Impact and Advances in the Role of Bacterial Extracellular Vesicles in Neurodegenerative Disease and Its Therapeutics
Abstract
1. Introduction
2. Offensive and Defensive Roles of Bacterial Extracellular Vesicles in Neurodegenerative Disease
3. Critical Networks of Bacterial Extracellular Vesicles in the Microbiome–Gut–Brain Axis
4. Role of Bacterial Extracellular Vesicles in Neuroimmune System and Their Crosstalk
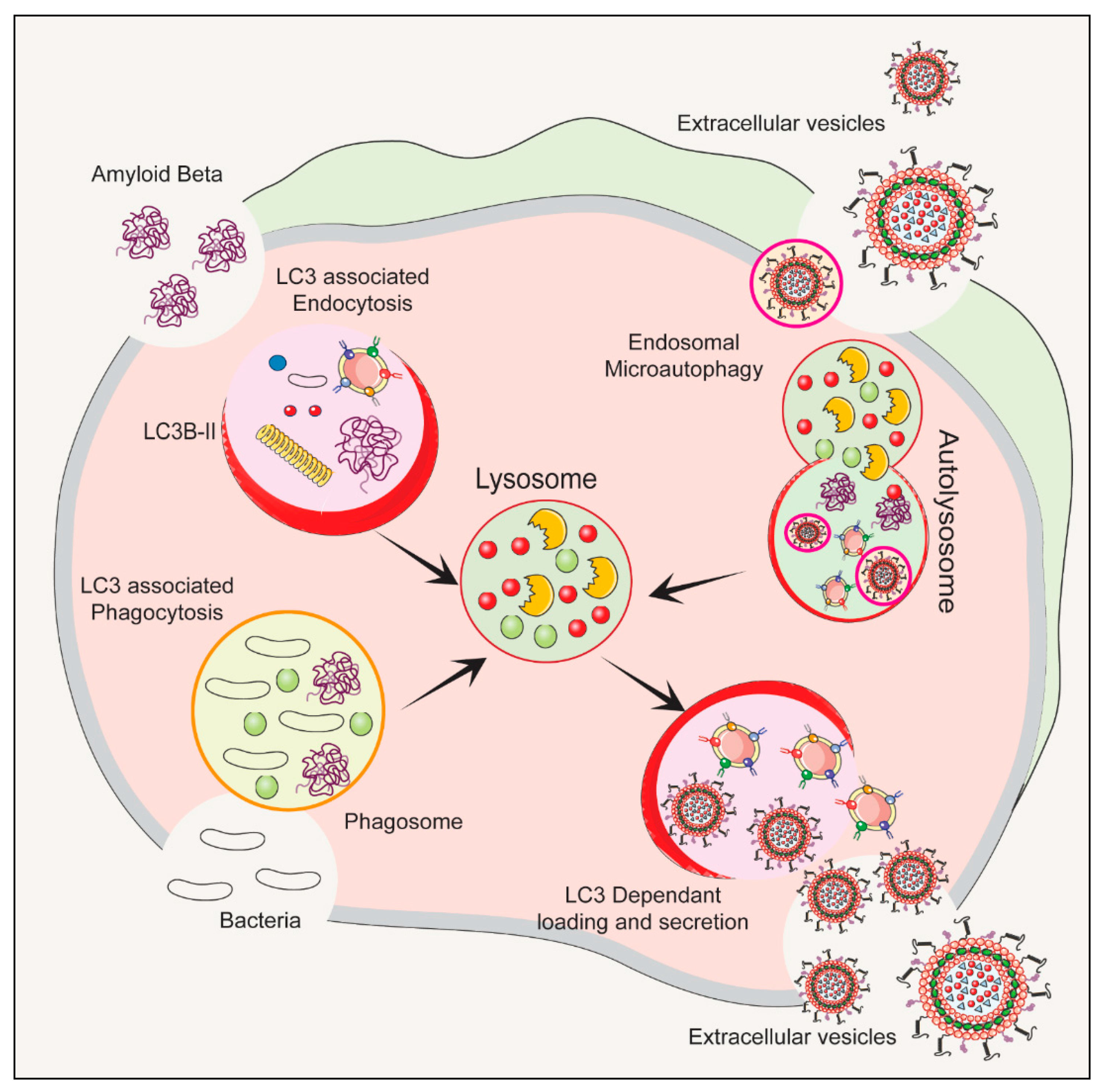
5. Risk Factors of Bacterial Extracellular Vesicles in Autophagy–Lysosomal Pathway
6. Therapeutic Applications of Bacterial Extracellular Vesicles
7. Future Outlook of Bacterial Extracellular Vesicles
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; Del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Andrews, S.J.; Fulton-Howard, B.; Goate, A. Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol. 2020, 19, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; DeCarli, C.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagust, W.; Landau, S.M.; et al. Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 824–857. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.M.; Guerri, C.; Urena, J.; Pascual, M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Badia, J.; Baldoma, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Thakur, A.; Qiu, G.; Xu, C.; Han, X.; Yang, T.; Ng, S.P.; Chan, K.W.Y.; Wu, C.M.L.; Lee, Y. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci. Adv. 2020, 6, eaaz6119. [Google Scholar] [CrossRef]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar] [CrossRef]
- Gaurav, I.; Wang, X.; Thakur, A.; Iyaswamy, A.; Thakur, S.; Chen, X.; Kumar, G.; Li, M.; Yang, Z. Peptide-Conjugated Nano Delivery Systems for Therapy and Diagnosis of Cancer. Pharmaceutics 2021, 13, 1433. [Google Scholar] [CrossRef]
- Thakur, A.; Ke, X.; Chen, Y.W.; Motallebnejad, P.; Zhang, K.; Lian, Q.; Chen, H.J. The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell 2022, 13, 631–654. [Google Scholar] [CrossRef]
- Zhao, G.; Jones, M.K. Role of Bacterial Extracellular Vesicles in Manipulating Infection. Infect. Immun. 2023, 91, e0043922. [Google Scholar] [CrossRef]
- Raghav, A.; Singh, M.; Jeong, G.B.; Giri, R.; Agarwal, S.; Kala, S.; Gautam, K.A. Extracellular vesicles in neurodegenerative diseases: A systematic review. Front. Mol. Neurosci. 2022, 15, 1061076. [Google Scholar] [CrossRef]
- Yang, Y.; Boza-Serrano, A.; Dunning, C.J.R.; Clausen, B.H.; Lambertsen, K.L.; Deierborg, T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflamm. 2018, 15, 168. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.B.; Kumar, S.; Bell, C.R.; Peoples, V.A.; Crenshaw, B.J.; Coats, M.T.; Scoffield, J.A.; Rowe, G.C.; Sims, B.; Matthews, Q.L. Effects of Pseudomonas aeruginosa on Microglial-Derived Extracellular Vesicle Biogenesis and Composition. Pathogens 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Choi, Y.; Lee, H.J.; Hwang, D.W.; Lee, D.S. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 2022, 20, 198. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, X.D.; Zhang, S.M.; Wang, H.W.; Wang, Y.L. Extracellular vesicles in neurodegenerative diseases: Insights and new perspectives. Genes Dis. 2021, 8, 124–132. [Google Scholar] [CrossRef]
