The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Assessment of Incontinence-Related Quality of Life
2.2.2. Intermittent Catheterization History
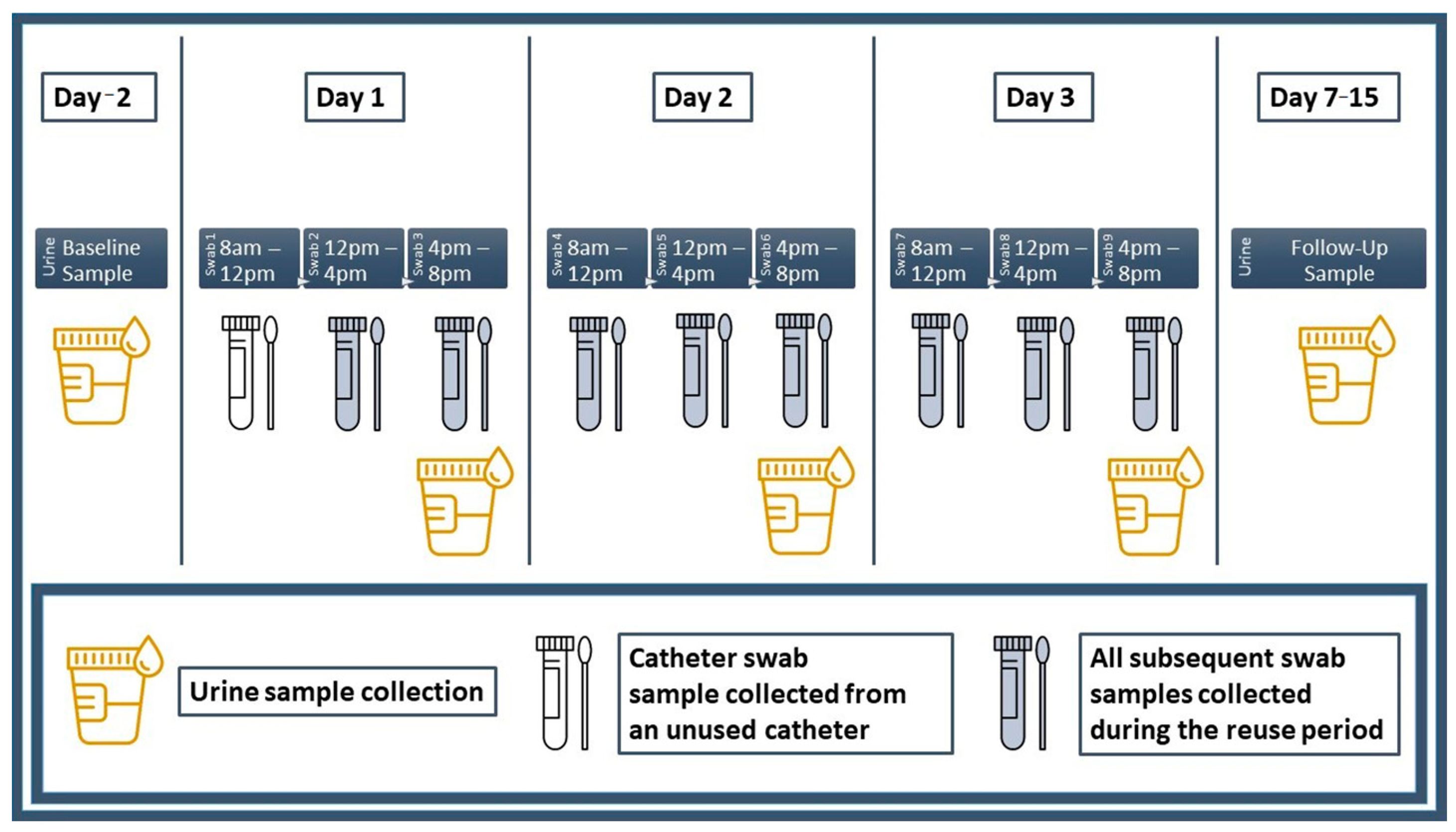
2.2.3. Urine, Swab Culture and Catheter Specimen Collection
2.2.4. Swab Gram Smear and Culture Analyses
2.2.5. Urinalysis
2.2.6. Scanning Electron Microscopy Imaging
2.2.7. X-ray Photoelectron Spectroscopy Analysis
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics and Intermittent Catheterization History
3.2. Catheter Swab Gram Smear, Culture and Sensitivity
3.3. Urinalysis
3.4. Scanning Electron Microscopy Images
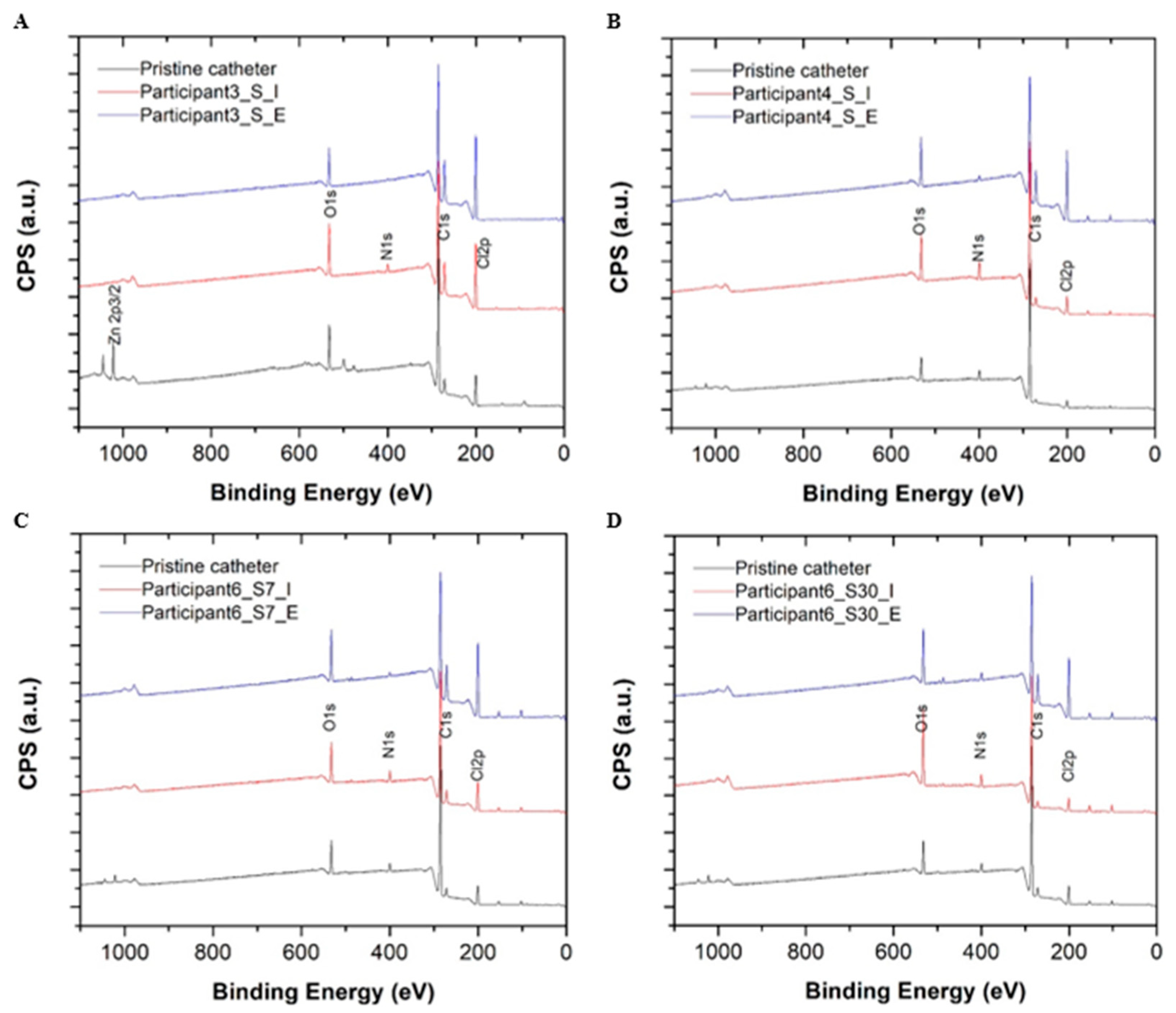
3.5. X-ray Photoelectron Spectroscopy Analysis
4. Discussion
4.1. Evidence Summary
4.2. Chemistry, Cellularity and Bacterial Growth
4.3. Catheter Surface Changes
4.4. Limitations
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groen, J.; Pannek, J.; Castro Diaz, D.; Del Popolo, G.; Gross, T.; Hamid, R.; Karsenty, G.; Kessler, T.M.; Schneider, M.; t Hoen, L.; et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur. Urol. 2016, 69, 324–333. [Google Scholar] [CrossRef]
- Persu, C.; Braschi, E.; Lavelle, J. A review of prospective Clinical Trials for neurogenic bladder: The place of surgery, experimental techniques and devices. Cent. Eur. J. Urol. 2014, 67, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taweel, W.A.; Seyam, R. Neurogenic bladder in spinal cord injury patients. Res. Rep. Urol. 2015, 7, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Fougere, R.; Zhou, M.W.; Nigro, M.K.; Krassioukov, A.V. Autonomic dysreflexia severity during urodynamics and cystoscopy in individuals with spinal cord injury. Spinal Cord 2013, 51, 863–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasell, R.W.; Arnold, J.M.O.; Krassioukov, A.; Delaney, G.A. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch. Phys. Med. Rehabil. 2000, 81, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Eltorai, I.; Kim, R.; Vulpe, M.; Kasravi, H.; Ho, W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: Case report and review. Spinal Cord 1992, 30, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.P.; Krassioukov, A.V. Autonomic dysreflexia and myocardial ischemia. Spinal Cord 2010, 48, 714–715. [Google Scholar] [CrossRef] [Green Version]
- Wan, D.; Krassioukov, A.V. Life-threatening outcomes associated with autonomic dysreflexia: A clinical review. J. Spinal Cord Med. 2014, 37, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef] [Green Version]
