Combined Band and Plate Fixation as a New Individual Option for Patients at Risk of Sternal Complications after Cardiac Surgery: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Patient Selection
3. Results
3.1. Preoperative Patient Characteristics
3.2. Operative Data
3.3. Postoperative Outcomes
3.4. Follow-Up Outcomes
4. Discussion
4.1. Individualized Therapy
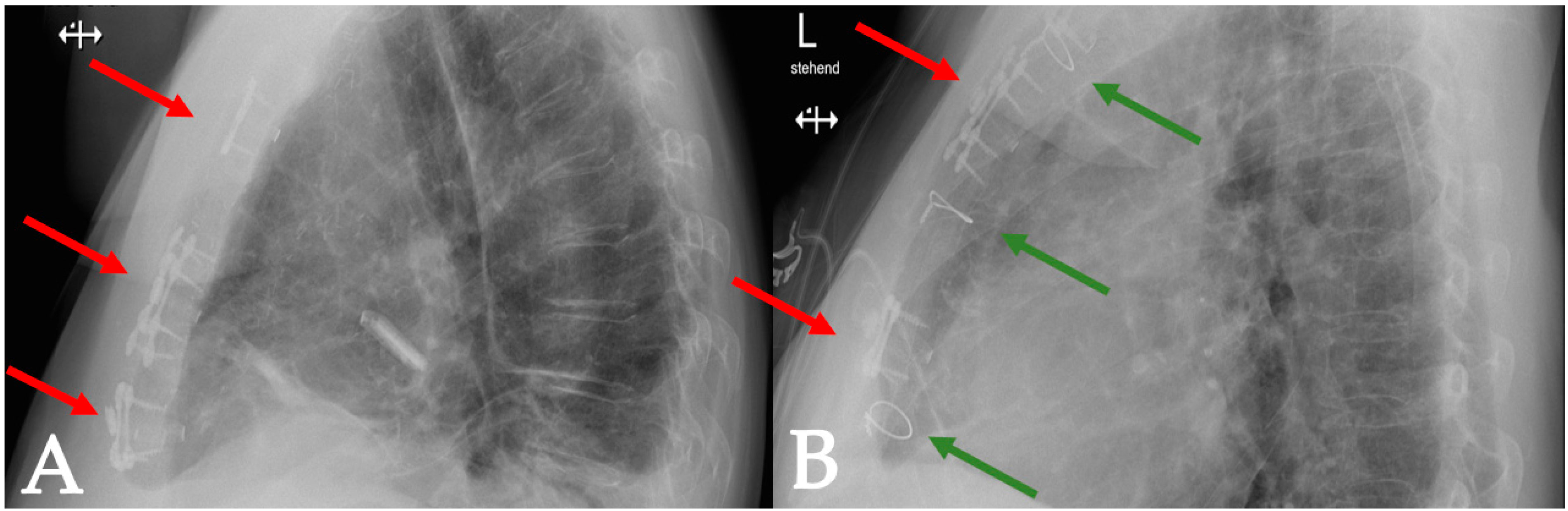
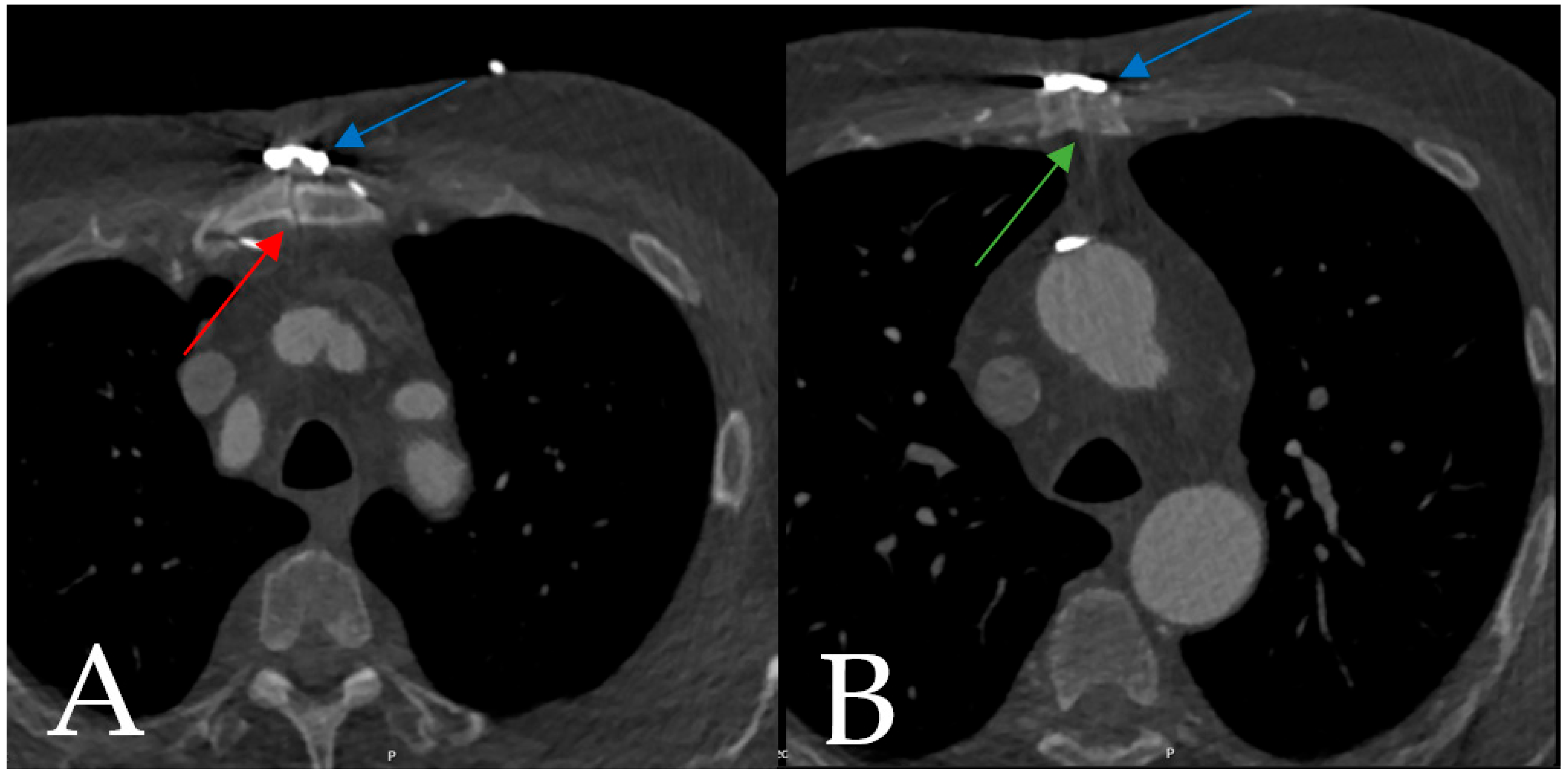
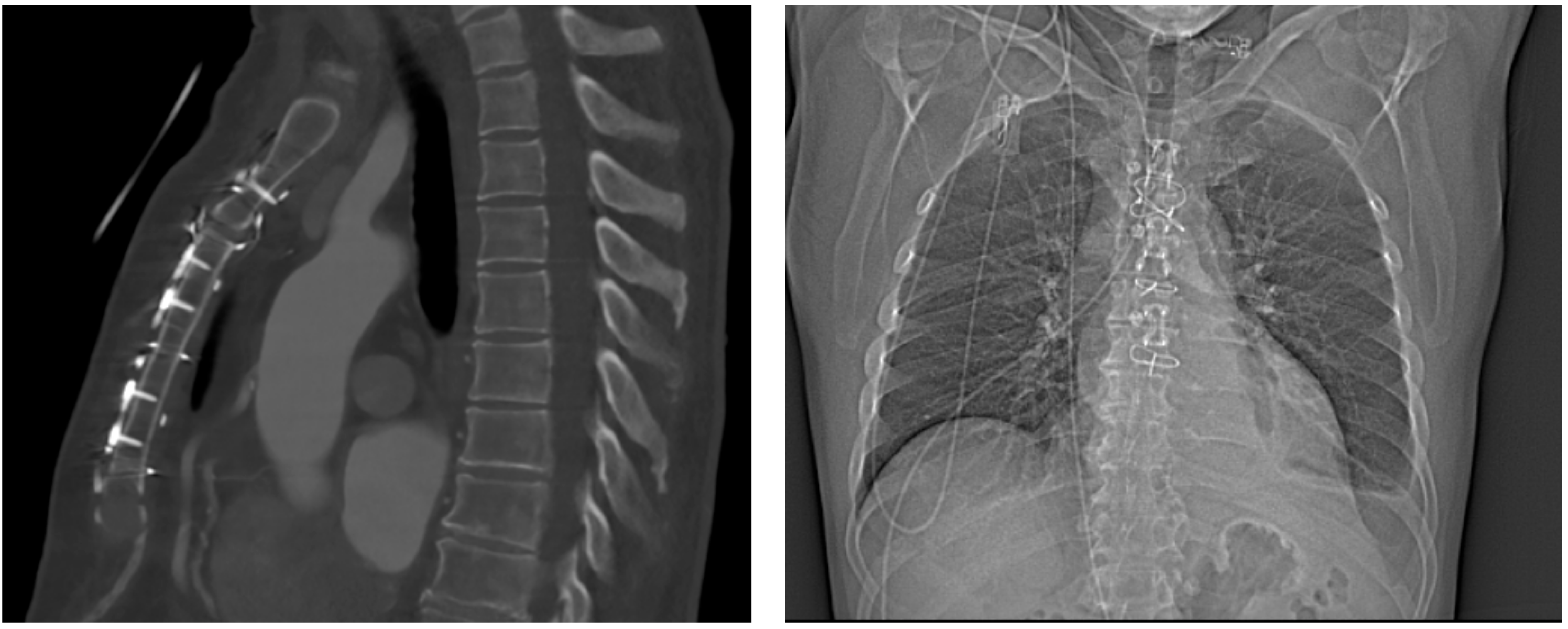
4.1.1. Case 1
4.1.2. Case 2
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milton, H. Mediastinal Surgery. Lancet 1897, 149, 872–875. [Google Scholar] [CrossRef]
- Heilmann, C.; Stahl, R.; Schneider, C.; Sukhodolya, T.; Siepe, M.; Olschewski, M.; Beyersdorf, F. Wound Complications after Median Sternotomy: A Single-Centre Study. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 643–648. [Google Scholar] [CrossRef]
- Meyerson, J.; Thelin, S.; Gordh, T.; Karlsten, R. The Incidence of Chronic Post-Sternotomy Pain after Cardiac Surgery—A Prospective Study. Acta Anaesthesiol. Scand. 2001, 45, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Aldea, G.S.; Bakaeen, F.G.; Pal, J.; Fremes, S.; Head, S.J.; Sabik, J.; Rosengart, T.; Kappetein, A.P.; Thourani, V.H.; Firestone, S.; et al. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2016, 101, 801–809. [Google Scholar] [CrossRef]
- Miclau, T.; Martin, R.E. The Evolution of Modern Plate Osteosynthesis. Injury 1997, 28, 3–6. [Google Scholar] [CrossRef]
- Robicsek, F.; Daugherty, H.K.; Cook, J.W. The Prevention and Treatment of Sternum Separation Following Open Heart Surgery. J. Thorac. Cardiovasc. Surg. 1977, 73, 267–268. [Google Scholar] [CrossRef]
- Schimmer, C.; Reents, W.; Berneder, S.; Eigel, P.; Sezer, O.; Scheld, H.; Sahraoui, K.; Gansera, B.; Deppert, O.; Rubio, A.; et al. Prevention of Sternal Dehiscence and Infection in High-Risk Patients: A Prospective Randomized Multicenter Trial. Ann. Thorac. Surg. 2008, 86, 1897–1904. [Google Scholar] [CrossRef]
