The Enigmatic CA2: Exploring the Understudied Region of the Hippocampus and Its Involvement in Parkinson’s Disease
Abstract
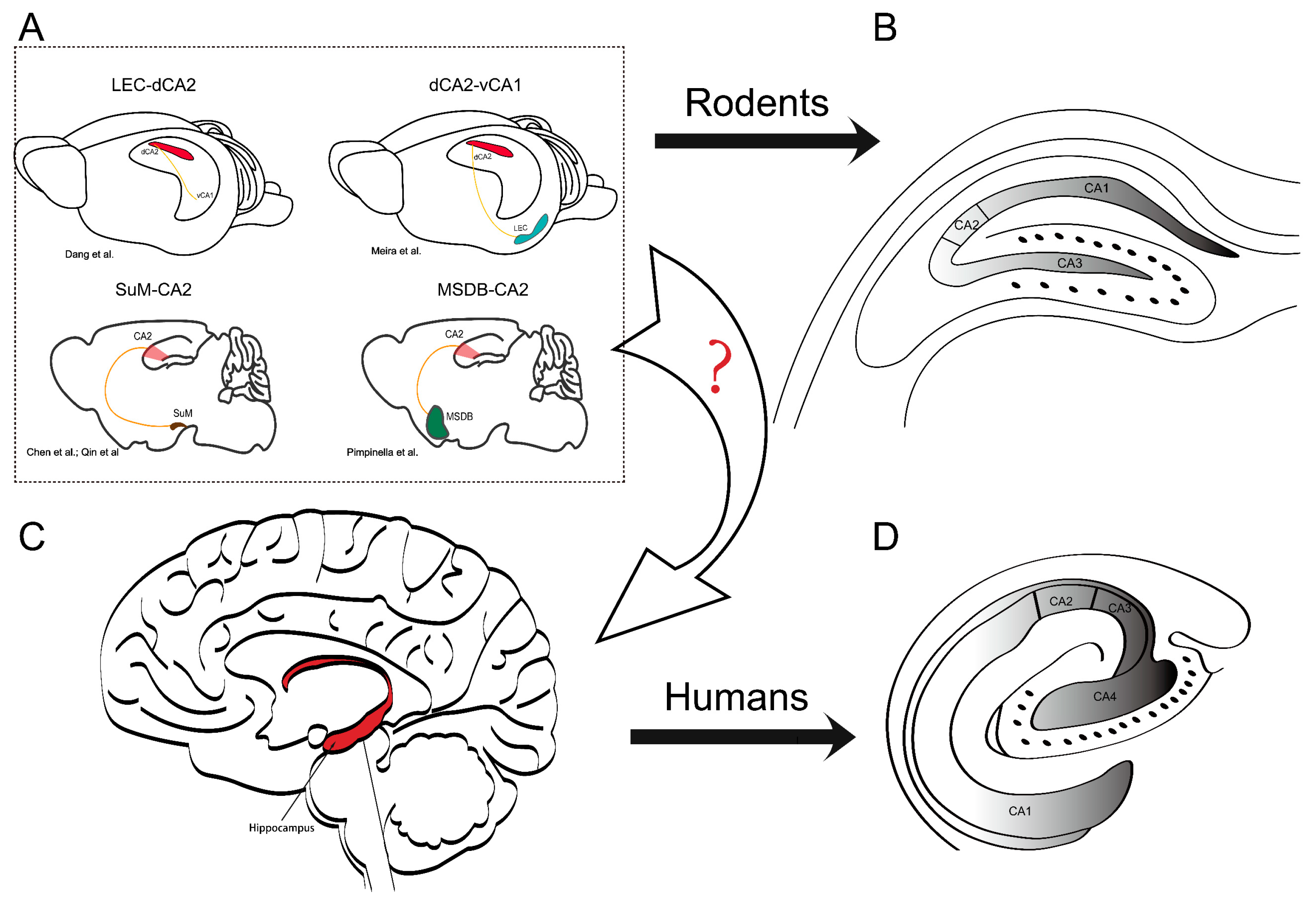
:1. Introduction
2. The Hippocampal Formation and the Unique Properties of the CA2 Region
3. Dopamine and Social Behavior in Parkinson’s Disease
4. Complex Changes in the Social Behavior of People with Parkinson’s Disease
5. Exploring the Role of CA2 in Parkinson’s Disease
| Medicine | Indication/Use | Effect on CA2 Region | Refs. |
|---|---|---|---|
| Allopathic Medicines | |||
| Vasopressin | Diabetes insipidus; cardiac arrest | LTP inhibition in EC-CA2 | [130] |
| Dantrolene | MH | Protection of ECS-induced apoptosis in CA2 | [131] |
| Ketamine | Anesthetics | Protect apoptosis induced by ECS in CA2 | [131] |
| Caffeine | N/A | Enhancement of synaptic transmission in CA2 | [39] |
| L-DOPA | PD | Restoration of CA2 volume in PD patients | [134] |
| Fluoxetine | Depression; OCD | Reduction in synaptic protein and GR expression in CA2 | [118] |
| Haloperidol | Schizophrenia; TS | Decrease in EAAT2 and NMDAR in the CA2 | [132,133] |
| Clozapine | Schizophrenia | Decrease in EAAT2 and NMDAR in the CA2 | [132,133] |
| Olanzapine | Schizophrenia | Decrease in NMDAR in the CA2 | [133] |
| Oxytocin | Delivery medication; autism | Rescue of social impairment in an autism model in association with SST neurons in CA2 | [41] |
| Traditional Chinese Medicines (TCMs) | |||
| CS 4-O-sulfation | N/A | Increase in PNNs and excitatory–inhibitory synapses in CA2 | [135] |
| NaoTaiFang | Activating blood and dissolving stasis | Protection of the CA2 neuronal population in cerebral ischemia | [137] |
| Dihydroartemisinin (extract from artemisinin) | Malaria | Protection against LPS-induced apoptosis in CA2 | [136] |
| For more information on specific herbs and TCM formulas for treating PD symptoms, please refer to the following reviews: | [138,139] | ||
6. Summary of the Role of CA2 in Non-Motor Symptoms of Parkinson’s Disease
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hague, S.M.; Klaffke, S.; Bandmann, O. Neurodegenerative disorders: Parkinson’s disease and Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat. Disord. 2016, 22 (Suppl. S1), S144–S148. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Buono, V.L.; Corallo, F.; Foti, M.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Nonmotor Symptoms in Parkinson Disease: A Descriptive Review on Social Cognition Ability. J. Geriatr. Psychiatry Neurol. 2017, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.W.; Smith-Petersen, G.A. Time perception and temporal order memory. Acta Psychol. 2014, 148, 173–180. [Google Scholar] [CrossRef]
- van Strien, N.M.; Cappaert, N.L.M.; Witter, M.P. The anatomy of memory: An interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 2009, 10, 272–282. [Google Scholar] [CrossRef]
- Goosens, K.A. Hippocampal regulation of aversive memories. Curr. Opin. Neurobiol. 2011, 21, 460–466. [Google Scholar] [CrossRef]
- Buzsáki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Felix-Ortiz, A.C.; Tye, K.M. Amygdala Inputs to the Ventral Hippocampus Bidirectionally Modulate Social Behavior. J. Neurosci. 2014, 34, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Fairént, A.; Regidort, J.; Kruger, L. The Cerebral Cortex of the Mouse (A First Contribution—The “Acoustic” Cortex). Somatosens. Mot. Res. 1992, 9, 3–36. [Google Scholar] [CrossRef]
- Dudek, S.M.; Alexander, G.M.; Farris, S. Rediscovering area CA2: Unique properties and functions. Nat. Rev. Neurosci. 2016, 17, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Lein, E.S.; Callaway, E.M.; Albright, T.D.; Gage, F.H. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J. Comp. Neurol. 2005, 485, 20426. [Google Scholar] [CrossRef]
- Insausti, R.; Muñoz-López, M.; Insausti, A.M. The CA2 hippocampal subfield in humans: A review. Hippocampus 2023, 33, 712–729. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Siegelbaum, S.A. Strong CA2 Pyramidal Neuron Synapses Define a Powerful Disynaptic Cortico-Hippocampal Loop. Neuron 2010, 66, 560–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohara, K.; Pignatelli, M.; Rivest, A.J.; Jung, H.-Y.; Kitamura, T.; Suh, J.; Frank, D.; Kajikawa, K.; Mise, N.; Obata, Y.; et al. Cell type–specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat. Neurosci. 2013, 17, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Simons, S.B.; Heldt, S.A.; Zhao, M.; Schroeder, J.P.; Vellano, C.P.; Cowan, D.P.; Ramineni, S.; Yates, C.K.; Feng, Y.; et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc. Natl. Acad. Sci. USA 2010, 107, 16994–16998. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Hosoya, A.; Yahagi, K.; Ferecskó, A.S.; Yaguchi, K.; Sík, A.; Itakura, M.; Takahashi, M.; Hirase, H. Hippocampal CA3 and CA2 have distinct bilateral innervation patterns to CA1 in rodents. Eur J Neurosci. 2012, 35, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.R.; Parra-Bueno, P.; Smirnov, M.S.; Lustberg, D.J.; Dudek, S.M.; Hepler, J.R.; Yasuda, R. RGS14 Restricts Plasticity in Hippocampal CA2 by Limiting Postsynaptic Calcium Signaling. eNeuro 2018, 5, ENEURO.0353-17. [Google Scholar] [CrossRef] [Green Version]