- Krassioukov, A.; Cragg, J.J.; West, C.; Voss, C.; Krassioukov-Enns, D. The good, the bad and the ugly of catheterization practices among elite athletes with spinal cord injury: A global perspective. Spinal Cord 2015, 53, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Larsen, L.D.; Chamberlin, D.A.; Khonsari, F.; Ahlering, T.E. Retrospective analysis of urologic complications in male patients with spinal cord injury managed with and without indwelling urinary catheters. Urology 1997, 50, 418–422. [Google Scholar] [CrossRef]
- Ruz, A.E.D.; Leoni, E.G.; Cabrera, R.H. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J. Urol. 2000, 164, 1285–1289. [Google Scholar] [CrossRef]
- Wyndaele, J.J.; Brauner, A.; Geerlings, S.E.; Bela, K.; Peter, T.; Bjerklund-Johanson, T.E. Clean intermittent catheterization and urinary tract infection: Review and guide for future research. BJU Int. 2012, 110, E910–E917. [Google Scholar] [CrossRef] [PubMed]
- Waller, L.; Jonsson, O.; Norlen, L.; Sullivan, L. Clean intermittent catheterization in spinal cord injury patients: Long-term follow up of a hydrophilic low friction technique. J. Urol. 1995, 153, 345–348. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamamoto, S.; Gotoh, M.; Saitoh, T.; Yokoyama, O.; Murata, T.; Takeda, M. Cost-Effectiveness Analysis of Long-Term Intermittent Self-Catheterization with Hydrophilic-Coated and Uncoated Catheters in Patients with Spinal Cord Injury in Japan. Low Urin. Tract Symptoms 2017, 9, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.V.; Umscheid, C.A.; Agarwal, R.K.; Kuntz, G.; Pegues, D.A. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Infect. Control. Hosp. Epidemiol. 2010, 31, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennelly, M.; Thiruchelvam, N.; Averbeck, M.A.; Konstatinidis, C.; Chartier-Kastler, E.; Trøjgaard, P.; Vaabengaard, R.; Krassioukov, A.; Jakobsen, B.P. Adult Neurogenic Lower Urinary Tract Dysfunction and Intermittent Catheterisation in a Community Setting: Risk Factors Model for Urinary Tract Infections. Adv. Urol. 2019, 2019, 2757862. [Google Scholar] [CrossRef] [Green Version]
- Håkansson, M.Å. Reuse versus single-use catheters for intermittent catheterization: What is safe and preferred? Review of current status. Spinal Cord 2014, 52, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Sherbondy Andrea, L.; Cooper Christopher, S.; Kalinowski Scott, E.; Boyt Margaret, A.; Hawtrey Charles, E. Variability in Catheter Microwave Sterilization Techniques in a Single Clinic Population. J. Urol. 2002, 168, 562–564. [Google Scholar] [CrossRef]
