [CrossRef] [Green Version]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Li, Z.H.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Ma, C.; Huang, X.; Yang, W.; Chen, L.; Bilotta, A.J.; Yao, S.; Cong, Y. Microbiota metabolites short-chain fatty acid butyrate conditions intestinal epithelial cells to promote development of Treg cells and T cell IL-10 production. J. Imminol. 2018, 200, 53-16. [Google Scholar] [CrossRef]
- Cresci, G.; Nagy, L.E.; Ganapathy, V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN J. Parenter Enteral. Nutr. 2013, 37, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Manguson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Dong, W. Role of hypoxia-inducible factor-1α in pathogenesis and disease evaluation of ulcerative colitis. Exp. Ther. Med. 2016, 11, 1330–1334. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, E.P.; Crean, D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017, 19, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Domselaar, G.V. A comparative study of the gut microbiota in immune-mediated inflammatory diseases—Does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Chen, N.; Wu, Z.; Song, Y.; Zhang, Y.; Wu, N.; Zhang, F.; Ren, X.; Liu, Y. 5-aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front. Microbiol. 2018, 9, 1274. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Wen, X.; Wang, H.G.; Zhang, M.N.; Zhang, M.H.; Wang, H.; Yang, X.Z. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J. Gastroenterol. 2021, 27, 2834–2849. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, G.; Li, B.; Du, X.; Sun, Z.; Sun, Y.; Jiang, X.F. Fecal microbiota transplantation (FMT) alleviates experimental colitis in mice by gut microbiota regulation. J. Microbio. Biotechnol. 2020, 30, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, M.; Wang, S.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus ruminis alleviates DSS-induced colitis by inflammatory cytokines and gut microbiota modulation. Foods 2021, 10, 1349. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Xia, Y.; Wang, F.; Sun, Y.; Yang, Y.; Ai, L. Probiotic yeast BR14 ameliorates DSS-induced colitis by restoring the gut barrier and adjusting the intestinal microbiota. Food. Funct. 2021, 12, 8386–8398. [Google Scholar] [CrossRef] [PubMed]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014, 64, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funke, G.; Aravena-Roman, M.; Frodl, R. First description of Curtobacterium spp. isolated from human clinical specimens. J. Clin. Microbiol. 2005, 43, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef] [Green Version]
- Vongsa, R.A.; Zimmerman, N.P.; Dwinell, M.B. CCR6 regulation of the actin cytoskeleton orchestrates human beta defendin-2 and CCL20-mediated restitution of colonic epithelial cells. J. Biol. Chem. 2009, 284, 10034–10045. [Google Scholar] [CrossRef] [Green Version]
- Zha, E.; Li, X.; Li, D.; Guo, X.; Gao, S.; Yue, X. Immunomodulatory effects of a 3.2kDa polypeptide from velvet antler of Cervus nippon Temminck. Int. Immunopharmacol. 2013, 16, 210–16213. [Google Scholar] [CrossRef]