- Wu, L.C.; Renucci, J.D.; Song, D.H. Sternal Nonunion: A Review of Current Treatments and a New Method of Rigid Fixation. Ann. Plast. Surg. 2005, 54, 55–58. [Google Scholar] [CrossRef]
- Raman, J.; Lehmann, S.; Zehr, K.; De Guzman, B.J.; Aklog, L.; Garrett, H.E.; MacMahon, H.; Hatcher, B.M.; Wong, M.S. Sternal Closure with Rigid Plate Fixation versus Wire Closure: A Randomized Controlled Multicenter Trial. Ann. Thorac. Surg. 2012, 94, 1854–1861. [Google Scholar] [CrossRef]
- Nishimura, T.; Kurihara, C.; Sakano, Y.; Kyo, S. Sternalock Plating System for Elderly Post-Sternotomy Patients. J. Artif. Organs 2014, 17, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.B.; Thourani, V.H.; Naka, Y.; Grubb, K.J.; Grehan, J.; Patel, N.; Guy, T.S.; Landolfo, K.; Gerdisch, M.; Bonnell, M.; et al. Randomized, Multicenter Trial Comparing Sternotomy Closure with Rigid Plate Fixation to Wire Cerclage. J. Thorac. Cardiovasc. Surg. 2017, 153, 888–896.e1. [Google Scholar] [CrossRef]
- Royse, A.G.; El-Ansary, D.; Hoang, W.; Lui, E.; McCusker, M.; Tivendale, L.; Yang, Y.; Canty, D.J.; Royse, C.F. A Randomized Trial Comparing the Effects of Sternal Band and Plate Fixation of the Sternum with That of Figure-of-8 Wires on Sternal Edge Motion and Quality of Recovery after Cardiac Surgery. Interact. Cardiovasc. Thorac. Surg. 2021, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, L.D.; Omer, S.; Rosengart, T.; Holman, W.L.; Bakaeen, F.G. Changes over Time in Risk Profiles of Patients Who Undergo Coronary Artery Bypass Graft Surgery: The Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surg. 2015, 150, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.W.; Kendall, S.; Goodwin, A.T.; Cooper, G.; Trivedi, U.; Page, R.; Jenkins, D.P. Trends and Outcomes for Cardiac Surgery in the United Kingdom from 2002 to 2016. JTCVS Open 2021, 7, 259–269. [Google Scholar] [CrossRef]