- Hill, T.C.; Baverstock, R.; Carlson, K.V.; Estey, E.P.; Gray, G.J.; Hill, D.C.; Ho, C.; McGinnis, R.H.; Moore, K.; Parmar, R. Best practices for the treatment and prevention of urinary tract infection in the spinal cord injured population: The Alberta context. Can. Urol. Assoc. J. 2013, 7, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.K.; New, P.W.; Heriseanu, R.; Petronis, S.; Håkansson, J.; Håkansson, M.Å.; Lee, B.B. Intermittent catheterization with single- or multiple-reuse catheters: Clinical study on safety and impact on quality of life. Int. Urol. Nephrol. 2020, 52, 1443–1451. [Google Scholar] [CrossRef]
- Sun, A.J.; Comiter, C.V.; Elliott, C.S. The cost of a catheter: An environmental perspective on single use clean intermittent catheterization. Neurourol. Urodyn. 2018, 37, 2204–2208. [Google Scholar] [CrossRef]
- Avery, M.; Prieto, J.; Okamoto, I.; Cullen, S.; Clancy, B.; Moore, K.N.; Macaulay, M.; Fader, M. Reuse of intermittent catheters: A qualitative study of IC users’ perspectives. BMJ Open 2018, 8, e021554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.N.; Alabastro, C.G.; Anger, J.T. Prevalence and Cost of Catheters to Manage Neurogenic Bladder. Curr. Bladder Dysfunct. Rep. 2018, 13, 215–223. [Google Scholar] [CrossRef]
- Walter, M.; Krassioukov, A.V. Single-use Versus Multi-use Catheters: Pro Single-use Catheters. Eur. Urol. Focus 2020, 6, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Kovindha, A.; Mai, W.N.C.; Madersbacher, H. Reused silicone catheter for clean intermittent catheterization (CIC): Is it safe for spinal cord-injured (SCI) men? Spinal Cord 2004, 42, 638–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christison, K.; Walter, M.; Wyndaele, J.-J.J.M.; Kennelly, M.; Kessler, T.M.; Noonan, V.K.; Fallah, N.; Krassioukov, A.V. Intermittent Catheterization: The Devil Is in the Details. J. Neurotrauma 2017, 35, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Grasdal, M.; Lai, R.; Walter, M.; Krassioukov, A.V. Short-term reuse of catheters is associated with microbiological and structural burden: A prospective pilot case series. Front. Urol. 2022, 2, 938968. [Google Scholar] [CrossRef]
- Khoury, A.E.; Lam, K.; Ellis, B.; Costerton, J.W. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992, 38, M174–M178. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, e83–e110. [Google Scholar] [CrossRef]
- Wagner, T.H.; Patrick, D.L.; Bavendam, T.G.; Martin, M.L.; Buesching, D.E. Quality of life of persons with urinary incontinence: Development of a new measure. Urology 1996, 47, 67–71. [Google Scholar] [CrossRef]