- Zha, E.H.; Gao, S.Y.; Pi, Y.Z.; Li, X.X.; Wang, Y.T.; Yue, X.Q. Wound healing by a 3.2 kDa recombinant polypeptide from velvet antler of Cervus nippon Temminck. Biotechnol. Lett. 2012, 34, 789–793. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Zhu, Z.H.; Zhu, Y.; Qian, D.W.; Liu, R.; Peng, Y.R.; Ding, Y.; Ouyang, Z.; Duan, J.A. Characterization of collagen peptides in Elaphuri Davidiani Cornu aqueous extract with proliferative activity on osteoblasts using nano-liquid chromatography in tandem with orbitrap mass spectrometry. Molecules 2017, 22, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, G.; Yurchenko, K.; Collette, C.; Rubio, M.; Villani, A.C.; Bitton, A.; Sarfati, M.; Franchimont, D. l-carnitine, a diet component and organic cation transporter OCTN ligand, displays immunosuppressive properties and abrogates intestinal inflammation. Clin. Exp. Immunol. 2009, 156, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Wang, R.X.; Alexeev, E.E.; Lanis, J.M.; Battista, K.D.; Glover, L.E.; Colgan, S.P. Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function. J. Biol. Chem. 2018, 293, 6039–6051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwers, H.; Jonasdottir, H.S.; Kuipers, M.E.; Kwekkeboom, J.C.; Auger, J.L.; Gonzalez-Torres, M.; López-Vicario, C.; Clària, J.; Freysdottir, J.; Hardardottir, I.; et al. Anti-inflammatory and proresolving effects of the Omega-6 polyunsaturated fatty acid adrenic acid. J. Immunol. 2020, 205, 2840–2849. [Google Scholar] [CrossRef]
- Nishitani, Y.; Okazaki, S.; Imabayashi, K.; Katada, R.; Umetani, K.; Yajima, H.; Matsumoto, H. Saturated and mono-unsaturated fatty acids increase interleukin-10 production in rat hepatocytes. Nihon Arukoru Yakubutsu Igakkai Zasshi 2007, 42, 32–35. [Google Scholar] [PubMed]
- Dinant, S.; Veteläinen, R.L.; Florquin, S.; van Vliet, A.K.; van Gulik, T.M. IL-10 attenuates hepatic I/R injury and promotes hepatocyte proliferation. J. Surg. Res. 2007, 141, 176–182. [Google Scholar] [CrossRef]
- Turer, E.; McAlpine, W.; Wang, K.W.; Lu, T.; Li, X.; Tang, M.; Zhan, X.; Wang, T.; Zhan, X.; Bu, C.H.; et al. Creatine maintains intestinal homeostasis and protects against colitis. Proc. Natl. Acad. Sci. USA 2017, 114, E1273–E1281. [Google Scholar] [CrossRef]
- Wallimann, T.; Hall, C.H.T.; Colgan, S.P.; Glover, L.E. Creatine supplementation for patients with inflammatory bowel diseases: A scientific rationale for a clinical trial. Nutrients 2021, 13, 1429. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.H.; Nie, S.P.; Yin, J.Y.; Xie, M.Y. Gamma-aminobutyric acid increases the production of short-chain fatty acids and decreases pH values in mouse colon. Molecules 2017, 22, 653. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, J.; de la Fuente, V.G.; García, M.T.F.; Śanchez, J.G.; Redondo, B.I.; Villar, C.J.; Lombó, F. A diet based on cured acorn-fed ham with oleic acid content promotes anti-inflammatory gut microbiota and prevents ulcerative colitis in an animal model. Lipids Health Dis. 2020, 19, 28. [Google Scholar] [CrossRef]
- Reddy, K.V.; Naidu, K.A. Oleic acid, hydroxytyrosol and n-3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies. Int. Immunopharmacol. 2016, 35, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Tsune, I.; Ikejima, K.; Hirose, M.; Yoshikawa, M.; Enomoto, N.; Takei, Y.; Sato, N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology 2003, 125, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, Y.; Ma, J.; Yin, J.; Chen, S. The effects of dietary glycine on the acetic acid-induced mouse model of colitis. Mediat. Inflamm. 2020, 2020, 5867627. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hwang, J.T.; Park, J.H.; Kim, M.S.; Wang, S.; Sung, M.J. Poly-γ-glutamic acid attenuates angiogenesis and inflammation in experimental colitis. Mediat. Inflamm. 