- Piskorowski, R.A.; Nasrallah, K.; Diamantopoulou, A.; Mukai, J.; Hassan, S.I.; Siegelbaum, S.A.; Gogos, J.A.; Chevaleyre, V. Age-Dependent Specific Changes in Area CA2 of the Hippocampus and Social Memory Deficit in a Mouse Model of the 22q11.2 Deletion Syndrome. Neuron 2016, 89, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Leroy, F.; Park, J.; Asok, A.; Brann, D.H.; Meira, T.; Boyle, L.M.; Buss, E.W.; Kandel, E.R.; Siegelbaum, S.A. A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 2018, 564, 213–218. [Google Scholar] [CrossRef]
- Young, W.; Li, J.; Wersinger, S.; Palkovits, M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 2006, 143, 1031–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVito, L.M.; Konigsberg, R.; Lykken, C.; Sauvage, M.; Young, W.S.; Eichenbaum, H. Vasopressin 1b Receptor Knock-Out Impairs Memory for Temporal Order. J. Neurosci. 2009, 29, 2676–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.S.; Avram, S.K.W.; Cymerblit-Sabba, A.; Song, J.; Young, W.S. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 2016, 21, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-T.; Hsieh, T.-Y.; Tsai, T.-C.; Chen, C.-C.; Huang, C.-C.; Hsu, K.-S. Conditional Deletion of Hippocampal CA2/CA3a Oxytocin Receptors Impairs the Persistence of Long-Term Social Recognition Memory in Mice. J. Neurosci. 2017, 38, 1218–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagani, J.H.; Zhao, M.; Cui, Z.; Avram, S.K.W.; Caruana, D.A.; Dudek, S.M.; Young, W.S. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 2014, 20, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.; Gerfen, C.R.; Young, W.S., 3rd. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 2013, 521, 1844–1866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hernández, V. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience 2013, 228, 139–162. [Google Scholar] [CrossRef]
- Chen, S.; He, L.; Huang, A.J.Y.; Boehringer, R.; Robert, V.; Wintzer, M.E.; Polygalov, D.; Weitemier, A.Z.; Tao, Y.; Gu, M.; et al. A hypothalamic novelty signal modulates hippocampal memory. Nature 2020, 586, 270–274. [Google Scholar] [CrossRef]
- Qin, H.; Fu, L.; Jian, T.; Jin, W.; Liang, M.; Li, J.; Chen, Q.; Yang, X.; Du, H.; Liao, X.; et al. REM sleep-active hypothalamic neurons may contribute to hippocampal social-memory consolidation. Neuron 2022, 110, 4000–4014.e6. [Google Scholar] [CrossRef]
- Dasgupta, A.; Baby, N.; Krishna, K.; Hakim, M.; Wong, Y.P.; Behnisch, T.; Soong, T.W.; Sajikumar, S. Substance P induces plasticity and synaptic tagging/capture in rat hippocampal area CA2. Proc. Natl. Acad. Sci. USA 2017, 114, E8741–E8749. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; Esclapez, M.; Alvarez, C.; Meyer, G.; Catala, M. Human and monkey fetal brain development of the supramammillary-hippocampal projections: A system involved in the regulation of theta activity. J. Comp. Neurol. 2000, 429, 515–529. [Google Scholar] [CrossRef]
- Meira, T.; Leroy, F.; Buss, E.W.; Oliva, A.; Park, J.; Siegelbaum, S.A. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 2018, 9, 4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, F.; Brann, D.H.; Meira, T.; Siegelbaum, S.A. Input-Timing-Dependent Plasticity in the Hippocampal CA2 Region and Its Potential Role in Social Memory. Neuron 2017, 95, 1089–1102.e5. [Google Scholar] [CrossRef]
- Alexander, G.M.; Brown, L.Y.; Farris, S.; Lustberg, D.; Pantazis, C.; Gloss, B.; Plummer, N.W.; Jensen, P.; Dudek, S.M. CA2 neuronal activity controls hippocampal low gamma and ripple oscillations. eLife. 2018, 7, e38052. [Google Scholar] [CrossRef]
- Oliva, A.; Fernández-Ruiz, A.; Leroy, F.; Siegelbaum, S.A. Hippocampal CA2 sharp-wave ripples reactivate and promote social memory. Nature 2020, 587, 264–269. [Google Scholar] [CrossRef]