- Aires, I.D.; Ribeiro-Rodrigues, T.; Boia, R.; Ferreira-Rodrigues, M.; Girao, H.; Ambrosio, A.F.; Santiago, A.R. Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading. Biomolecules 2021, 11, 770. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 53. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Z.; Yang, Z.; Zhang, Y.; Chen, H.; Yang, X.; Fang, X.; Zhu, Y.; Zhang, J.; Ouyang, F.; et al. Lactobacillus plantarum-derived extracellular vesicles protect against ischemic brain injury via the microRNA-101a-3p/c-Fos/TGF-beta axis. Pharmacol. Res. 2022, 182, 106332. [Google Scholar] [CrossRef]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef]
- Haney, M.S.; Bohlen, C.J.; Morgens, D.W.; Ousey, J.A.; Barkal, A.A.; Tsui, C.K.; Ego, B.K.; Levin, R.; Kamber, R.A.; Collins, H.; et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat. Genet. 2018, 50, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, P.; Groeger, D.; Ziegler, M.; Frei, R.; Ferstl, R.; Shanahan, F.; Quigley, E.M.; Kiely, B.; Akdis, C.A.; O’Mahony, L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut 2012, 61, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, E.J.; Bae, I.H.; Myoung, K.; Kim, S.T.; Park, P.J.; Lee, K.H.; Pham, A.V.Q.; Ko, J.; Oh, S.H.; et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 2020, 9, 1793514. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Huang, H.J.; Chen, J.L.; Liao, J.F.; Chen, Y.H.; Chieu, M.W.; Ke, Y.Y.; Hsu, C.C.; Tsai, Y.C.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 2021, 21, 259. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Wang, H.; Long, T.; You, J.; Li, P.; Xu, Q. Bibliometric Visualization Analysis of Microbiome-Gut-Brain Axis from 2004 to 2020. Med. Sci. Monit. 2022, 28, e936037. [Google Scholar] [CrossRef]
- Claudino Dos Santos, J.C.; Lima, M.P.P.; Brito, G.A.C.; Viana, G.S.B. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2023, 84, 101812. [Google Scholar] [CrossRef]
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Neuropsychological and Gastrointestinal Functional Disorders. ACS Med. Chem. Lett. 2023, 14, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, G.; Byun, M.S.; Lee, J.H.; Yi, D.; Park, H.; Lee, D.Y.; Group, K.R. Gut microbiome alterations in preclinical Alzheimer’s disease. PLoS ONE 2022, 17, e0278276. [Google Scholar] [CrossRef]
- Chu, C.Q.; Yu, L.L.; Qi, G.Y.; Mi, Y.S.; Wu, W.Q.; Lee, Y.K.; Zhai, Q.X.; Tian, F.W.; Chen, W. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: Exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022, 135, 104556. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Roig-Carles, D.; Willms, E.; Fontijn, R.D.; Martinez-Pacheco, S.; Mager, I.; de Vries, H.E.; Hirst, M.; Sharrack, B.; Male, D.K.; Hawkes, C.A.; et al. Endothelial-Derived Extracellular Vesicles Induce Cerebrovascular Dysfunction in Inflammation. Pharmaceutics 2021, 13, 1525. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Sterling, K.G.; Dodd, G.K.; Alhamdi, S.; Asimenios, P.G.; Dagda, R.K.; De Meirleir, K.L.; Hudig, D.; Lombardi, V.C. Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int. J. Mol. Sci. 2022, 23, 13328. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Patrycy, M.; Chodkowski, M.; Krzyzowska, M. Role of Microglia in Herpesvirus-Related Neuroinflammation and Neurodegeneration. Pathogens 2022, 11, 809. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Guo, M.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium breve intervention combined with environmental enrichment alleviates cognitive impairment by regulating the gut microbiota and microbial metabolites in Alzheimer’s disease mice. Front. Immunol. 2022, 13, 1013664. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine 2022, 76, 103798. [Google Scholar] [CrossRef]
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Haas-Neill, S.; Forsythe, P. A Budding Relationship: Bacterial Extracellular Vesicles in the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2020, 21, 8899. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Reparaz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef]
- Eskandari, S.K.; Sulkaj, I.; Melo, M.B.; Li, N.; Allos, H.; Alhaddad, J.B.; Kollar, B.; Borges, T.J.; Eskandari, A.S.; Zinter, M.A.; et al. Regulatory T cells engineered with TCR signaling-responsive IL-2 nanogels suppress alloimmunity in sites of antigen encounter. Sci. Transl. Med. 2020, 12, eaaw4744. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The role of the probiotic Akkermansia muciniphila in brain functions: Insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2023, 49, 151–176. [Google Scholar] [CrossRef]
- Ferreira, T.G.; Trindade, C.; Bell, P.; Teixeira-Ferreira, A.; Perales, J.E.; Vommaro, R.C.; Domingues, R.; Ferreira, E.O. Identification of the alpha-enolase P46 in the extracellular membrane vesicles of Bacteroides fragilis. Mem. Inst. Oswaldo Cruz 2018, 113, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Liu, J.; Wang, Z.; Tong, B.C.; Song, J.; Lu, J.; et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 728. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- Spencer, N.; Yeruva, L. Role of bacterial infections in extracellular vesicles release and impact on immune response. Biomed. J. 2021, 44, 157–164. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Su, C.; Iyaswamy, A.; Krishnamoorthi, S.K.; Zhu, Z.; Yang, S.; Tong, B.C.; Liu, J.; Sreenivasmurthy, S.G.; Guan, X.; et al. Celastrol enhances transcription factor EB (TFEB)-mediated autophagy and mitigates Tau pathology: Implications for Alzheimer’s disease therapy. Acta Pharm. Sin. B 2022, 12, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Malovic, E.; Harischandra, D.S.; Ngwa, H.A.; Ghosh, A.; Hogan, C.; Rokad, D.; Zenitsky, G.; Jin, H.; Anantharam, V.; et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 2018, 64, 204–218. [Google Scholar] [CrossRef]
