Comparative Study of the Cytokine Profiles of Serum and Tissues from Patients with the Ossification of the Posterior Longitudinal Ligament
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Spinal Ligament and Serum Samples
2.3. Histologic Examination
2.4. Cytokine Arrays
2.5. Comparison of Serum and Tissue Levels
2.6. Western Blotting
2.7. Immunohistochemistry
2.8. Statistical Analysis
3. Results
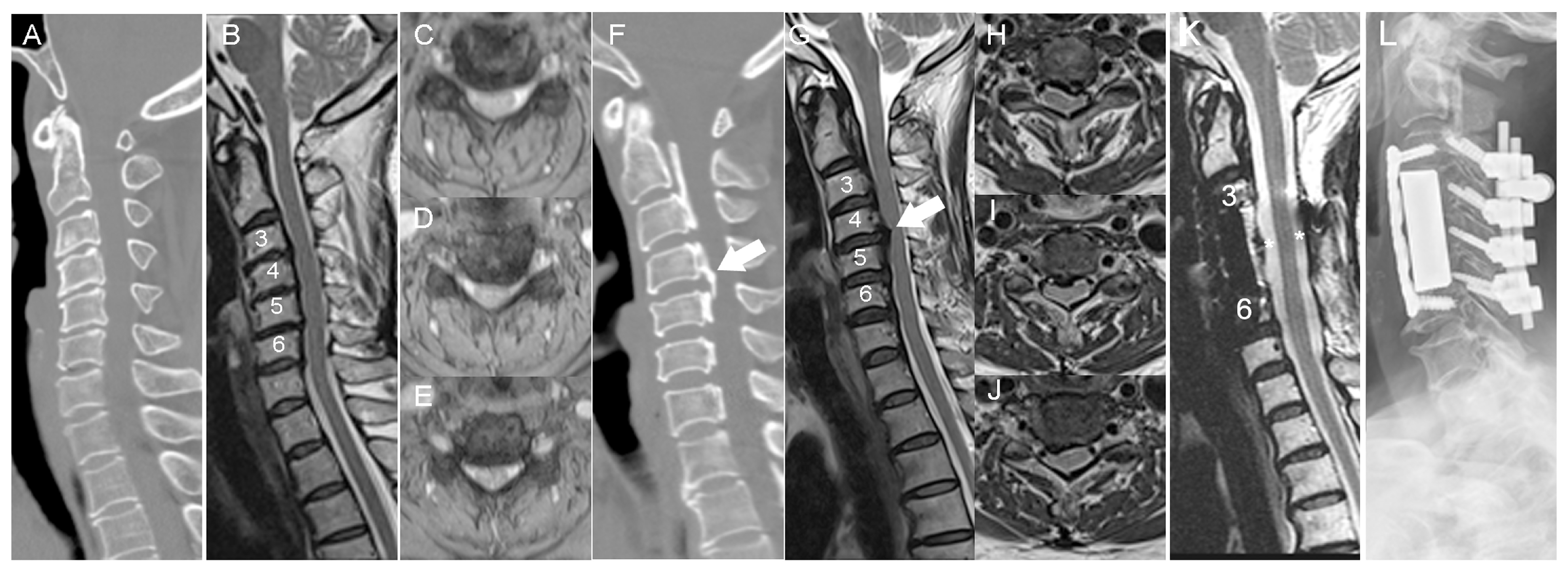
3.1. Patient Demographics
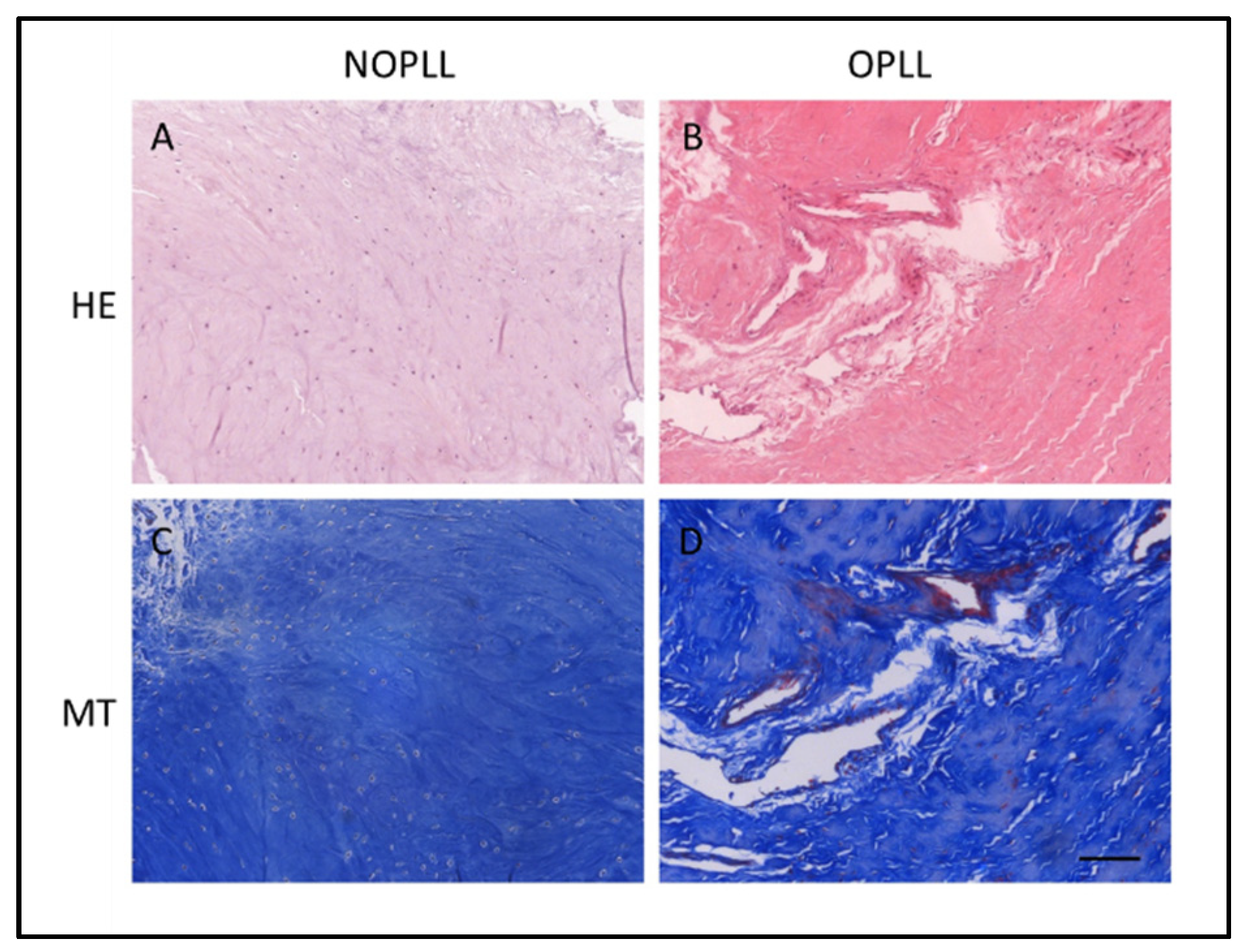
3.2. Histological Staining
3.3. Serum Cytokine
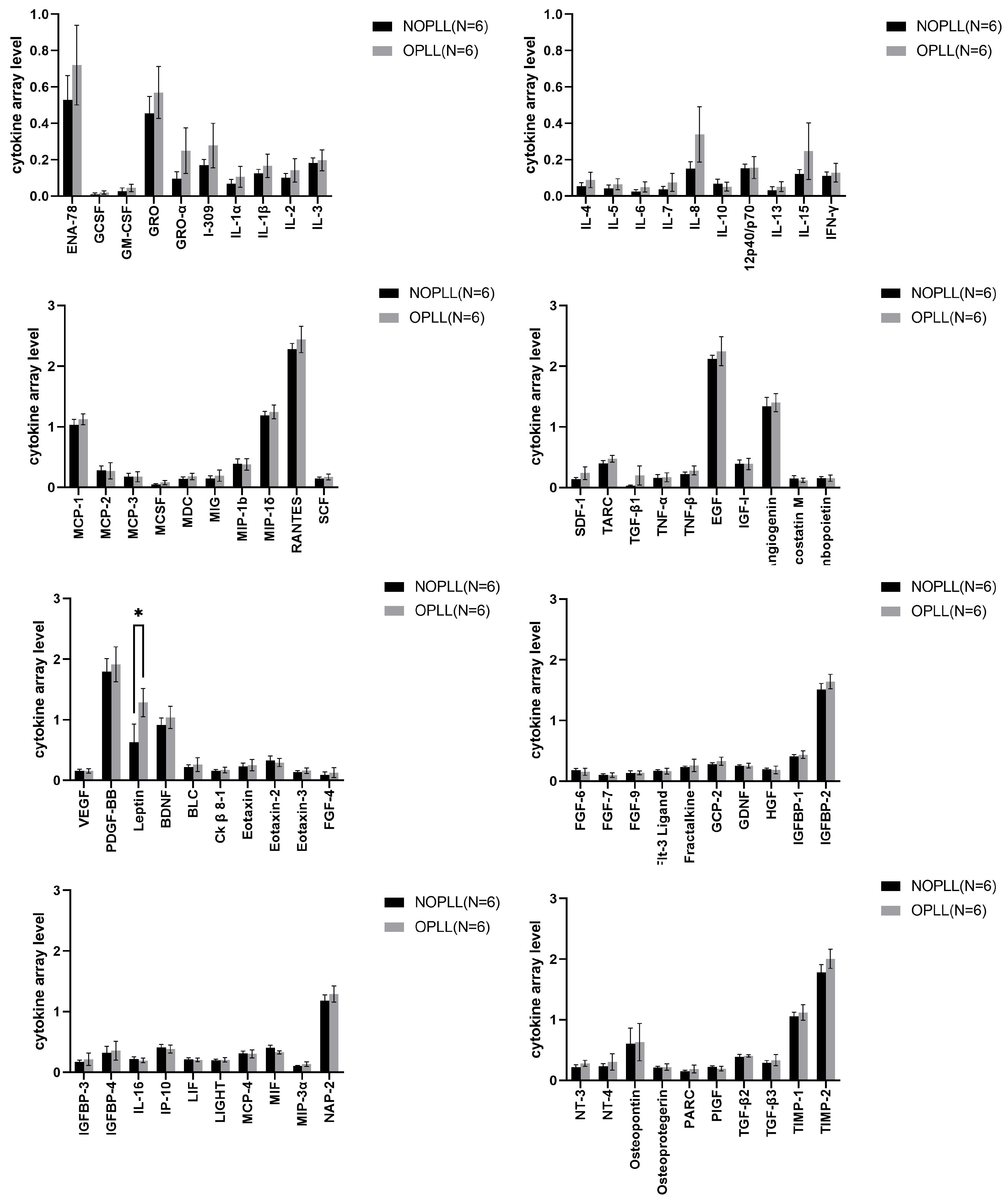
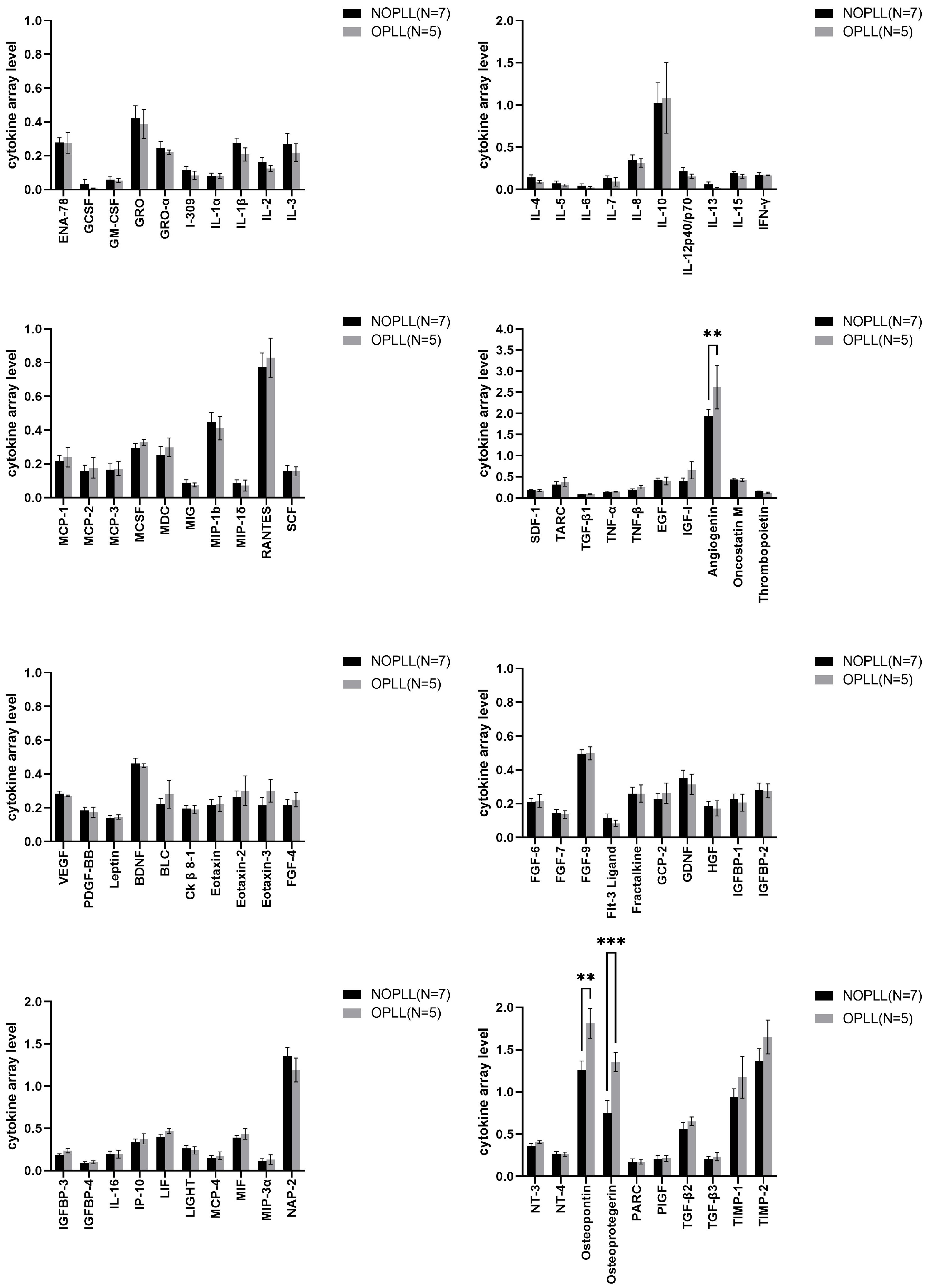
3.4. Tissue Cytokine
3.5. Level of Proteins in OPLL vs. NOPLL
3.6. Levels of Proteins in Serum vs. Tissue: Serum/Tissue > 2
3.7. Levels of Proteins in Serum vs. Tissue: Tissue/Serum > 2
3.8. Verification of Key Protein Expression with Western Blot Methods
3.9. Immunohistochemical Stain
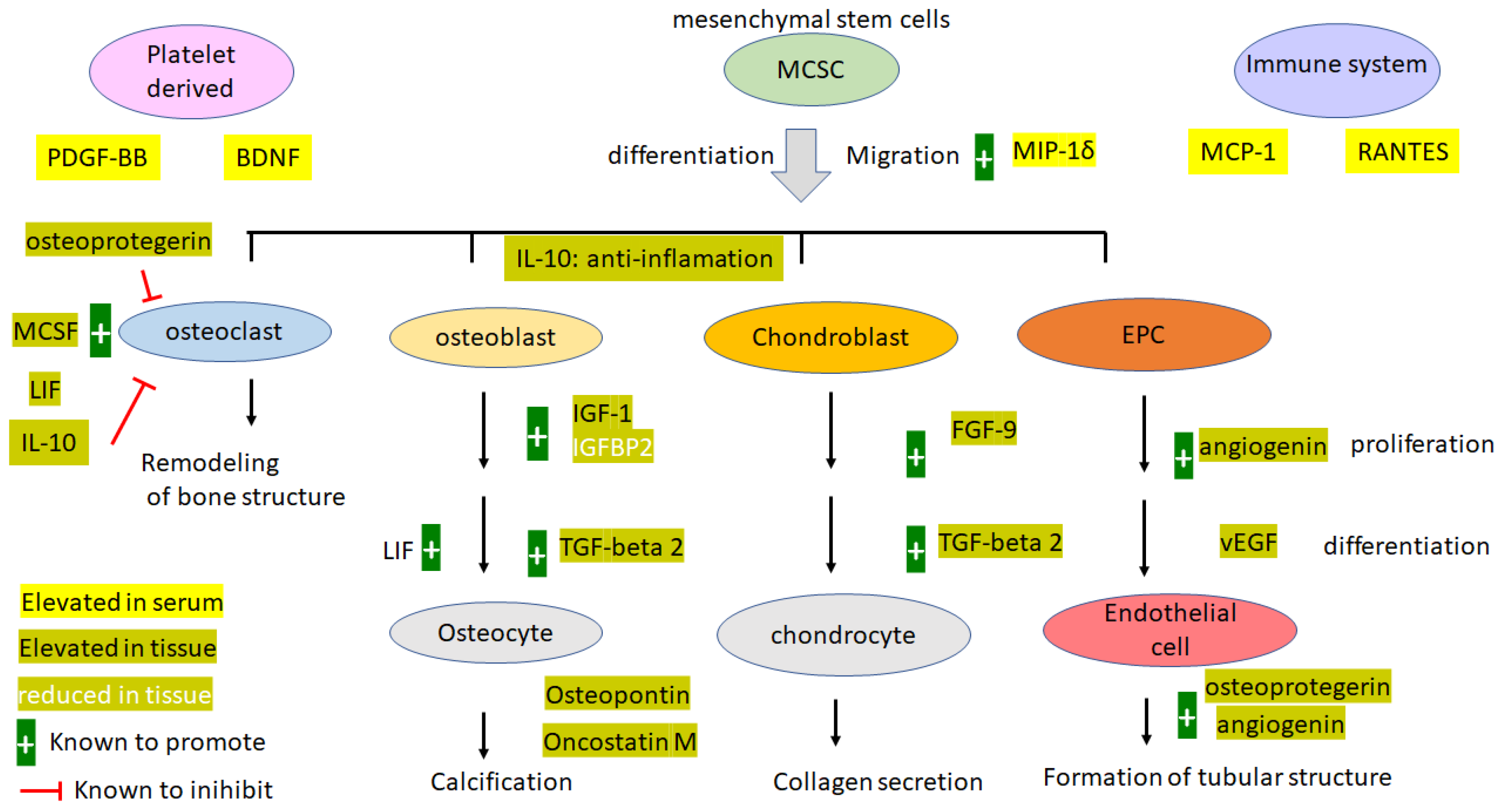
4. Discussion
4.1. Pathology and Etiology
4.2. Severity of the Disease and Importance of the Study
4.3. Leptin
4.4. Angiogenesis Factors: ANG, vEGF, and Osteopretegerin
4.5. Systemic Inflammation cf Local Inflammation
4.6. Platelet-Derived EGF, PDGF-BB, and BDNF
4.7. Bone Formation-Promoting Proteins
4.8. Osteopontin (OPN)
4.9. Osteoprotegerin OPG
4.10. IGF-1 and IGFBPs
4.11. IL-6
4.12. Concern of Limitations and Future Advances
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.F.; Tu, T.H.; Chen, Y.C.; Wu, J.C.; Chang, P.Y.; Liu, L.; Huang, W.C.; Lo, S.S.; Cheng, H. Risk of spinal cord injury in patients with cervical spondylotic myelopathy and ossification of posterior longitudinal ligament: A national cohort study. Neurosurg. Focus 2016, 40, E4. [Google Scholar] [CrossRef]
- Wu, J.C.; Chen, Y.C.; Huang, W.C. Ossification of the Posterior Longitudinal Ligament in Cervical Spine: Prevalence, Management, and Prognosis. Neurospine 2018, 15, 33–41. [Google Scholar] [CrossRef]