- Srinivas, K.V.; Buss, E.W.; Sun, Q.; Santoro, B.; Takahashi, H.; Nicholson, D.A.; Siegelbaum, S.A. The Dendrites of CA2 and CA1 Pyramidal Neurons Differentially Regulate Information Flow in the Cortico-Hippocampal Circuit. J Neurosci. 2017, 37, 3276–3293. [Google Scholar] [CrossRef]
- McCann, K.E.; Lustberg, D.J.; Shaughnessy, E.K.; Carstens, K.E.; Farris, S.; Alexander, G.M.; Radzicki, D.; Zhao, M.; Dudek, S.M. Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Mol. Psychiatry 2019, 26, 350–364. [Google Scholar] [CrossRef]
- Simons, S.B.; Caruana, D.A.; Zhao, M.; Dudek, S.M. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat. Neurosci. 2011, 15, 23–25. [Google Scholar] [CrossRef]
- Caruana, D.; Dudek, S.M. Adenosine A1 Receptor-Mediated Synaptic Depression in the Developing Hippocampal Area CA2. Front. Synaptic Neurosci. 2020, 12, 21. [Google Scholar] [CrossRef]
- Bertoni, A.; Schaller, F.; Tyzio, R.; Gaillard, S.; Santini, F.; Xolin, M.; Diabira, D.; Vaidyanathan, R.; Matarazzo, V.; Medina, I.; et al. Oxytocin administration in neonates shapes hippocampal circuitry and restores social behavior in a mouse model of autism. Mol. Psychiatry 2021, 26, 7582–7595. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Lim, Y.J.; Kumar, K.; Baby, N.; Pang, K.L.K.; Benoy, A.; Behnisch, T.; Sajikumar, S. Group III metabotropic glutamate receptors gate long-term potentiation and synaptic tagging/capture in rat hippocampal area CA2. eLife 2020, 9, 55344. [Google Scholar] [CrossRef]
- Robert, V.; Therreau, L.; Davatolhagh, M.F.; Bernardo-Garcia, F.J.; Clements, K.N.; Chevaleyre, V.; Piskorowski, R.A. The mechanisms shaping CA2 pyramidal neuron action potential bursting induced by muscarinic acetylcholine receptor activation. J. Gen. Physiol. 2020, 152, 12462. [Google Scholar] [CrossRef] [PubMed]
- Benoy, A.; Bin Ibrahim, M.Z.; Behnisch, T.; Sajikumar, S. Metaplastic Reinforcement of Long-Term Potentiation in Hippocampal Area CA2 by Cholinergic Receptor Activation. J. Neurosci. 2021, 41, 9082–9098. [Google Scholar] [CrossRef]
- Nouraei, N.; Mason, D.M.; Miner, K.M.; Carcella, M.A.; Bhatia, T.N.; Dumm, B.K.; Soni, D.; Johnson, D.A.; Luk, K.C.; Leak, R.K. Critical appraisal of pathology transmission in the α-synuclein fibril model of Lewy body disorders. Exp. Neurol. 2017, 299, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Grossman, M.; Weintraub, D.; I Hurtig, H.; Duda, J.E.; Xie, S.X.; Lee, E.B.; Van Deerlin, V.M.; Lopez, O.L.; Kofler, J.K.; et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017, 16, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, D.H.; Roy, S.; Salmon, D.P.; Galasko, D.R.; Hansen, L.A.; Masliah, E.; Gage, F.H. Hippocampal α-Synuclein in Dementia with Lewy Bodies Contributes to Memory Impairment and Is Consistent with Spread of Pathology. J. Neurosci. 2016, 37, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Churchyard, A.; Lees, A.J. The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson’s disease. Neurology 1997, 49, 1570–1576. [Google Scholar] [CrossRef]
- Trudler, D.; Sanz-Blasco, S.; Eisele, Y.S.; Ghatak, S.; Bodhinathan, K.; Akhtar, M.W.; Lynch, W.P.; Piña-Crespo, J.C.; Talantova, M.; Kelly, J.W.; et al. α-Synuclein Oligomers Induce Glutamate Release from Astrocytes and Excessive Extrasynaptic NMDAR Activity in Neurons, Thus Contributing to Synapse Loss. J. Neurosci. 2021, 41, 2264–2273. [Google Scholar] [CrossRef]
- Kalaitzakis, M.; Pearce, R.; Gentleman, S. Clinical correlates of pathology in the claustrum in Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 2009, 461, 12–15. [Google Scholar] [CrossRef]
- Flores-Cuadrado, A.; Ubeda-Bañon, I.; Saiz-Sanchez, D.; de la Rosa-Prieto, C.; Martinez-Marcos, A. Hippocampal α-synuclein and interneurons in Parkinson’s disease: Data from human and mouse models. Mov. Disord. 2016, 31, 979–988. [Google Scholar] [CrossRef]
- Maki, R.A.; Holzer, M.; Motamedchaboki, K.; Malle, E.; Masliah, E.; Marsche, G.; Reynolds, W.F. Human myeloperoxidase (hMPO) is expressed in neurons in the substantia nigra in Parkinson’s disease and in the hMPO-α-synuclein-A53T mouse model, correlating with increased nitration and aggregation of α-synuclein and exacerbation of motor impairment. Free. Radic. Biol. Med. 2019, 141, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Reyes, S.; Landeck, N.; Bye, C.; Leanza, G.; Double, K.; Thompson, L.; Halliday, G.; Kirik, D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 2014, 137, 2493–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.K.L.; Chau, T.W.; Lim, E.J.; Ahmed, I.; Chang, R.C.-C.; Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K.B. Hippocampal CA2 Lewy pathology is associated with cholinergic degeneration in Parkinson’s disease with cognitive decline. Acta Neuropathol. Commun. 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Zinn, C.G.; Clairis, N.; Cavalcante, L.E.S.; Furini, C.R.G.; Myskiw, J.D.C.; Izquierdo, I. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc. Natl. Acad. Sci. USA 2016, 113, 1609883113. [Google Scholar] [CrossRef]