- Gaurav, I.; Thakur, A.; Kumar, G.; Long, Q.; Zhang, K.; Sidu, R.K.; Thakur, S.; Sarkar, R.K.; Kumar, A.; Iyaswamy, A.; et al. Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker. Nanomaterials 2023, 13, 1306. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Extracellular Vesicles miRNA Cargo for Microglia Polarization in Traumatic Brain Injury. Biomolecules 2020, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Go, V.; Bowley, B.G.E.; Pessina, M.A.; Zhang, Z.G.; Chopp, M.; Finklestein, S.P.; Rosene, D.L.; Medalla, M.; Buller, B.; Moore, T.L. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. GeroScience 2020, 42, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Singh, S.; Chemparathy, D.T.; Chivero, E.T.; Gordon, L.; Buch, S. Astrocytes & Astrocyte derived Extracellular Vesicles in Morphine Induced Amyloidopathy: Implications for Cognitive Deficits in Opiate Abusers. Aging Dis. 2021, 12, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Stentz, R.; Telatin, A.; Savva, G.M.; Booth, C.; Baker, D.; Rudder, S.; Knight, S.C.; Noble, A.; Carding, S.R. The Origin of Plasma-Derived Bacterial Extracellular Vesicles in Healthy Individuals and Patients with Inflammatory Bowel Disease: A Pilot Study. Genes 2021, 12, 1636. [Google Scholar] [CrossRef]
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Wang, X.; Krishnamoorthi, S.; Kaliamoorthy, V.; Sreenivasmurthy, S.G.; Kumar Durairajan, S.S.; Song, J.X.; Tong, B.C.; Zhu, Z.; Su, C.F.; et al. Theranostic F-SLOH mitigates Alzheimer’s disease pathology involving TFEB and ameliorates cognitive functions in Alzheimer’s disease models. Redox Biol. 2022, 51, 102280. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Vasudevan, K.; Jayaraman, S.; Jaganathan, R.; Thakur, A.; Chang, R.C.; Yang, C. Editorial: Advances in Alzheimer’s disease diagnostics, brain delivery systems, and therapeutics. Front. Mol. Biosci. 2023, 10, 1162879. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, Y.; Zhang, S.; Xiao, Y.; Shi, S. Salmonella Interacts With Autophagy to Offense or Defense. Front. Microbiol. 2020, 11, 721. [Google Scholar] [CrossRef]
- Keller, M.D.; Torres, V.J.; Cadwell, K. Autophagy and microbial pathogenesis. Cell Death Differ. 2020, 27, 872–886. [Google Scholar] [CrossRef]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Lu, M.S.; Hsieh, C.C.; Chen, W.C. Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma. Cell. Oncol. 2021, 44, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Daniello, V.; Conese, M. Extracellular Vesicles’ Role in the Pathophysiology and as Biomarkers in Cystic Fibrosis and COPD. Int. J. Mol. Sci. 2022, 24, 228. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Choi, J.H.; Moon, C.M.; Shin, T.S.; Kim, E.K.; McDowell, A.; Jo, M.K.; Joo, Y.H.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.; Kim, S.R.; Choi, D.S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef]
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675. [Google Scholar] [CrossRef]
- Mombelli, L.; Fidanza, M.A.; Roncari, G. Evaluation of a new method of determination of glomerular filtration rate by use of radioactive methylglucamine iothalamate. Acta Isot. 1968, 8, 21–29. [Google Scholar]