- Wu, J.C.; Chen, Y.C.; Liu, L.; Huang, W.C.; Chen, T.J.; Lo, S.S.; Thien, P.F.; Cheng, H. Conservatively treated ossification of the posterior longitudinal ligament increases the risk of spinal cord injury: A nationwide cohort study. J. Neurotrauma 2012, 29, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Sakou, T. Ossification of the posterior longitudinal ligament of the cervical spine: Etiology and natural history. Spine 2012, 37, E309–E314. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takahashi, N.; Kobayashi, Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J. Mol. Med. 2013, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Rawadi, G.; Roman-Roman, S. Wnt signalling pathway: A new target for the treatment of osteoporosis. Expert Opin. Ther. Targets 2005, 9, 1063–1077. [Google Scholar] [CrossRef]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Niu, C.C.; Lin, S.S.; Yuan, L.J.; Chen, L.H.; Yang, C.Y.; Chung, A.N.; Lu, M.L.; Tsai, T.T.; Lai, P.L.; Chen, W.J. Correlation of blood bone turnover biomarkers and Wnt signaling antagonists with AS, DISH, OPLL, and OYL. BMC Musculoskelet. Disord. 2017, 18, 61. [Google Scholar] [CrossRef]
- Wu, J.C.; Liu, L.; Chen, Y.C.; Huang, W.C.; Chen, T.J.; Cheng, H. Ossification of the posterior longitudinal ligament in the cervical spine: An 11-year comprehensive national epidemiology study. Neurosurg. Focus 2011, 30, E5. [Google Scholar] [CrossRef]
- Inamasu, J.; Guiot, B.H.; Sachs, D.C. Ossification of the posterior longitudinal ligament: An update on its biology, epidemiology, and natural history. Neurosurgery 2006, 58, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Kurokawa, T.; Hoshino, Y.; Kawahara, H.; Ogata, E.; Matsumoto, T. Immunohistochemical demonstration of bone morphogenetic protein-2 and transforming growth factor-beta in the ossification of the posterior longitudinal ligament of the cervical spine. Spine 1992, 17, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nakajima, A.; Aiba, A.; Koda, M.; Okawa, A.; Takahashi, K.; Yamazaki, M. Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur. Spine J. 2011, 20, 1450–1458. [Google Scholar] [CrossRef]