- The SternaLock ® 360 Study; Zimmer Biomet: Jacksonville, FL, USA, 2018.
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Hota, P.; Dass, C.; Erkmen, C.; Donuru, A.; Kumaran, M. Poststernotomy Complications: A Multimodal Review of Normal and Abnormal Postoperative Imaging Findings. Am. J. Roentgenol. 2018, 211, 1194–1205. [Google Scholar] [CrossRef]
- Kaul, P. Sternal Reconstruction after Post-Sternotomy Mediastinitis. J. Cardiothorac. Surg. 2017, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Ayaon Albarrán, A.; Blázquez González, J.A.; Hernández Cabrero, T.; González Villegas, E. Internal Mammary Artery Pseudoaneurysm Following a Robicsek Sternal Closure. J. Card. Surg. 2017, 32, 264–265. [Google Scholar] [CrossRef]
- Andreas, M.; Muckenhuber, M.; Hutschala, D.; Kocher, A.; Thalhammer, F.; Vogt, P.; Fleck, T.; Laufer, G. Direct Sternal Administration of Vancomycin and Gentamicin during Closure Prevents Wound Infection. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 6–10. [Google Scholar] [CrossRef]
- Nooh, E.; Griesbach, C.; Rösch, J.; Weyand, M.; Harig, F. Development of a New Sternal Dehiscence Prediction Scale for Decision Making in Sternal Closure Techniques after Cardiac Surgery. J. Cardiothorac. Surg. 2021, 16, 174. [Google Scholar] [CrossRef]
- Tam, D.Y.; Nedadur, R.; Yu, M.; Yanagawa, B.; Fremes, S.E.; Friedrich, J.O. Rigid Plate Fixation Versus Wire Cerclage for Sternotomy After Cardiac Surgery: A Meta-Analysis. Ann. Thorac. Surg. 2018, 106, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.S.C. Post-Sternotomy Mediastinitis in the Modern Era. J. Card. Surg. 2017, 32, 556–566. [Google Scholar] [CrossRef]
- Kalso, E.; Mennander, S.; Tasmuth, T.; Nilsson, E. Chronic Post-Sternotomy Pain. Acta Anaesthesiol. Scand. 2001, 45, 935–939. [Google Scholar] [CrossRef]
- Cogan, J. Pain Management after Cardiac Surgery. Semin. Cardiothorac. Vasc. Anesth. 2010, 14, 201–204. [Google Scholar] [CrossRef]