- Patrick, D.L.; Martin, M.L.; Bushnell, D.M.; Yalcin, I.; Wagner, T.H.; Buesching, D.P. Quality of life of women with urinary incontinence: Further development of the incontinence quality of life instrument (I-QOL). Urology 1999, 53, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Schurch, B.; Denys, P.; Kozma, C.M.; Reese, P.R.; Slaton, T.; Barron, R. Reliability and validity of the Incontinence Quality of Life questionnaire in patients with neurogenic urinary incontinence. Arch. Phys. Med. Rehabil. 2007, 88, 646–652. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, P.-J.; Verleysen, E.; Lammertyn, J.; Mast, J. Size measurement uncertainties of near-monodisperse, near-spherical nanoparticles using transmission electron microscopy and particle-tracking analysis. J. Nanoparticle Res. 2014, 16, 2628. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Comparisons of the Mann-Whitney, Student’s “t”, and Alternate “t” Tests for Means of Normal Distributions. J. Exp. Educ. 1991, 59, 258. [Google Scholar] [CrossRef]
- Portney, L.G. Foundations of Clinical Research: Applications to Practice, 3rd ed.; F.A. Davis Company: Philadelphia, PA, USA, 2015. [Google Scholar]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. Open Forum Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef]
- Garshick, E.; Kelley, A.; Cohen, S.A.; Garrison, A.; Tun, C.G.; Gagnon, D.; Brown, R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005, 43, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Rish, B.L.; Dilustro, J.F.; Salazar, A.M.; Schwab, K.A.; Brown, H.R. Spinal cord injury: A 25-year morbidity and mortality study. Mil. Med. 1997, 162, 141–148. [Google Scholar]
- Shergill, I.S.; Arya, M.; Hamid, R.; Khastgir, J.; Patel, H.R.H.; Shah, P.J.R. The importance of autonomic dysreflexia to the urologist. BJU Int. 2004, 93, 923–926. [Google Scholar] [CrossRef]
- Eldahan, K.C.; Rabchevsky, A.G. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton. Neurosci. 2018, 209, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Khasriya, R.; Sathiananthamoorthy, S.; Ismail, S.; Kelsey, M.; Wilson, M.; Rohn, J.L.; Malone-Lee, J. Spectrum of Bacterial Colonization Associated with Urothelial Cells from Patients with Chronic Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2013, 51, 2054–2062. [Google Scholar] [CrossRef] [Green Version]
- Horsley, H.; Malone-Lee, J.; Holland, D.; Tuz, M.; Hibbert, A.; Kelsey, M.; Kupelian, A.; Rohn, J.L. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS ONE 2013, 8, e83637. [Google Scholar] [CrossRef] [PubMed]