- Tahara, M.; Aiba, A.; Yamazaki, M.; Ikeda, Y.; Goto, S.; Moriya, H.; Okawa, A. The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine 2005, 30, 877–880, discussion 881. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Weiss, S.; Zimmermann, G.; Baumgart, R.; Kasten, P.; Bidlingmaier, M.; Henle, P. Systemic regulation of angiogenesis and matrix degradation in bone regeneration--distraction osteogenesis compared to rigid fracture healing. Bone 2005, 37, 781–790. [Google Scholar] [CrossRef]
- Malyankar, U.M.; Scatena, M.; Suchland, K.L.; Yun, T.J.; Clark, E.A.; Giachelli, C.M. Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J. Biol. Chem. 2000, 275, 20959–20962. [Google Scholar] [CrossRef]
- Benslimane-Ahmim, Z.; Heymann, D.; Dizier, B.; Lokajczyk, A.; Brion, R.; Laurendeau, I.; Bièche, I.; Smadja, D.M.; Galy-Fauroux, I.; Colliec-Jouault, S.; et al. Osteoprotegerin, a new actor in vasculogenesis, stimulates endothelial colony-forming cells properties. J. Thromb. Haemost. JTH 2011, 9, 834–843. [Google Scholar] [CrossRef]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef]
- Xu, L.L.; Warren, M.K.; Rose, W.L.; Gong, W.; Wang, J.M. Human recombinant monocyte chemotactic protein and other c-c chemokines bind and induce directional migration of dendritic cells in vitro. J. Leukoc. Biol. 1996, 60, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Day, C.J.; Morrison, N.A. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J. Biol. Chem. 2005, 280, 16163–16169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Chen, L.L.; Deng, X.L.; Song, H.J.; Liao, Y.F.; Zeng, T.S.; Zheng, J.; Li, H.Q. Methylation status of CpG sites in the MCP-1 promoter is correlated to serum MCP-1 in Type 2 diabetes. J. Endocrinol. Investig. 2012, 35, 585–589. [Google Scholar] [CrossRef]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Song, A.; Nikolcheva, T.; Krensky, A.M. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol. Rev. 2000, 177, 236–245. [Google Scholar] [CrossRef]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef] [PubMed]
