Nano-Catechin Gel as a Sustained Release Antimicrobial Agent against Clinically Isolated Porphyromonas gingivalis for Promising Treatment of Periodontal Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Catechin-Loaded Chitosan Nanoparticles
2.2.2. Nanoparticle Characterization
2.2.3. Nano-Catechin Gel Preparation
2.2.4. The Release Outline
2.2.5. Cytotoxicity Test
2.2.6. Sampling of P. gingivalis
2.2.7. Cultivation of P. gingivalis
2.2.8. The Antimicrobial Activity of Nanoparticles
2.2.9. Statistical Analysis
3. Results
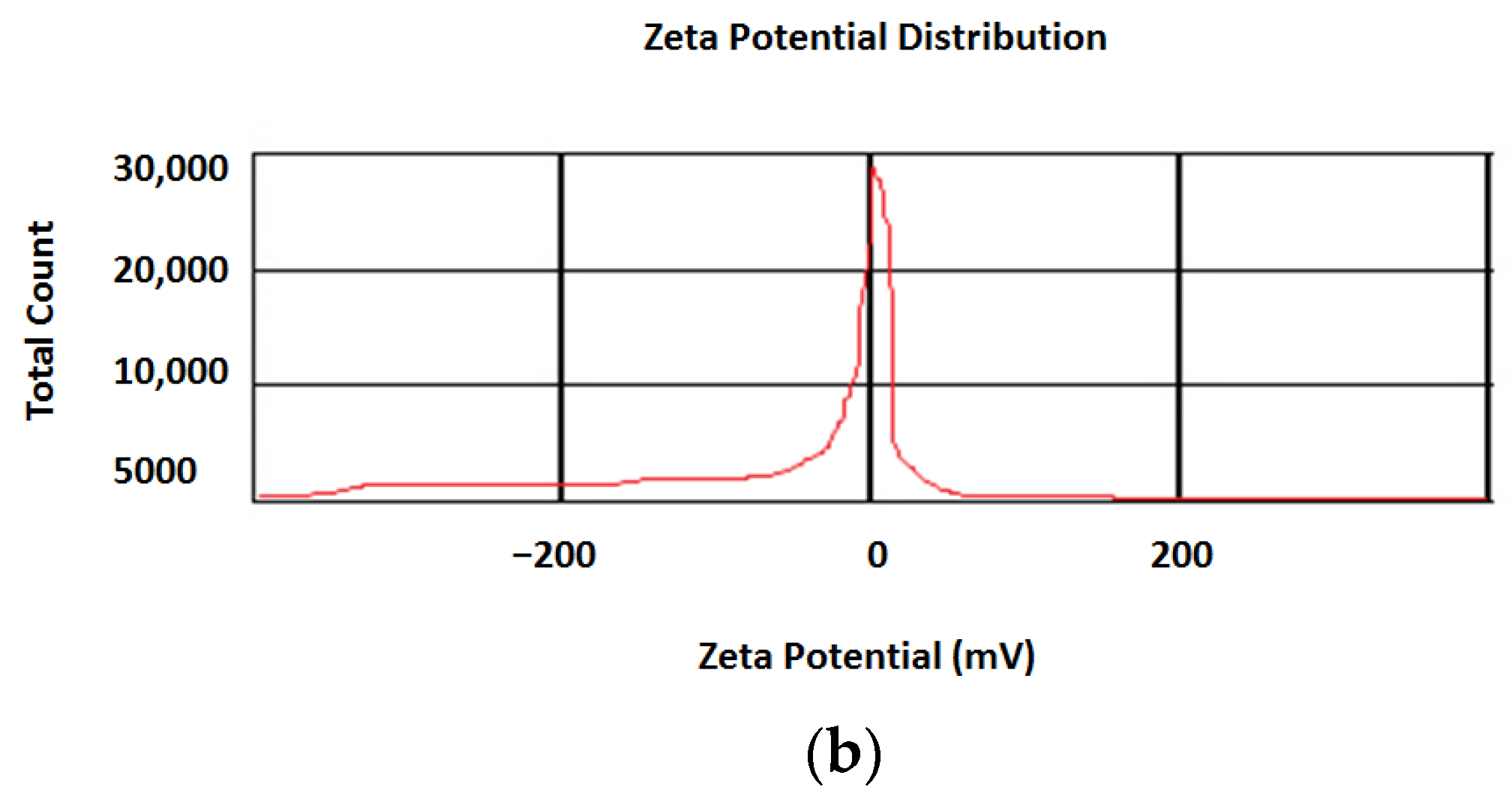
3.1. Nanoparticle Characterization
3.2. Cytotoxicity Test
3.3. Antimicrobial Action
3.4. Release Results
4. Discussion
4.1. Nanoparticle Characterization
4.2. Cytotoxicity
4.3. Antimicrobial Effects
5. The Strengths and Limitations
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brun, A.; Moignot, N.; Colombier, M.L.; Dursun, E. Emerging Nanotechnology in Non-Surgical Periodontal Therapy in Animal Models: A Systematic Review. Nanomaterials 2020, 10, 1414. [Google Scholar] [CrossRef]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflam. 2018, 15, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Shirai, M.; Murakami, M.; Mizusawa, T.; Hagimoto, A.; Wada, K.; Nomura, R.; Nakano, K.; Ooshima, T.; Asai, F. Molecular detection of human periodontal pathogens in oral swab specimens from dogs in Japan. J. Vet. Dent. 2011, 28, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Bhatt, P.; Sutariya, V.; Florez, F.L.E.; Pathak, Y.V. Application of nanoparticles in treating periodontitis: Preclinical and clinical overview. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 467–480. [Google Scholar]
- Fernandes, P.; Martens, E. Antibiotics in late clinical development. Biochem. Pharmacol. 2017, 133, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Maleki Dizaj, S.; Sharifi, S.; Jahangiri, A. Electrospun nanofibers as versatile platform in antimicrobial delivery: Current state and perspectives. Pharm. Dev. Technol. 2019, 24, 1187–1199. [Google Scholar] [CrossRef]
- Negahdari, R.; Ghavimi, M.A.; Barzegar, A.; Memar, M.Y.; Balazadeh, L.; Bohlouli, S.; Maleki Dizaj, S. Antibacterial effect of nanocurcumin inside the implant fixture: An in vitro study. Clin. Exp. Dent. Res. 2021, 7, 163–169. [Google Scholar] [CrossRef]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J. Drug. Deliv. 2011, 2011, 418316. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of Phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate. Pharm. Dev. Technol. 2018, 23, 787–793. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, Y.; Yu, Q.; Li, D.; Li, F. Synthesis, characterization of catechin-loaded folate-conjugated chitosan nanoparticles and their anti-proliferative effect. CyTA-J. Food 2018, 16, 868–876. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular Dynamics Study on the Biophysical Interactions of Seven Green tea Catechins with Lipid Bilayers of Cell Membranes. J. Agric. Food Chememical. 2008, 2008, 7750–7758. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Sarabandi, S.; Khameneh, B.; Hosseinzadeh, H. Effect of catechins, green tea extract and methylxanthines in combination with gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa:-combination therapy against resistant bacteria. J. Pharmacopunct. 2016, 19, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Tahani, B.; Mostajeran, E.; Faghihian, R.; Tavakoli, F.; Ehteshami, A.; Ziaei, S. Effects of Green Tea Products in Controlling and Decreasing the Periodontal Disease and Dental Caries-A Systematic Review. J. Mashhad Dent. Sch. 2014, 38, 169–184. [Google Scholar]