- Schmiemann, G.; Kniehl, E.; Gebhardt, K.; Matejczyk, M.M.; Hummers-Pradier, E. The diagnosis of urinary tract infection: A systematic review. Dtsch. Arztebl. Int. 2010, 107, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Siegman-Igra, Y. The significance of urine culture with mixed flora. Curr. Opin. Nephrol. Hypertens. 1994, 3, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, T.; Pandey, M.; Varma, M.; Bhatambare, G. Mixed flora in the urine of hospitalized and elderly patients: Contamination or True infection? Niger. J. Exp. Clin. Biosci. 2014, 2, 20–27. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Yazdanbod, H.; Aghapour, R.; Akbari Cheshmeh, M.; Salehian Omran, D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica 2010, 5, 111–115. [Google Scholar]
- Baraboutis, I.G.; Tsagalou, E.P.; Lepinski, J.L.; Papakonstantinou, I.; Papastamopoulos, V.; Skoutelis, A.T.; Johnson, S. Primary Staphylococcus aureus urinary tract infection: The role of undetected hematogenous seeding of the urinary tract. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- van der Kolk, J.H.; Endimiani, A.; Graubner, C.; Gerber, V.; Perreten, V. Acinetobacter in veterinary medicine, with an emphasis on Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2019, 16, 59–71. [Google Scholar] [CrossRef]
- Newman, J.W.; Floyd, R.V.; Fothergill, J.L. The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett. 2017, 364, fnx124. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Bogaert, G.A.; Goeman, L.; de Ridder, D.; Wevers, M.; Ivens, J.; Schuermans, A. The physical and antimicrobial effects of microwave heating and alcohol immersion on catheters that are reused for clean intermittent catheterisation. Eur. Urol. 2004, 46, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Wilks, S.A.; Morris, N.S.; Thompson, R.; Prieto, J.A.; Macaulay, M.; Moore, K.N.; Keevil, C.W.; Fader, M. An effective evidence-based cleaning method for the safe reuse of intermittent urinary catheters: In vitro testing. Neurourol. Urodyn. 2020, 39, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasdal, M.; Walter, M.; Krassioukov, A.V. The microbiological and physical properties of catheters for intermittent catheterization: A systematic review on the impact of reuse and cleaning. Spinal Cord 2022, 60, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Mazzoli, S.; Mondaini, N.; Meacci, F.; Nesi, G.; D’Elia, C.; Malossini, G.; Boddi, V.; Bartoletti, R. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: To treat or not to treat? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, G.; Bolter, L.-M.; Sluka, R.; Höller, Y.; Bathke, A.C.; Thomschewski, A.; Leis, S.; Lattanzi, S.; Brigo, F.; Trinka, E. Sample sizes and statistical methods in interventional studies on individuals with spinal cord injury: A systematic review. J. Evid.-Based Med. 2019, 12, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| (N = 10) | |