- Lejmi, E.; Perriraz, N.; Clément, S.; Morel, P.; Baertschiger, R.; Christofilopoulos, P.; Meier, R.; Bosco, D.; Bühler, L.H.; Gonelle-Gispert, C. Inflammatory Chemokines MIP-1δ and MIP-3α Are Involved in the Migration of Multipotent Mesenchymal Stromal Cells Induced by Hepatoma Cells. Stem Cells Dev. 2015, 24, 1223–1235. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Seki, S.; Suzuki, K.; Yahara, Y.; Makino, H.; Kitajima, I.; Kimura, T. Serum biomarkers in patients with ossification of the posterior longitudinal ligament (OPLL): Inflammation in OPLL. PLoS ONE 2017, 12, e0174881. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Oncostatin M, a multifunctional cytokine. Rev. Physiol. Biochem. Pharmacol. 2003, 149, 39–52. [Google Scholar] [CrossRef]
- Sims, N.A.; Quinn, J.M. Osteoimmunology: Oncostatin M as a pleiotropic regulator of bone formation and resorption in health and disease. BoneKEy Rep. 2014, 3, 527. [Google Scholar] [CrossRef]
- Walker, E.C.; McGregor, N.E.; Poulton, I.J.; Solano, M.; Pompolo, S.; Fernandes, T.J.; Constable, M.J.; Nicholson, G.C.; Zhang, J.G.; Nicola, N.A.; et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J. Clin. Investig. 2010, 120, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Manabe, T.; Matsumoto, M.; Kajiume, T.; Matsumoto, M.; Yuge, L. LIF-free embryonic stem cell culture in simulated microgravity. PLoS ONE 2009, 4, e6343. [Google Scholar] [CrossRef]
- Williams, R.L.; Hilton, D.J.; Pease, S.; Willson, T.A.; Stewart, C.L.; Gearing, D.P.; Wagner, E.F.; Metcalf, D.; Nicola, N.A.; Gough, N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988, 336, 684–687. [Google Scholar] [CrossRef]
- Du, J.; Yang, J.; He, Z.; Cui, J.; Yang, Y.; Xu, M.; Qu, X.; Zhao, N.; Yan, M.; Li, H.; et al. Osteoblast and Osteoclast Activity Affect Bone Remodeling Upon Regulation by Mechanical Loading-Induced Leukemia Inhibitory Factor Expression in Osteocytes. Front. Mol. Biosci. 2020, 7, 585056. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L.; Guo, H.; Wang, J.; Zhang, S.; Yang, X.; Yang, L.; Jin, Q. Osteoclasts secrete leukemia inhibitory factor to promote abnormal bone remodeling of subchondral bone in osteoarthritis. BMC Musculoskelet. Disord. 2022, 23, 87. [Google Scholar] [CrossRef]