- Lee, J.Y.; Kim, H.S. Extracellular Vesicles in Neurodegenerative Diseases: A Double-Edged Sword. Tissue Eng. Regen. Med. 2017, 14, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Keighron, C.N.; Avazzadeh, S.; Goljanek-Whysall, K.; McDonagh, B.; Howard, L.; Ritter, T.; Quinlan, L.R. Extracellular Vesicles, Cell-Penetrating Peptides and miRNAs as Future Novel Therapeutic Interventions for Parkinson’s and Alzheimer’s Disease. Biomedicines 2023, 11, 728. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernandez-Sapiens, M.A.; Gutierrez-Mercado, Y.K.; Sandoval-Avila, S.; Gomez-Pinedo, U.; Marquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Ruan, J.; Miao, X.; Schluter, D.; Lin, L.; Wang, X. Extracellular vesicles in neuroinflammation: Pathogenesis, diagnosis, and therapy. Mol. Ther. 2021, 29, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, J.; You, T.; Shen, W.; Xu, W.; Dong, Q.; Cui, M. Extracellular Vesicle-Based Therapeutics in Neurological Disorders. Pharmaceutics 2022, 14, 2652. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Bernuz, M.; Puerta, R.; Esteban de Antonio, E.; Sanchez-Lopez, E.; Souto, E.B.; Camins, A.; Marti, M.; Pividori, M.I.; et al. Extracellular vesicles, the emerging mirrors of brain physiopathology. Int. J. Biol. Sci. 2023, 19, 721–743. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Sun, J.; Shen, H.M.; Wang, J.; Yang, C. Impairment of the autophagy-lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm. Sin. B 2022, 12, 1019–1040. [Google Scholar] [CrossRef] [PubMed]


| BEV Source | Disease Type | Key Findings | Ref. |
|---|---|---|---|
| Bifidobacterium bifidum | AD | BEVs reduced neuroinflammation and improved cognitive function | [24,25,46] |
| Akkermansia muciniphila | PD | BEVs reduced neuroinflammation by decreasing pro-inflammatory cytokine levels while raising anti-inflammatory cytokine levels | [26,27,53] |
| Bacteroides fragilis | MS | BEVs promoted the expansion of regulatory T cells, dampening immune responses and preventing autoimmunity | [24,52,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyaswamy, A.; Lu, K.; Guan, X.-J.; Kan, Y.; Su, C.; Liu, J.; Jaganathan, R.; Vasudevan, K.; Paul, J.; Thakur, A.; et al. Impact and Advances in the Role of Bacterial Extracellular Vesicles in Neurodegenerative Disease and Its Therapeutics. Biomedicines 2023, 11, 2056. https://doi.org/10.3390/biomedicines11072056
Iyaswamy A, Lu K, Guan X-J, Kan Y, Su C, Liu J, Jaganathan R, Vasudevan K, Paul J, Thakur A, et al. Impact and Advances in the Role of Bacterial Extracellular Vesicles in Neurodegenerative Disease and Its Therapeutics. Biomedicines. 2023; 11(7):2056. https://doi.org/10.3390/biomedicines11072056
Chicago/Turabian StyleIyaswamy, Ashok, Kejia Lu, Xin-Jie Guan, Yuxuan Kan, Chengfu Su, Jia Liu, Ravindran Jaganathan, Karthick Vasudevan, Jeyakumari Paul, Abhimanyu Thakur, and et al. 2023. "Impact and Advances in the Role of Bacterial Extracellular Vesicles in Neurodegenerative Disease and Its Therapeutics" Biomedicines 11, no. 7: 2056. https://doi.org/10.3390/biomedicines11072056
APA StyleIyaswamy, A., Lu, K., Guan, X.-J., Kan, Y., Su, C., Liu, J., Jaganathan, R., Vasudevan, K., Paul, J., Thakur, A., & Li, M. (2023). Impact and Advances in the Role of Bacterial Extracellular Vesicles in Neurodegenerative Disease and Its Therapeutics. Biomedicines, 11(7), 2056. https://doi.org/10.3390/biomedicines11072056