|---|---|
| Demographics | |
| Age (years) | 61.6 ± 9.6 |
| Age—range (years) | 50–79 |
| Height (cm) | 173.5 ± 5.9 |
| Weight (kg) | 71.9 ± 17.6 |
| BMI (kg/m2) | 24.0 ± 6.7 |
| Injury Characteristics | |
| Time since injury (years) | 35.6 ± 9.8 |
| Time since injury—range (years) | 17–50 |
| Neurological level of injury—range | C6–L3 |
| Motor—complete, AIS (A/B) | 5/0 |
| Motor—incomplete, AIS (C/D) | 1/4 |
| Daily Catheterization | |
| Average | 5.2 ± 1.7 |
| Average—range | 3–9 |
| Minimum | 3.6 ± 1.4 |
| Minimum—range | 1–6 |
| Maximum | 7.9 ± 1.9 |
| Maximum—range | 5–10 |
| Catheter Reuse | |
| Reuse history (years) | 29.4 ± 10.7 |
| Reuse history—range (years) | 11–44 |
| Shortest reuse time (single catheter) (days) | 10.0 ± 14.9 |
| Shortest reuse time—range (single catheter) (days) | 1–42 |
| Longest reuse time (single catheter) (days) | 71.1 ± 106.4 |
| Longest reuse time—range (single catheter) (days) | 1–336 |
| Incontinence | |
| I-QoL—scaled Domain 1 (0–100) | 77.5 ± 14.5 |
| I-QoL—scaled Domain 2 (0–100) | 86.9 ± 11.2 |
| I-QoL—scaled Domain 3 (0–100) | 73.0 ± 20.6 |
| I-QoL—scaled total (0–100) | 80.3 ± 12.6 |
| Variable | Day −2 (Baseline) | Day 1 | Day 2 | Day 3 | Day 7–15 (Follow-Up) | χ2 | H | p |
|---|---|---|---|---|---|---|---|---|
| Swab Gram Smear, Culture and Sensitivity | ||||||||
| Gram stain cellularity † (swab # 1/2/3), n (0–10) | - | 3/2/1 | 2/1/5 | 7/1/0 | - | 12.000 | - | 0.062 |
| Positive culture results (swab # 1/2/3), n (0–10) | - | 4/2/0 | 1/2/5 | 0/3/4 | - | 10.819 | - | 0.094 |
| Antibiotic resistance, n (0–10) | - | 0 | 2 | 0 | - | 4.286 | - | 0.117 |
| Urine Chemistry | ||||||||
| Appearance (clear/cloudy/turbid), n (0–10) | 6/3/1 | 8/1/1 | 6/3/1 | 7/2/1 | 6/3/1 | 1.818 | - | 0.986 |
| Urine pH (5.0–8.0) | 7.10 ± 1.02 | 6.55 ± 0.98 | 6.10 ± 0.77 | 6.55 ± 0.98 | 6.10 ± 0.77 | - | 6.679 | 0.154 |
| Specific gravity (1.003–1.035) | 1.012 ± 0.004 | 1.012 ± 0.006 | 1.014 ± 0.007 | 1.013 ± 0.008 | 1.015 ± 0.005 | - | 1.616 | 0.806 |
| Hemoglobin (mg/L) | 2.53 ± 7.89 | 2.50 ± 7.90 | 2.53 ± 7.89 | 5.03 ± 10.52 | 10.56 ± 25.62 | - | 2.725 | 0.605 |
| Leukocyte (WBC/μL) | 250.00 ± 220.47 | 182.00 ± 172.37 | 196.00 ± 213.90 | 164.00 ± 183.58 | 189.00 ± 219.55 | - | 1.542 | 0.819 |
| Nitrite (positive), n (0–10) | 2 | 3 | 4 | 6 | 6 | 5.255 | - | 0.272 |