- Singh, K.; Anderson, E.; Harper, J.G. Overview and Management of Sternal Wound Infection. Semin. Plast. Surg. 2011, 25, 25–33. [Google Scholar] [CrossRef]
- Gummert, J.F.; Barten, M.J.; Hans, C.; Kluge, M.; Doll, N.; Walther, T.; Hentschel, B.; Schmitt, D.V.; Mohr, F.W.; Diegeler, A. Mediastinitis and Cardiac Surgery—An Updated Risk Factor Analysis in 10,373 Consecutive Adult Patients. Thorac. Cardiovasc. Surg. 2002, 50, 87–91. [Google Scholar] [CrossRef]
- Lo Torto, F.; Turriziani, G.; Donato, C.; Marcasciano, M.; Redi, U.; Greco, M.; Miraldi, F.; Ribuffo, D. Deep Sternal Wound Infection Following Cardiac Surgery: A Comparison of the Monolateral with the Bilateral Pectoralis Major Flaps. Int. Wound J. 2020, 17, 683–691. [Google Scholar] [CrossRef]
- Finkelstein, R.; Rabino, G.; Mashiah, T.; Bar-El, Y.; Adler, Z.; Kertzman, V.; Cohen, O.; Milo, S. Surgical Site Infection Rates Following Cardiac Surgery: The Impact of a 6-Year Infection Control Program. Am. J. Infect. Control 2005, 33, 450–454. [Google Scholar] [CrossRef]
- Silverborn, M.; Heitmann, L.A.; Sveinsdottir, N.; Rögnvaldsson, S.; Kristjansson, T.T.; Gudbjartsson, T. Non-Infectious Sternal Dehiscence after Coronary Artery Bypass Surgery. J. Cardiothorac. Surg. 2022, 17, 249. [Google Scholar] [CrossRef]
- Coltro, P.S.; Farina, J.A., Jr. The Role of the Unilateral Pectoralis Major Muscle Flap in the Treatment of Deep Sternal Wound Infection and Dehiscence. J. Card. Surg. 2022, 37, 2315–2316. [Google Scholar] [CrossRef] [PubMed]
- Olbrecht, V.A.; Barreiro, C.J.; Bonde, P.N.; Williams, J.A.; Baumgartner, W.A.; Gott, V.L.; Conte, J.V. Clinical Outcomes of Noninfectious Sternal Dehiscence After Median Sternotomy. Ann. Thorac. Surg. 2006, 82, 902–907. [Google Scholar] [CrossRef]
- Almdahl, S.M.; Halvorsen, P.; Veel, T.; Rynning, S.E. Avoidance of Noninfectious Sternal Dehiscence: Figure-of-8 Wiring Is Superior to Straight Wire Closure. Scand. Cardiovasc. J. 2013, 47, 247–250. [Google Scholar] [CrossRef]