- Kahnt, T.; Tobler, P.N. Dopamine regulates stimulus generalization in the human hippocampus. eLife 2016, 5, e12678. [Google Scholar] [CrossRef] [Green Version]
- Trezza, V.; Vanderschuren, L.J.M.J. Divergent Effects of Anandamide Transporter Inhibitors with Different Target Selectivity on Social Play Behavior in Adolescent Rats. Experiment 2008, 328, 343–350. [Google Scholar] [CrossRef]
- Achterberg, M.; Van Kerkhof, L.W.M.; Servadio, M.; van Swieten, M.; Houwing, D.J.; Aalderink, M.; Driel, N.V.; Trezza, V.; Vanderschuren, L.J.M.J. Contrasting Roles of Dopamine and Noradrenaline in the Motivational Properties of Social Play Behavior in Rats. Neuropsychopharmacology 2015, 41, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Barik, J.; Marti, F.; Morel, C.; Fernandez, S.P.; Lanteri, C.; Godeheu, G.; Tassin, J.-P.; Mombereau, C.; Faure, P.; Tronche, F. Chronic Stress Triggers Social Aversion via Glucocorticoid Receptor in Dopaminoceptive Neurons. Science 2013, 339, 332–335. [Google Scholar] [CrossRef]
- Gabriel, P.; Mastracchio, T.-A.; Bordner, K.; Jeffrey, R. Impact of enriched environment during adolescence on adult social behavior, hippocampal synaptic density and dopamine D2 receptor expression in rats. Physiol. Behav. 2020, 226, 113133. [Google Scholar] [CrossRef] [PubMed]
- Locke, T.M.; Soden, M.E.; Miller, S.M.; Hunker, A.; Knakal, C.; Licholai, J.A.; Dhillon, K.S.; Keene, C.D.; Zweifel, L.S.; Carlson, E.S. Dopamine D1 Receptor–Positive Neurons in the Lateral Nucleus of the Cerebellum Contribute to Cognitive Behavior. Biol. Psychiatry 2018, 84, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bariselli, S.; Tzanoulinou, S.; Glangetas, C.; Prévost-Solié, C.; Pucci, L.; Viguié, J.; Bezzi, P.; O’Connor, E.C.; Georges, F.; Lüscher, C.; et al. SHANK3 controls maturation of social reward circuits in the VTA. Nat. Neurosci. 2016, 19, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, G.A.; Nieh, E.H.; Vander Weele, C.M.; Halbert, S.A.; Pradhan, R.V.; Yosafat, A.S.; Glober, G.F.; Izadmehr, E.M.; Thomas, R.E.; Lacy, G.D.; et al. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 2016, 164, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; He, Y.; Chen, X.; Tian, Y.; Cheng, K.; Zhang, L.; Wang, Y.; Yang, D.; Wang, H.; Wu, Z.; et al. Validation of the targeted metabolomic pathway in the hippocampus and comparative analysis with the prefrontal cortex of social defeat model mice. J. Neurochem. 2018, 149, 799–810. [Google Scholar] [CrossRef]
- Hjorth, O.R.; Frick, A.; Gingnell, M.; Hoppe, J.M.; Faria, V.; Hultberg, S.; Alaie, I.; Månsson, K.N.T.; Wahlstedt, K.; Jonasson, M.; et al. Expression and co-expression of serotonin and dopamine transporters in social anxiety disorder: A multitracer positron emission tomography study. Mol. Psychiatry 2021, 26, 3970–3979. [Google Scholar] [CrossRef]
- Xing, B.; Mack, N.R.; Guo, K.-M.; Zhang, Y.-X.; Ramirez, B.; Yang, S.-S.; Lin, L.; Wang, D.V.; Li, Y.-C.; Gao, W.-J. A Subpopulation of Prefrontal Cortical Neurons Is Required for Social Memory. Biol. Psychiatry 2020, 89, 521–531. [Google Scholar] [CrossRef]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. S1), 14–20. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.-Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef]
- Wen, M.; Chan, L.L.; Tan, L.C.S.; Tan, E.K. Depression, anxiety, and apathy in Parkinson’s disease: Insights from neuroimaging studies. Eur. J. Neurol. 2016, 23, 1001–1019. [Google Scholar] [CrossRef] [Green Version]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Channon, S. Frontal lobe dysfunction and everyday problem-solving: Social and non-social contributions. Acta Psychol. 2004, 115, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.G.; Millett, A.C. The closing of the theory of mind: A critique of perspective-taking. Psychon. Bull. Rev. 2019, 26, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Tsuruya, N.; Kobayakawa, M.; Kawamura, M. Is “reading mind in the eyes” impaired in Parkinson’s disease? Park. Relat. Disord. 2011, 17, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Mckinlay, A.; Albicini, M.; Kavanagh, P.S. The effect of cognitive status and visuospatial performance on affective theory of mind in Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2013, 9, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Poletti, M.; Vergallo, A.; Ulivi, M.; Sonnoli, A.; Bonuccelli, U. Affective theory of mind in patients with Parkinson’s disease. Psychiatry Clin. Neurosci. 2013, 67, 273–276. [Google Scholar] [CrossRef]