| Urine Culture and Sensitivity | ||||||||
| Positive culture results, n (0–10) | 9 | 9 | 10 | 10 | 10 | 3.125 | - | 0.537 |
| Antibiotic resistance, n (0–10) | 6 | 6 | 7 | 6 | 6 | 0.340 | - | 0.987 |
| Sample No. | Participant—Catheter Sample | † C% | N% | O% | ‡ Carbon | |||
|---|---|---|---|---|---|---|---|---|
| C-C | C-NH2, C-N | -C-O, =C-NH, NH2-C-COOH | O=C-OH | |||||
| 284.6 eV | 285.6 eV | 286.5 eV | 288.5–289 eV | |||||
| S1 | Participant 3—pristine control | 82.59 | 0 | 8.69 | 87.1 | 0 | 9.12 | 3.78 |
| S2 | Participant 4—pristine control | 87.14 | 3.32 | 5.99 | 93.15 | 0 | 3.72 | 3.13 |
| S3 | Participant 6—pristine control | 82.77 | 2.47 | 7.28 | 90.38 | 0 | 6.42 | 3.19 |
| S4 | Participant 3—reused catheter (3 days, intralaminar) | 72.02 | 2.35 | 9.96 | 61.78 | 14.41 | 20.64 | 3.17 |
| S5 | Participant 3—reused catheter (3 days, extraluminal) | 72.83 | 0.15 | 7.40 | 66.26 | 5.83 | 25.14 | 2.77 |
| S6 | Participant 4—reused catheter (3 days, intralaminar) | 80.45 | 4.8 | 8.76 | 72.85 | 14.25 | 8.1 | 4.8 |
| S7 | Participant 4—reused catheter (3 days, extraluminal) | 69.92 | 1.25 | 9.35 | 62.76 | 16.22 | 19.17 | 1.85 |
| S8 | Participant 6—reused catheter (3 days, intralaminar) | 77.71 | 3.02 | 8.93 | 77.59 | 9.34 | 11.3 | 1.76 |
| S9 | Participant 6—reused catheter (3 days, extraluminal) | 68.67 | 1.29 | 9.57 | 64.94 | 8.1 | 25.39 | 1.58 |
| S10 | Participant 6—reused catheter (30 days, intralaminar) | 74.22 | 3.42 | 15.30 | 68.7 | 8.71 | 15.93 | 6.66 |
| S11 | Participant 6—reused catheter (30 days, extraluminal) | 70.15 | 2.12 | 10.30 | 57.41 | 16.94 | 23.42 | 2.23 |
 | Intralaminar catheter surface |  | Extraluminal catheter surface | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, T.; Lange, D.; Kizhakkedathu, J.N.; Yu, K.; Felix, D.; Samejima, S.; Shackleton, C.; Malik, R.N.; Sachdeva, R.; Walter, M.; et al. The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study. Biomedicines 2023, 11, 1929. https://doi.org/10.3390/biomedicines11071929
Miller T, Lange D, Kizhakkedathu JN, Yu K, Felix D, Samejima S, Shackleton C, Malik RN, Sachdeva R, Walter M, et al. The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study. Biomedicines. 2023; 11(7):1929. https://doi.org/10.3390/biomedicines11071929
Chicago/Turabian StyleMiller, Tiev, Dirk Lange, Jayachandran N. Kizhakkedathu, Kai Yu, Demian Felix, Soshi Samejima, Claire Shackleton, Raza N. Malik, Rahul Sachdeva, Matthias Walter, and et al. 2023. "The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study" Biomedicines 11, no. 7: 1929. https://doi.org/10.3390/biomedicines11071929