- Kristensen, K.L.; Rauer, L.J.; Mortensen, P.E.; Kjeldsen, B.J. Reoperation for Bleeding in Cardiac Surgery. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 709–713. [Google Scholar] [CrossRef]
- Bianco, V.; Kilic, A.; Gleason, T.G.; Aranda-Michel, E.; Habertheuer, A.; Wang, Y.; Navid, F.; Kacin, A.; Sultan, I. Reoperative Cardiac Surgery Is a Risk Factor for Long-Term Mortality. Ann. Thorac. Surg. 2020, 110, 1235–1242. [Google Scholar] [CrossRef]
- Kamiya, H.; Al-Maisary, S.S.A.; Akhyari, P.; Ruhparwar, A.; Kallenbach, K.; Lichtenberg, A.; Karck, M. The Number of Wires for Sternal Closure Has a Significant Influence on Sternal Complications in High-Risk Patients. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 665–670. [Google Scholar] [CrossRef]


| Patient Characteristics | Total (N = 17) |
|---|---|
| Age, years | 71 (6.2) |
| Female | 11 (65%) |
| Diabetes | 5 (29%) |
| No insulin | 2 (12%) |
| Insulin | 3 (18%) |
| HbA1c, % | 5.7 (5.4 to 7.2) |
| BMI kg/m2 | 27 (24 to 31) |
| BMI > 35 kg/m2 | 3 (18%) |
| BMI 25–35 kg/m2 | 9 (53%) |
| 3-Vessel CAD | 8 (47%) |
| Left main CAD | 2 (12%) |
| Peripheral artery disease | 1 (6%) |
| Preoperative stroke | 3 (18%) |
| Renal disease | 1 (6%) |
| Dialysis | 0 (0%) |
| COPD | 2 (12%) |
| Prior myocardial infarction | 12 (71%) |
| Hypertension | 13 (76%) |
| Hypercholesteremia | 7 (41%) |
| Diagnosed with osteoporosis or osteopenia | 2 (12%) |
| Smoker | 4 (24%) |
| Current | 3 (18%) |
| Former | 1 (6%) |
| Previous cardiac operations > 6 months | 2 (12%) |
| MUST-Score | 4 (3.0 to 6.0) |
| Dyspnea grade NYHA | |
| n/a | 1 (6%) |
| I | 14 (82%) |
| II | 2 (12%) |
| Dyspnea NYHA III or IV | 0 (0%) |
| Preoperative AF | 2 (12%) |
| Left ventricular ejection fraction, % | 52 (12) |
| Operative Data | Total (N = 17) |
|---|---|
| CABG and Valve(s) | 3 (18%) |
| CABG only | 9 (53%) |
| Valve(s) only | 1 (6%) |
| Aorta | 2 (12%) |
| LVAD | 1 (6%) |
| Pericardial disease | 1 (6%) |
| Emergency | 1 (6%) |
| Re-exploration | 2 (12%) |