- Park-Min, K.H.; Ji, J.D.; Antoniv, T.; Reid, A.C.; Silver, R.B.; Humphrey, M.B.; Nakamura, M.; Ivashkiv, L.B. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J. Immunol. 2009, 183, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Laitala-Leinonen, T. Osteoclast lineage and function. Arch. Biochem. Biophys. 2008, 473, 132–138. [Google Scholar] [CrossRef]
- Merry, K.; Dodds, R.; Littlewood, A.; Gowen, M. Expression of osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone. J. Cell Sci. 1993, 104 Pt 4, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Guo, H.; Cai, C.Q.; Schroeder, R.A.; Kuo, P.C. Osteopontin is a negative feedback regulator of nitric oxide synthesis in murine macrophages. J. Immunol. 2001, 166, 1079–1086. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Phadke, S.A.; Batlle, D.; Sahai, A. Hypoxia and high glucose cause exaggerated mesangial cell growth and collagen synthesis: Role of osteopontin. Am. J. Physiol. Ren. Physiol. 2001, 280, F667–F674. [Google Scholar] [CrossRef]
- McKee, M.D.; Addison, W.N.; Kaartinen, M.T. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs 2005, 181, 176–188. [Google Scholar] [CrossRef]
- Giachelli, C.M. Ectopic calcification: Gathering hard facts about soft tissue mineralization. Am. J. Pathol. 1999, 154, 671–675. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; Murshed, M.; Karsenty, G.; McKee, M.D. Osteopontin upregulation and polymerization by transglutaminase 2 in calcified arteries of Matrix Gla protein-deficient mice. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 375–386. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, S.; Abdelnaby, R.; Khachab, A.; Bläsius, K.; Tingart, M.; Rath, B. Osteopontin as a biochemical marker and severity indicator for idiopathic hip osteoarthritis. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2016, 26, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vitovski, S.; Phillips, J.S.; Sayers, J.; Croucher, P.I. Investigating the interaction between osteoprotegerin and receptor activator of NF-kappaB or tumor necrosis factor-related apoptosis-inducing ligand: Evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J. Biol. Chem. 2007, 282, 31601–31609. [Google Scholar] [CrossRef]