2013, 2013, 982383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prossomariti, A.; Scaioli, E.; Piazzi, G.; Fazio, C.; Bellanova, M.; Biagi, E.; Candela, M.; Brigidi, P.; Consolandi, C.; Balbi, T.; et al. Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci. Rep. 2017, 7, 7458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaioli, E.; Sartini, A.; Bellanova, M.; Campieri, M.; Festi, D.; Bazzoli, F.; Belluzzi, A. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1268–1275.e2. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Beltzer, A.; Kaulisch, T.; Bluhmki, T.; Schoenberger, T.; Stierstorfer, B.; Stiller, D. Evaluation of quantitative imaging biomarker in the DSS colitis model. Mol. Imaging Biol. 2016, 18, 697–704. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH mage to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Torri, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef] [PubMed]




| Rank | SVAE | RVAE |
|---|---|---|
| 1 | Palmitic acid | Palmitic acid |
| 2 | l-Carnitine | l-Carnitine |
| 3 | Hypoxanthine | Hypoxanthine |
| 4 | Creatinine | Creatinine |
| 5 | TranexamicAcid; (S)-omostachydrine; Homostachydrine; Lentiginosine; Tranexamic acid | TES; N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid |
| 6 | Acetylcarnitine | 2-Methylbutyroylcarnitine |
| 7 | 2-Methylbutyroylcarnitine | Acetylcarnitine |
| 8 | TES; N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid | Gamma-Aminobutyryl-lysine |
| 9 | Gamma-Aminobutyryl-lysine; | Porson; Gingerenone B; Isogingerenone B; Burseran; (+)-Burseran; (+)-Eudesmin; Pinoresinol dimethyl ether |
| 10 | PC(16:0/18:1(9Z)); PC(14:0/20:1(11Z)); PC(20:0/14:1(9Z)); PE(22:1(13Z)/15:0); PC(18:1(9Z)/16:0); PC(20:1(11Z)/14:0); PC(18:0/16:1(9Z)); PC(18:1(11Z)/16:0); PC(14:1(9Z)/20:0); PC(16:0/18:1(11Z)); PC(16:1(9Z)/18:0); PE(15:0/22:1(13Z)); | (13R,14R)-7-Labdene-13,14,15-triol; (13R,14R)-8-Labdene-13,14,15-triol; (Z)-15-Oxo-11-eicosenoic acid |
| 11 | Glycerophosphocholine | Thioetheramide-PC |
| 12 | Porson; Gingerenone B; Isogingerenone B; Burseran; (+)-Burseran; (+)-Eudesmin; Pinoresinol dimethyl ether | PC(16:0/18:1(9Z)); PC(14:0/20:1(11Z)); PC(20:0/14:1(9Z)); PE(22:1(13Z)/15:0); PC(18:1(9Z)/16:0); PC(20:1(11Z)/14:0); PC(18:0/16:1(9Z)); PC(18:1(11Z)/16:0); PC(14:1(9Z)/20:0); PC(16:0/18:1(11Z)); PC(16:1(9Z)/18:0); PE(15:0/22:1(13Z)); |
| 13 | Erucamide | Glycerophosphocholine |
| 14 | Thioetheramide-PC | 3-(2-Hydroxyethyl)indole |
| 15 | N1-(3-Aminopropyl)agmatine; N1-Aminopropylagmatine | 4-O-Methylmelleolide; Clausarinol; Eplerenone; Armillarin; Armillaripin; Magnoshinin; Eplerenone; Estra-1,3,5(10)-triene-3,6alpha,17beta-triol triacetate; |
| 16 | 4-O-Methylmelleolide; Clausarinol; Eplerenone; Armillarin; Armillaripin; Magnoshinin; Eplerenone; Estra-1,3,5(10)-triene-3,6alpha,17beta-triol triacetate; Estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate | l-Leucine |
| 17 | O-Phosphotyrosine; Phosphotyrosine; Phosphonotyrosine | Isobutylpropylamine |
| 18 | Isobutylpropylamine | 1-Methylhistidine |
| 19 | (3-Carboxypropyl)trimethylammonium cation | Threoninyl-Lysine; Lysyl-Threonine |
| 20 | Threoninyl-Lysine; Lysyl-Threonine | Cytidine |
| Rank | SVAE | RVAE |
|---|---|---|
| 1 | Oleic acid | Oleic acid |
| 2 | Palmitic acid | cis-9-Palmitoleic acid |
| 3 | Arachidonic Acid (peroxide free) | Palmitic acid |
| 4 | cis-9-Palmitoleic acid | Arachidonic Acid (peroxide free) |
| 5 | Suberenone; Graveolone; Eriobofuran; (2E,11Z)-Wyerone acid; 9,10-Dihydro-2,3,5,7-Phenanthrenetetrol; 3,3′,4′5-Tetrahydroxystilbene; (R)-Apiumetin; Piceatannol; 3,3′,4′5-Tetrahydroxystilbene; Wyerone acid; Eriobofuran; 2,4-Dimethoxydibenzofuran-3-ol; Fulvoplumierin; Oxyresveratrol; Methylstyrylpyron; 2,2′-Dihydroxy-4-methoxybenzophenone; Dioxybenzone | Uracil |