| EuroSCORE II | 3.3 (1.4 to 6.6) |
| Duration of operation | 223 (177 to 278) |
| Duration of surgery > 300 min | 1 (6%) |
| Perfusion time, min | 108 (82 to 133) |
| Aortic clamping time, min | 75 (45 to 87) |
| Indications for SL360 | |
| MUST-Score > 8 | 4 (24%) |
| Intraoperative osteoporosis/ | |
| pathological sternal compressibility test | 8 (47%) |
| BIMA and diabetes [4] | 1 (6%) |
| Surgeon’s preference | 2 (12%) |
| Sternal instability | 3 (18%) |
| Secondary sternal closure | 3 (18%) |
| SL360 only | 2 (12%) |
| SL360 and sternal wires | 15 (88%) |
| SL360 at index operation | 14 (82%) |
| SL360 as secondary procedure | 3 (18%) |
| Patient | History of Diabetes | Preoperative HbA1 (%) | BIMA | History of Osteoporosis or Osteopenia | BMI (kg/m2) | Secondary Sternal Closure | Pathological Sternal Compressibility Test | Surgeon’s Preference for SL360 | Age > 60 Years |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NIDDM | 7.3 | X | 32 | X | ||||
| 2 | 5.4 | 28 | X | X | |||||
| 3 | 6.7 | 21 | X | X | |||||
| 4 | IDDM | 6 | 26 | X | |||||
| 5 | 5.7 | X | X | 28 | X | X | |||
| 6 | n.a. | X | 40 | X | X | ||||
| 7 | n.a. | n.a. | X | X | X | ||||
| 8 | IDDM | 7.6 | 39 | X | |||||
| 9 | NIDDM | 7.8 | 29 | X | X | ||||
| 10 | 5.7 | 31 | X | X | |||||
| 11 | n.a. | 22 | X | ||||||
| 12 | 5.1 | X | 26 | X | X | ||||
| 13 | 5.2 | 27 | X | X | |||||
| 14 | IDDM | 9.5 | 37 | X | |||||
| 15 | 5.5 | 24 | X | X | |||||
| 16 | 5.3 | 20 | X | X | |||||
| 17 | 5.6 | 21 | X | X |
| Postoperative Outcomes | Total (N = 17) |
|---|---|
| Operative mortality | 0 (0%) |
| ICU stay, d | 2.0 (1.0 to 2.0) |
| Reoperation for bleeding | 0 (0%) |
| Postoperative myocardial infarction | 1 (6%) |
| Postoperative stroke | 2 (12%) |
| Time to postoperative mobilization into standing position, d | 2 (2 to 3) |
| Atrial fibrillation at discharge | 3 (18%) |
| Permanent pacemaker | 0 (0%) |
| Sternal infection | 0 (0%) |
| Postoperative renal failure | 2 (12%) |