- Dixit, M.; Poudel, S.B.; Yakar, S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell. Endocrinol. 2021, 519, 111052. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Lee, E.J.; Kim, K.R.; Cha, B.S.; Song, Y.D.; Lim, S.K.; Lee, H.C.; Huh, K.B. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1997, 21, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Azar, W.J.; Yau, S.W.; Sabin, M.A.; Werther, G.A. IGFBP-2: The dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2015, 26, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Hedbacker, K.; Birsoy, K.; Wysocki, R.W.; Asilmaz, E.; Ahima, R.S.; Farooqi, I.S.; Friedman, J.M. Antidiabetic Effects of IGFBP2, a Leptin-Regulated Gene. Cell Metab. 2010, 11, 11–22. [Google Scholar] [CrossRef]
- Saito, H.; Yayama, T.; Mori, K.; Kumagai, K.; Fujikawa, H.; Chosei, Y.; Imai, S. Increased Cellular Expression of Interleukin-6 in Patients With Ossification of the Posterior Longitudinal Ligament. Spine 2023, 48, E78–E86. [Google Scholar] [CrossRef]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef]
- Yayama, T.; Mori, K.; Saito, H.; Fujikawa, H.; Kitagawa, M.; Okumura, N.; Nishizawa, K.; Nakamura, A.; Kumagai, K.; Mimura, T.; et al. Cytokine Profile From the Ligamentum Flavum in Patients with Ossification of the Posterior Longitudinal Ligament in the Cervical Spine. Spine 2022, 47, 277–285. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Stegmaier, S.; Moghaddam, A.; Hänsch, G.M. The osteoblast as an inflammatory cell: Production of cytokines in response to bacteria and components of bacterial biofilms. BMC Musculoskelet. Disord. 2016, 17, 243. [Google Scholar] [CrossRef]


| NOPLL | OPLL | p Value | |
|---|---|---|---|
| patient numbers | 33 | 18 | |
| age | 54.7 ± 10.2 | 59.7 ± 9.7 | 0.09 |
| male (%) | 15 (45.5) | 13 (72.2) | 0.08 |
| lesion level # | |||
| one | 12 | 6 | |
| two | 10 | 11 | |