| 6 | Isoplumbagin; 1-Hydroxy-2-phthoate; 1-Hydroxy-2-phthoic acid; 1-phthol-2-carboxylic acid; Plumbagin; Ramentaceone; 7-Methyljuglone; 3-Hydroxy-2-phthoate | Hypoxanthine |
| 7 | Hypoxanthine | Myristoleic acid |
| 8 | Pyridine N-oxide glucuronide; 16-Hydroxypalmitate; 16-Hydroxypalmitic acid | Isoplumbagin; 1-Hydroxy-2-phthoate; 1-Hydroxy-2-phthoic acid; 1-phthol-2-carboxylic acid; Plumbagin; Ramentaceone; 7-Methyljuglone; 3-Hydroxy-2-phthoate |
| 9 | Isoferulic acid 3-sulfate; Ferulic acid 4-sulfate | Suberenone; Graveolone; Eriobofuran; (2E,11Z)-Wyerone acid; 9,10-Dihydro-2,3,5,7-Phenanthrenetetrol; 3,3′,4′5-Tetrahydroxystilbene; (R)-Apiumetin;Piceatannol; 3,3′,4′5-Tetrahydroxystilbene; Wyerone acid;Eriobofuran; 2,4-Dimethoxydibenzofuran-3-ol; Fulvoplumierin; Oxyresveratrol; Methylstyrylpyron; 2,2′-Dihydroxy-4-methoxybenzophenone; Dioxybenzone |
| 10 | Dihomo-gamma-Linolenic Acid | Isoferulic acid 3-sulfate; Ferulic acid 4-sulfate |
| 11 | dl-lactate | Atenolol; Practolol; Tributyl phosphate; TBP |
| 12 | 7Z, 10Z, 13Z, 16Z, 19Z-Docosapentaenoic acid | Dihomo-gamma-Linolenic Acid |
| 13 | Pentadecanoic Acid | dl-lactate |
| 14 | Uracil | 9R,10S-EpOME |
| 15 | Pristimerin | 7Z, 10Z, 13Z, 16Z, 19Z-Docosapentaenoic acid |
| 16 | 3alpha-Hydroxy-3,5-dihydromocolin L acid | 3alpha-Hydroxy-3,5-dihydromocolin L acid |
| 17 | Atenolol; Practolol; Tributyl phosphate; TBP | 12(R)-HETE |
| 18 | Adrenic Acid | 2-Oxoadipic acid |
| 19 | 12(R)-HETE | Pentadecanoic Acid |
| 20 | Bromobenzene | Adrenic Acid |
| Rank | SVAE | RVAE |
|---|---|---|
| 1 | lactic acid | lactic acid |
| 2 | alanine | alanine |
| 3 | palmitic acid | urea |
| 4 | glycine | methylamine |
| 5 | methylamine | galactose |
| 6 | urea | glycine |
| 7 | proline | palmitic acid |
| 8 | stearic acid | proline |
| 9 | galactose | isoleucine |
| 10 | isoleucine | oxoproline |
| 11 | oxoproline | valine |
| 12 | valine | stearic acid |
| 13 | glutamic acid | glutamic acid |
| 14 | isoleucine | glycine 1 |
| 15 | glycine 1 | isoleucine |
| 16 | hypoxanthine | uracil |
| 17 | serine | 3-hydroxybutyric acid |
| 18 | glycolic acid | hypoxanthine |
| 19 | aminomalonate | glycolic acid |
| 20 | glyceric acid | glyceric acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-K.; Ho, S.-T.; Kuo, C.-Y.; Chen, M.-J. Multiomics Strategy Reveals the Mechanism of Action and Ameliorating Effect of Deer Velvet Antler Water Extracts on DSS-Induced Colitis. Biomedicines 2023, 11, 1913. https://doi.org/10.3390/biomedicines11071913
Hung Y-K, Ho S-T, Kuo C-Y, Chen M-J. Multiomics Strategy Reveals the Mechanism of Action and Ameliorating Effect of Deer Velvet Antler Water Extracts on DSS-Induced Colitis. Biomedicines. 2023; 11(7):1913. https://doi.org/10.3390/biomedicines11071913
Chicago/Turabian StyleHung, Ying-Kai, Shang-Tse Ho, Ching-Yun Kuo, and Ming-Ju Chen. 2023. "Multiomics Strategy Reveals the Mechanism of Action and Ameliorating Effect of Deer Velvet Antler Water Extracts on DSS-Induced Colitis" Biomedicines 11, no. 7: 1913. https://doi.org/10.3390/biomedicines11071913
APA StyleHung, Y.-K., Ho, S.-T., Kuo, C.-Y., & Chen, M.-J. (2023). Multiomics Strategy Reveals the Mechanism of Action and Ameliorating Effect of Deer Velvet Antler Water Extracts on DSS-Induced Colitis. Biomedicines, 11(7), 1913. https://doi.org/10.3390/biomedicines11071913