- Hirasawa, M.; Takada, K.; Makimura, M.; Otake, S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J. Periodontal. Res. 2002, 37, 433–438. [Google Scholar] [CrossRef]
- Tamura, M.; Ochiai, K. Exploring the possible applications of catechin (gel) for oral care of the elderly and disabled individuals. Jpn. Dent. Sci. Rev. 2012, 48, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef]
- Ballard, J.; Brockson, R.; De, S.; Gray, V.; Robinson, D. Intrinsic dissolution performance testing of the USP dissolution apparatus 2 (rotating paddle) using modified salicylic acid calibrator tablets: Proof of principle. Dissolution Technol. 2003, 10, 6–15. [Google Scholar]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Shah, R.M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Stability mechanisms for microwave-produced solid lipid nanoparticles. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 643, 128774. [Google Scholar] [CrossRef]
- Kim, S.; Requejo, K.I.; Nakamatsu, J.; Gonzales, K.N.; Torres, F.G.; Cavaco-Paulo, A. Modulating antioxidant activity and the controlled release capability of laccase mediated catechin grafting of chitosan. Process. Biochem. 2017, 59, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Lagha, A.B.; Groeger, S.; Meyle, J.; Grenier, D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog. Dis. 2018, 76, fty030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.A.; Song, Y.R.; Park, M.H.; Chung, H.Y.; Na, H.S.; Chung, J. Catechin ameliorates Porphyromonas gingivalis-induced inflammation via the regulation of TLR2/4 and inflammasome signaling. J. Periodontol. 2020, 91, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef]
- Fournier-Larente, J.; Morin, M.-P.; Grenier, D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch. Oral Biol. 2016, 65, 35–43. [Google Scholar] [CrossRef]
- Takashiba, S.; Naruishi, K.; Murayama, Y. Perspective of cytokine regulation for periodontal treatment: Fibroblast biology. J. Periodontol. 2003, 74, 103–110. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Mukherjee, I.; Ghosh, S.; Dey, A.; Banerjee, R.; Ray, R.R. Catechin as the most efficient bioactive compound from Azadirachta indica with antibiofilm and anti-quorum sensing activities against dental biofilm: An in vitro and in silico study. Appl. Biochem. Biotechnol. 2021, 193, 1617–1630. [Google Scholar] [CrossRef]
- Venkateswara, B.; Sirisha, K.; Chava, V.K. Green tea extract for periodontal health. J. Indian Soc. Periodontol. 2011, 15, 18. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javadkhani, A.; Shokouhi, B.; Mosayebzadeh, A.; Safa, S.; Fahimi, M.; Sharifi, S.; Maleki Dizaj, S.; Salatin, S. Nano-Catechin Gel as a Sustained Release Antimicrobial Agent against Clinically Isolated Porphyromonas gingivalis for Promising Treatment of Periodontal Diseases. Biomedicines 2023, 11, 1932. https://doi.org/10.3390/biomedicines11071932
Javadkhani A, Shokouhi B, Mosayebzadeh A, Safa S, Fahimi M, Sharifi S, Maleki Dizaj S, Salatin S. Nano-Catechin Gel as a Sustained Release Antimicrobial Agent against Clinically Isolated Porphyromonas gingivalis for Promising Treatment of Periodontal Diseases. Biomedicines. 2023; 11(7):1932. https://doi.org/10.3390/biomedicines11071932
Chicago/Turabian StyleJavadkhani, Anahita, Behnaz Shokouhi, Amin Mosayebzadeh, Samira Safa, Mahsa Fahimi, Simin Sharifi, Solmaz Maleki Dizaj, and Sara Salatin. 2023. "Nano-Catechin Gel as a Sustained Release Antimicrobial Agent against Clinically Isolated Porphyromonas gingivalis for Promising Treatment of Periodontal Diseases" Biomedicines 11, no. 7: 1932. https://doi.org/10.3390/biomedicines11071932