| Renal substitution therapy | 0 (0%) |
| Pulmonary infection | 0 (0%) |
| MACCE | 3 (18%) |
| Sepsis | 1 (6%) |
| Length of in-hospital stay, d | 9.0 (8.0 to 11) |
| Outcomes at Follow-Up | Total (N = 17) |
|---|---|
| Available follow-up | 14 (82%) |
| Follow-up time, days | 141 (47.8 to 511.5) |
| Deep wound infection | 0 (0%) |
| Superficial wound infection | 1 (6%) |
| Sternal instability | 0 (0%) |
| Sternal pain | 0 (0%) |
| Revision linked to SL360 | 0 (0%) |
| CT scan | 6 (35%) |
| Sternal dehiscence | 0 (0%) |
| Sternal mal-union | 1 (6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miazza, J.; Vasiloi, I.; Koechlin, L.; Gahl, B.; Reuthebuch, O.; Eckstein, F.S.; Santer, D. Combined Band and Plate Fixation as a New Individual Option for Patients at Risk of Sternal Complications after Cardiac Surgery: A Single-Center Experience. Biomedicines 2023, 11, 1946. https://doi.org/10.3390/biomedicines11071946
Miazza J, Vasiloi I, Koechlin L, Gahl B, Reuthebuch O, Eckstein FS, Santer D. Combined Band and Plate Fixation as a New Individual Option for Patients at Risk of Sternal Complications after Cardiac Surgery: A Single-Center Experience. Biomedicines. 2023; 11(7):1946. https://doi.org/10.3390/biomedicines11071946
Chicago/Turabian StyleMiazza, Jules, Ion Vasiloi, Luca Koechlin, Brigitta Gahl, Oliver Reuthebuch, Friedrich S. Eckstein, and David Santer. 2023. "Combined Band and Plate Fixation as a New Individual Option for Patients at Risk of Sternal Complications after Cardiac Surgery: A Single-Center Experience" Biomedicines 11, no. 7: 1946. https://doi.org/10.3390/biomedicines11071946
APA StyleMiazza, J., Vasiloi, I., Koechlin, L., Gahl, B., Reuthebuch, O., Eckstein, F. S., & Santer, D. (2023). Combined Band and Plate Fixation as a New Individual Option for Patients at Risk of Sternal Complications after Cardiac Surgery: A Single-Center Experience. Biomedicines, 11(7), 1946. https://doi.org/10.3390/biomedicines11071946