| three | 10 | 1 | |
| four | 1 | 0 | |
| K-line | |||
| positive | n/a | 13 | |
| negative | n/a | 5 | |
| OPLL type | |||
| segmental | n/a | 6 | |
| continuous | n/a | 2 | |
| mixed | n/a | 10 | |
| JOA score ## | |||
| pre-op | 13.8 ± 2.5 | 12.9 ± 3.3 | 0.42 |
| 1 mo | 14.4 ± 2.5 ** | 11.3 ± 4.7 | 0.06 |
| 3 mo | 14.5 ± 2.3 ** | 11.8 ± 3.4 | 0.05 |
| 6 mo | 14.5 ± 3.1 ** | 13.5 ± 2.7 | 0.07 |
| p Value | |||
|---|---|---|---|
| Sample Type | NOPLL | OPLL | |
| Serum leptin | 0.40 ± 0.25 | 1.23 ± 0.23 | 0.0138 * |
| Tissue leptin | 0.141 ± 0.037 | 0.146± 0.028 | 0.8248 |
| Tissue angiogenin | 1.94 ± 0.15 | 3.0 ± 0.50 | 0.0012 ** |
| Tissue osteopontin | 1.27 ± 0.10 | 1.81 ± 0.18 | 0.0038 ** |
| Tissue osteoprotegerin | 0.75 ± 0.15 | 1.35 ± 0.11 | 0.0006 ** |
| Protein Name | Level (Serum) | Level (Tissue) | Ratio (Serum/Tissue) | p Value | Comments |
|---|---|---|---|---|---|
| MCP-1 | 1.13 | 0.24 | 4.69 | <0.0001 | injury elicite, recruite lymphocyte |
| MIP-1δ | 1.25 | 0.07 | 17.3 | <0.0001 | enhances osteoclastogenesis |
| EGF | 2.25 | 0.40 | 5.62 | <0.0001 | platelet-derived factors |
| RANTES | 2.44 | 0.83 | 2.94 | 0.0002 | T cell-regulated, late expression, for maitaining inflammation |
| PDGF-BB | 1.92 | 0.17 | 11.0 | 0.0004 | platelete-derived factors |
| Leptin | 1.28 | 0.15 | 8.79 | 0.0012 | serum leptin high = OPLL marker |
| BDNF | 1.04 | 0.45 | 2.32 | 0.0168 | platelet-derived factors |
| IGFBP-1 | 0.44 | 0.21 | 2.12 | 0.0206 | regulate IGF-1 bioavaialbility |
| IGFBP-2 | 1.64 | 0.28 | 5.95 | <0.0001 | regulate IGF-1 bioavaialbility |
| Protein Name | Level (Serum) | Level (Tissue) | Ratio (Tissue/Serum) | p Value | Comments |
|---|---|---|---|---|---|
| IL-10 | 0.05 | 1.08 | 20.98 | 0.02 | cytokine for general repair, inhibit osteoclastogenesis |
| MCSF | 0.08 | 0.328 | 4.10 | 0.0002 | promote osteoclastgenesis |
| Angiogenin | 1.40 | 2.62 | 1.87 | 0.0353 | promoting angiogenesis |
| Oncostatin M | 0.12 | 0.42 | 3.50 | 0.0002 | pleiotropic regulator of bone formation and resorption |
| VEGF | 0.16 | 0.27 | 1.74 | 0.0153 | essential growth factor for vascular endothelial cells |