- Bodden, M.E.; Mollenhauer, B.; Trenkwalder, C.; Cabanel, N.; Eggert, K.M.; Unger, M.M.; Oertel, W.H.; Kessler, J.; Dodel, R.; Kalbe, E. Affective and cognitive theory of mind in patients with parkinson’s disease. Park. Relat. Disord. 2010, 16, 466–470. [Google Scholar] [CrossRef]
- Yu, R.-L.; Wu, R.-M.; Chiu, M.-J.; Tai, C.-H.; Lin, C.-H.; Hua, M.-S. Advanced Theory of Mind in patients at early stage of Parkinson’s disease. Park. Relat. Disord. 2012, 18, 21–24. [Google Scholar] [CrossRef]
- Xi, C.; Zhu, Y.; Mu, Y.; Chen, B.; Dong, B.; Cheng, H.; Hu, P.; Zhu, C.; Wang, K. Theory of mind and decision-making processes are impaired in Parkinson’s disease. Behav. Brain Res. 2015, 279, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Mimura, M.; Oeda, R.; Kawamura, M. Impaired decision-making in Parkinson’s disease. Parkinsonism Relat. Disord. 2006, 12, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, M.; Koyama, S.; Mimura, M.; Kawamura, M. Decision making in Parkinson’s disease: Analysis of behavioral and physiological patterns in the Iowa gambling task. Mov. Disord. 2007, 23, 547–552. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.; Durso, R.; Brown, A.; Lynch, A. Counterfactual cognitive deficit in persons with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Rosen, J.B.; Brand, M.; Polzer, C.; Ebersbach, G.; Kalbe, E. Moral decision-making and theory of mind in patients with idiopathic Parkinson’s disease. Neuropsychology 2013, 27, 562–572. [Google Scholar] [CrossRef]
- Starcke, K.; Polzer, C.; Wolf, O.T.; Brand, M. Does stress alter everyday moral decision-making? Psychoneuroendocrinology 2011, 36, 210–219. [Google Scholar] [CrossRef]
- Anderson, R.J.; Simpson, A.C.; Channon, S.; Samuel, M.; Brown, R.G. Social problem solving, social cognition, and mild cognitive impairment in Parkinson’s disease. Behav. Neurosci. 2013, 127, 184–192. [Google Scholar] [CrossRef]
- Santangelo, G.; Vitale, C.; Trojano, L.; Errico, D.; Amboni, M.; Barbarulo, A.M.; Grossi, D.; Barone, P. Neuropsychological correlates of theory of mind in patients with early Parkinson’s disease. Mov. Disord. 2011, 27, 98–105. [Google Scholar] [CrossRef]
- Esteves, S.; Gleichgerrcht, E.; Torralva, T.; Chade, A.; Arévalo, G.G.; Gershanik, O.; Manes, F.; Roca, M. Performance of Patients with Early Parkinson Disease on an Executive and Social Cognition Battery. Cogn. Behav. Neurol. 2018, 31, 142–150. [Google Scholar] [CrossRef]
- De Risi, M.; Di Gennaro, G.; Picardi, A.; Casciato, S.; Grammaldo, L.G.; D’Aniello, A.; Lanni, D.; Meletti, S.; Modugno, N. Facial emotion decoding in patients with Parkinson’s disease. Int. J. Neurosci. 2017, 128, 71–78. [Google Scholar] [CrossRef]
- Multani, N.; Taghdiri, F.; Anor, C.J.; Varriano, B.; Misquitta, K.; Tang-Wai, D.F.; Keren, R.; Fox, S.; Lang, A.E.; Vijverman, A.C.; et al. Association Between Social Cognition Changes and Resting State Functional Connectivity in Frontotemporal Dementia, Alzheimer’s Disease, Parkinson’s Disease, and Healthy Controls. Front. Neurosci. 2019, 13, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narme, P.; Bonnet, A.-M.; Dubois, B.; Chaby, L. Understanding facial emotion perception in Parkinson’s disease: The role of configural processing. Neuropsychologia 2011, 49, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Pietschnig, J.; Schröder, L.; Ratheiser, I.; Kryspin-Exner, I.; Pflüger, M.; Moser, D.; Auff, E.; Pirker, W.; Pusswald, G.; Lehrner, J. Facial emotion recognition and its relationship to cognition and depressive symptoms in patients with Parkinson’s disease. Int. Psychogeriatrics 2016, 28, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Sedda, A.; Petito, S.; Guarino, M.; Stracciari, A. Identification and intensity of disgust: Distinguishing visual, linguistic and facial expressions processing in Parkinson disease. Behav. Brain Res. 2017, 330, 30–36. [Google Scholar] [CrossRef]
- Lawrence, A.D.; Goerendt, I.K.; Brooks, D.J. Impaired recognition of facial expressions of anger in Parkinson’s disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia 2007, 45, 65–74. [Google Scholar] [CrossRef]
- Cohen, H.; Gagné, M.-H.; Hess, U.; Pourcher, E. Emotion and object processing in Parkinson’s disease. Brain Cogn. 2010, 72, 457–463. [Google Scholar] [CrossRef]