| FGF-9 | 0.14 | 0.50 | 3.60 | <0.0001 | promote chondrogenesis and osteogenesis |
| LIF | 0.21 | 0.47 | 2.29 | 0.0002 | action on skeletal cells, osteoclasts secrete LIF to promote abnormal bone remodeling |
| Osteopontin | 0.63 | 1.81 | 2.86 | 0.0122 | organic component of bone, mineralization, bone remodeling |
| Osteoprotegerin | 0.22 | 1.35 | 6.15 | <0.0001 | a decoy receptor for RANKL, suppress osteoclastogenesis and bone resorption |
| TGF-β2 | 0.41 | 0.65 | 1.61 | 0.0009 | important in bone homeostasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fay, L.-Y.; Kuo, C.-H.; Chang, H.-K.; Yeh, M.-Y.; Chang, C.-C.; Ko, C.-C.; Tu, T.-H.; Kuo, Y.-H.; Hsu, W.-Y.; Hung, C.-H.; et al. Comparative Study of the Cytokine Profiles of Serum and Tissues from Patients with the Ossification of the Posterior Longitudinal Ligament. Biomedicines 2023, 11, 2021. https://doi.org/10.3390/biomedicines11072021
Fay L-Y, Kuo C-H, Chang H-K, Yeh M-Y, Chang C-C, Ko C-C, Tu T-H, Kuo Y-H, Hsu W-Y, Hung C-H, et al. Comparative Study of the Cytokine Profiles of Serum and Tissues from Patients with the Ossification of the Posterior Longitudinal Ligament. Biomedicines. 2023; 11(7):2021. https://doi.org/10.3390/biomedicines11072021
Chicago/Turabian StyleFay, Li-Yu, Chao-Hung Kuo, Hsuan-Kan Chang, Mei-Yin Yeh, Chih-Chang Chang, Chin-Chu Ko, Tsung-Hsi Tu, Yi-Hsuan Kuo, Wang-Yu Hsu, Chien-Hui Hung, and et al. 2023. "Comparative Study of the Cytokine Profiles of Serum and Tissues from Patients with the Ossification of the Posterior Longitudinal Ligament" Biomedicines 11, no. 7: 2021. https://doi.org/10.3390/biomedicines11072021
APA StyleFay, L.-Y., Kuo, C.-H., Chang, H.-K., Yeh, M.-Y., Chang, C.-C., Ko, C.-C., Tu, T.-H., Kuo, Y.-H., Hsu, W.-Y., Hung, C.-H., Chen, C.-J., Wu, J.-C., Tsai, M.-J., Huang, W.-C., Cheng, H., & Lee, M.-J. (2023). Comparative Study of the Cytokine Profiles of Serum and Tissues from Patients with the Ossification of the Posterior Longitudinal Ligament. Biomedicines, 11(7), 2021. https://doi.org/10.3390/biomedicines11072021