- Péron, J.; Vicente, S.; Leray, E.; Drapier, S.; Drapier, D.; Cohen, R.; Biseul, I.; Rouaud, T.; Le Jeune, F.; Sauleau, P.; et al. Are dopaminergic pathways involved in theory of mind? A study in Parkinson’s disease. Neuropsychologia 2009, 47, 406–414. [Google Scholar] [CrossRef]
- Roca, M.; Torralva, T.; Gleichgerrcht, E.; Chade, A.; Arévalo, G.G.; Gershanik, O.; Manes, F. Impairments in Social Cognition in Early Medicated and Unmedicated Parkinson Disease. Cogn. Behav. Neurol. 2010, 23, 152–158. [Google Scholar] [CrossRef]
- Heinrichs, M.; von Dawans, B.; Domes, G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocr. 2009, 30, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Montanez-Miranda, C.; Bramlett, S.N.; Hepler, J.R. RGS14 expression in CA2 hippocampus, amygdala, and basal ganglia: Implications for human brain physiology and disease. Hippocampus 2022, 33, 166–181. [Google Scholar] [CrossRef]
- Quiroga, R.Q. Concept cells: The building blocks of declarative memory functions. Nat. Rev. Neurosci. 2012, 13, 587–597. [Google Scholar] [CrossRef]
- Cao, R.; Lin, C.; Brandmeir, N.J.; Wang, S. A human single-neuron dataset for face perception. Sci. Data 2022, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Montagrin, A.; Saiote, C.; Schiller, D. The social hippocampus. Hippocampus 2017, 28, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Trinkler, I.; King, J.A.; Doeller, C.F.; Rugg, M.D.; Burgess, N. Neural bases of autobiographical support for episodic recollection of faces. Hippocampus 2009, 19, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Banker, S.M.; Pagliaccio, D.; Ramphal, B.; Thomas, L.; Dranovsky, A.; Margolis, A.E. Altered structure and functional connectivity of the hippocampus are associated with social and mathematical difficulties in nonverbal learning disability. Hippocampus 2020, 31, 79–88. [Google Scholar] [CrossRef]
- Raam, T.; McAvoy, K.M.; Besnard, A.; Veenema, A.H.; Sahay, A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun. 2017, 8, 2001. [Google Scholar] [CrossRef] [Green Version]
- Diethorn, E.J.; Gould, E. Postnatal development of hippocampal CA2 structure and function during the emergence of social recognition of peers. Hippocampus 2022, 33, 208–222. [Google Scholar] [CrossRef]
- Raghuraman, R.; Navakkode, S.; Sajikumar, S. Alteration of hippocampal CA2 plasticity and social memory in adult rats impacted by juvenile stress. Hippocampus 2023, 33, 745–758. [Google Scholar] [CrossRef]
- Maletta, T.; Palummieri, M.; Correa, J.; Holahan, M.R. Preadolescent exposure to a sexually mature, unrelated male rat reduces postadolescent social recognition memory and CA2 c-Fos labeling. Front. Behav. Neurosci. 2023, 17, 1104866. [Google Scholar] [CrossRef]
- Lisgaras, C.P.; Oliva, A.; Mckenzie, S.; LaFrancois, J.; Siegelbaum, S.A.; Scharman, H.E. Hippocampal area CA2 controls seizure dynamics, interictal EEG abnormalities and social comorbidity in mouse models of temporal lobe epilepsy. BioRxiv 2023. [Google Scholar] [CrossRef]
- Rey, C.C.; Robert, V.; Bouisset, G.; Loisy, M.; Lopez, S.; Cattaud, V.; Lejards, C.; Piskorowski, R.A.; Rampon, C.; Chevaleyre, V.; et al. Altered inhibitory function in hippocampal CA2 contributes in social memory deficits in Alzheimer’s mouse model. iScience 2022, 25, 103895. [Google Scholar] [CrossRef] [PubMed]
- Carstens, K.E.; Lustberg, D.J.; Shaughnessy, E.K.; McCann, K.E.; Alexander, G.M.; Dudek, S.M. Perineuronal net degradation rescues CA2 plasticity in a mouse model of Rett syndrome. J. Clin. Investig. 2021, 131, jci137221. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, S.; Rey, C.C.; Therreau, L.; Fanton, A.; Massotte, D.; Verret, L.; Piskorowski, R.A.; Chevaleyre, V. Maturation of PNN and ErbB4 Signaling in Area CA2 during Adolescence Underlies the Emergence of PV Interneuron Plasticity and Social Memory. Cell Rep. 2019, 29, 1099–1112.e4. [Google Scholar] [CrossRef] [Green Version]
- Wersinger, S.R.; Kelliher, K.R.; Zufall, F.; Lolait, S.J.; O’Carroll, A.-M.; Young, W.S. Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm. Behav. 2004, 46, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Rojas, J.; de Solis, C.A.; Leroy, F.; Kandel, E.R.; Siegelbaum, S.A. A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron 2022, 110, 1559–1572.e4. [Google Scholar] [CrossRef]
- Cope, E.C.; Wang, S.H.; Waters, R.C.; Gore, I.R.; Vasquez, B.; Laham, B.J.; Gould, E. Activation of the CA2-ventral CA1 pathway reverses social discrimination dysfunction in Shank3B knockout mice. Nat. Commun. 2023, 14, 1750. [Google Scholar] [CrossRef]
- Gemmel, M.; Hazlett, M.; Bögi, E.; De Lacalle, S.; Hill, L.A.; Kokras, N.; Hammond, G.L.; Dalla, C.; Charlier, T.D.; Pawluski, J.L. Perinatal fluoxetine effects on social play, the HPA system, and hippocampal plasticity in pre-adolescent male and female rats: Interactions with pre-gestational maternal stress. Psychoneuroendocrinology 2017, 84, 159–171. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Piskorowski, R. Hippocampal Area CA2: An Overlooked but Promising Therapeutic Target. Trends Mol. Med. 2016, 22, 645–655. [Google Scholar] [CrossRef]
- Nagano-Saito, A.; Habak, C.; Mejía-Constaín, B.; Degroot, C.; Monetta, L.; Jubault, T.; Bedetti, C.; Lafontaine, A.-L.; Chouinard, S.; Soland, V.; et al. Effect of mild cognitive impairment on the patterns of neural activity in early Parkinson’s disease. Neurobiol. Aging 2014, 35, 223–231. [Google Scholar] [CrossRef]
- Pang, C.C.; Kiecker, C.; O’Brien, J.T.; Noble, W.; Chang, R.C. Ammon’s Horn 2 (CA2) of the Hippocampus: A Long-Known Region with a New Potential Role in Neurodegeneration. Neurosci. 2018, 25, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Cinar, E.; Yalcin-Cakmakli, G.; Saka, E.; Ulusoy, A.; Yuruker, S.; Elibol, B.; Tel, B.C. Modelling cognitive deficits in Parkinson’s disease: Is CA2 a gateway for hippocampal synucleinopathy? Exp. Neurol. 2020, 330, 113357. [Google Scholar] [CrossRef]
- Norwood, B.A.; Bumanglag, A.V.; Osculati, F.; Sbarbati, A.; Marzola, P.; Nicolato, E.; Fabene, P.F.; Sloviter, R.S. Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J. Comp. Neurol. 2010, 518, 3381–3407. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, W.L.; Dhillon, K.; Harper, L.; Espin, J.; MacIntosh, T.K.; Smith, D.H.; Graham, D.I. There Is Differential Loss of Pyramidal Cells from the Human Hippocampus with Survival after Blunt Head Injury. J. Neuropathol. Exp. Neurol. 2003, 62, 272–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steve, T.A.; Jirsch, J.D.; Gross, D.W. Quantification of subfield pathology in hippocampal sclerosis: A systematic review and meta-analysis. Epilepsy Res. 2014, 108, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.B.; Escobedo, Y.; Yasuda, R.; Dudek, S.M. Regional differences in hippocampal calcium handling provide a cellular mechanism for limiting plasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14080–14084. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Zhou, Y.; Zhang, Y.; Liu, D.; Wu, M.; Liu, A.; Jia, Z.; Xie, W. Regulation of Social Memory by Lateral Entorhinal Cortical Projection to Dorsal Hippocampal CA2. Neurosci. Bull. 2022, 38, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Pimpinella, D.; Mastrorilli, V.; Giorgi, C.; Coemans, S.; Lecca, S.; Lalive, A.L.; Ostermann, H.; Fuchs, E.C.; Monyer, H.; Mele, A.; et al. Septal cholinergic input to CA2 hippocampal region controls social novelty discrimination via nicotinic receptor-mediated disinhibition. eLife 2021, 10, 65580. [Google Scholar] [CrossRef]
- Duvernoy, H.; Françoise, C.; Fatterpekar, G.; Naidich, T.H. The Human Hippocampus, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chafai, M.; Corbani, M.; Guillon, G.; Desarménien, M.G. Vasopressin Inhibits LTP in the CA2 Mouse Hippocampal Area. PLoS ONE 2012, 7, e49708. [Google Scholar] [CrossRef] [Green Version]
- Gursoy, I.D.; Barun, S.; Erdem, R.; Keskin, U.; Kiziltas, M.; Atilla, P.; Muftuoglu, S.; Yuce, D.; Narin, F.; Ertunc, M.; et al. Investigation of the possible protective effects of ketamine and dantrolene on the hippocampal apoptosis and spatial learning in rats exposed to electroconvulsive seizures as a model of status epilepticus. Turk. Neurosurg. 2020, 30, 871–884. [Google Scholar] [CrossRef]
- Schmitt, A.; Zink, M.; Petroianu, G.; May, B.; Braus, D.F.; Henn, F.A. Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain. Neurosci. Lett. 2003, 347, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Krzystanek, M.; Bogus, K.; Palasz, A.; Krzystanek, E.; Worthington, J.J.; Wiaderkiewicz, R. Effects of long-term treatment with the neuroleptics haloperidol, clozapine and olanzapine on immunoexpression of NMDA receptor subunits NR1, NR2A and NR2B in the rat hippocampus. Pharmacol. Rep. 2015, 67, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Györfi, O.; Nagy, H.; Bokor, M.; Moustafa, A.A.; Rosenzweig, I.; Kelemen, O.; Kéri, S. Reduced CA2–CA3 Hippocampal Subfield Volume Is Related to Depression and Normalized by l-DOPA in Newly Diagnosed Parkinson’s Disease. Front. Neurol. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Joffrin, A.M.; Zhao, Y.; Miller, G.M.; Zhang, G.C.; Oka, Y.; Hsieh-Wilson, L.C. Chondroitin 4- O -sulfation regulates hippocampal perineuronal nets and social memory. Acad. Sci. 2023, 120, e2301312120. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, M.; Zhong, S.; Feng, C.; Nwobodo, A.K.; Chen, B.; Song, Y.; Wang, Y. Dihydroartemisinin ameliorates LPS-induced neuroinflammation by inhibiting the PI3K/AKT pathway. Metab. Brain Dis. 2020, 35, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xia, X.; Wang, G.-Z.; Shi, Y.-M.; Ge, J.-W. Naotaifang extract treatment results in increased ferroportin expression in the hippocampus of rats subjected to cerebral ischemia. Mol. Med. Rep. 2015, 11, 4047–4052. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zhang, J.; Wang, C.; Chai, Y.-H.; Wu, A.-G.; Huang, N.-Y.; Wang, L. The pathogenesis and treatment mechanism of Parkinson’s disease from the perspective of traditional Chinese medicine. Phytomedicine 2022, 100, 154044. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Huang, P.; Luo, Y.; Shi, Y.; Ma, P. The potential applications of traditional Chinese medicine in Parkinson’s disease: A new opportunity. Biomed. Pharmacother. 2022, 149, 112866. [Google Scholar] [CrossRef]
| Name | Function | References |
|---|---|---|
| PCP4 | Identification of the DG and CA2 regions | [12,15] |
| RGS14 | Restriction of CA2 synaptic plasticity | [15,16,18] |
| STEP | LTP inhibition at EC-CA2 synapses | [15] |
| A1R | LTD enhancement at SC-CA2 synapses | [39,40] |
| AVPR1B | Enhancement of synaptic potentiation at SC-CA2 synapses Facilitation of social behavior | [21,24,26] |
| OXTR | Enhancement of synaptic potentiation at SC-CA2 synapses Facilitation of social behavior | [26,41] |
| MRs; | Facilitation of CA2-dependent behaviors | [38] |
| group III mGluRs; | Restriction of CA2 synaptic plasticity | [42] |
| cholinergic receptors | Induction of LTD at SC and EC CA2 synapses | [43,44] |
| Related to PD | ||
| Substance P | Induction of SC and EC-CA2 synaptic plasticity | [31] |
| α-synuclein | Controversial | [45,46,47,48,49,50,51,52,53,54] |
| Social Symptoms | Related Molecules or Factors | Species | References |
|---|---|---|---|
| Social Behavior and CA2 | |||
| Social recognition memory | OXT/OXTR | Mouse | [25,41,107] |
| Ageing | Mouse | [108] | |
| AVPR1B | Mouse | [24] | |
| CA2 pyramidal neurons | Mouse | [22] | |
| Juvenile stress | Rat; mouse | [109,110] | |
| High-frequency oscillations in CA2 neurons | Mouse | [111] | |
| PV interneurons and PNN | Mouse | [112,113,114] | |
| Social novelty recognition | SuM-CA2 synapse | Mouse | [29] |
| Social aggression | AVPR1B | Mouse | [20,26] |
| CA2-LS | Mouse | [20] | |
| Social motivation | AVPR1B | Mouse | [115] |
| Social discrimination | LEC-CA2 | Mouse | [116] |
| Shank3B | Mouse | [117] | |
| Sociability and social interaction | Juvenile stress | Rat | [109] |
| Perinatal fluoxetine | Mouse | [118] | |
| Social cognition | Schizophrenia patients; CA2 PV+ interneurons | Human; mouse | [119] |
| Human Hippocampus and Social Behavior | |||
| Social recognition | Healthy people | Human | [104,105] |
| Social function | Connectivity of hippocampus in NVLD patients | Human | [106] |
| Face perception | Healthy people | Human | [103] |
| Social memory: familiar face and name recognition deficits | Case study | Human | [102] |
| Human Social Behavior and CA2-Related Molecular Targets | |||
| Social stress and anxiety; social cognition and social approach; social behavior | Oxytocin; vasopressin | Human | [99,100] |
| Social anxiety; social discrimination; social behavior; social memory | RGS14 | Human | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Behnisch, T. The Enigmatic CA2: Exploring the Understudied Region of the Hippocampus and Its Involvement in Parkinson’s Disease. Biomedicines 2023, 11, 1996. https://doi.org/10.3390/biomedicines11071996
Zhao F, Behnisch T. The Enigmatic CA2: Exploring the Understudied Region of the Hippocampus and Its Involvement in Parkinson’s Disease. Biomedicines. 2023; 11(7):1996. https://doi.org/10.3390/biomedicines11071996
Chicago/Turabian StyleZhao, Fang, and Thomas Behnisch. 2023. "The Enigmatic CA2: Exploring the Understudied Region of the Hippocampus and Its Involvement in Parkinson’s Disease" Biomedicines 11, no. 7: 1996. https://doi.org/10.3390/biomedicines11071996
APA StyleZhao, F., & Behnisch, T. (2023). The Enigmatic CA2: Exploring the Understudied Region of the Hippocampus and Its Involvement in Parkinson’s Disease. Biomedicines, 11(7), 1996. https://doi.org/10.3390/biomedicines11071996





