Dietary Supplementation with 20-Hydroxyecdysone Ameliorates Hepatic Steatosis and Reduces White Adipose Tissue Mass in Ovariectomized Rats Fed a High-Fat, High-Fructose Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Tissue Collection
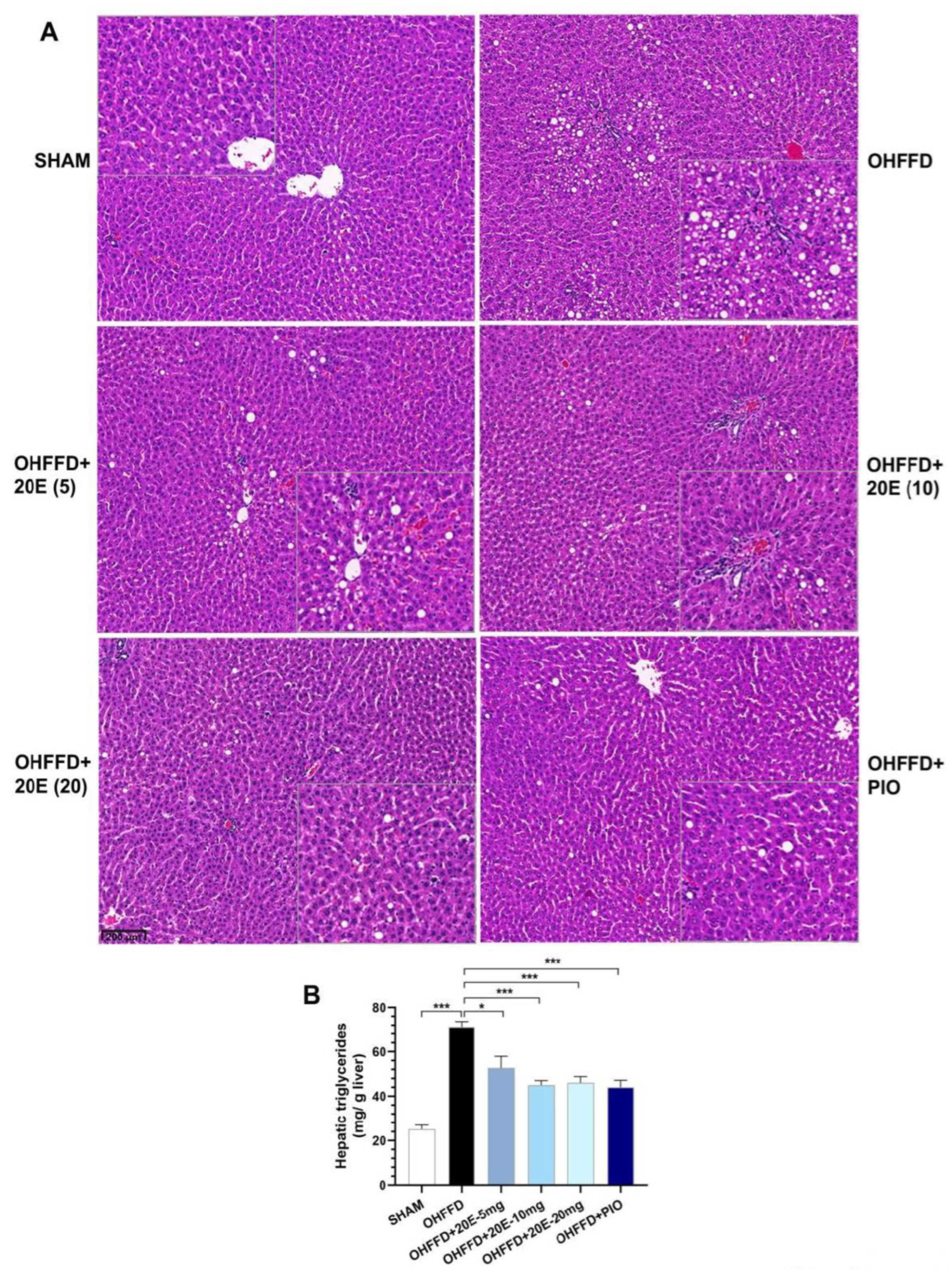
2.3. Histopathological Analysis
2.4. Liver Triglyceride Content
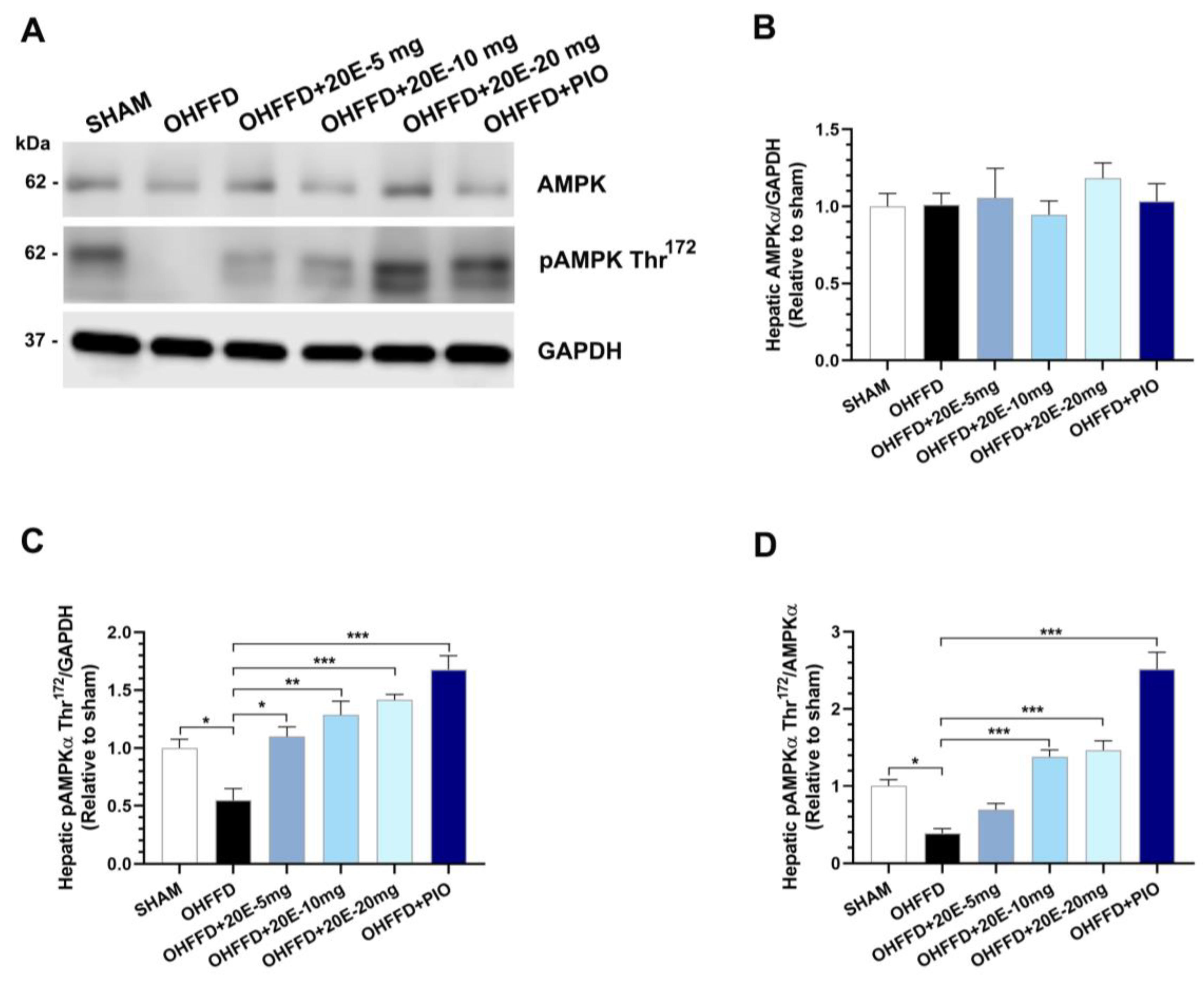
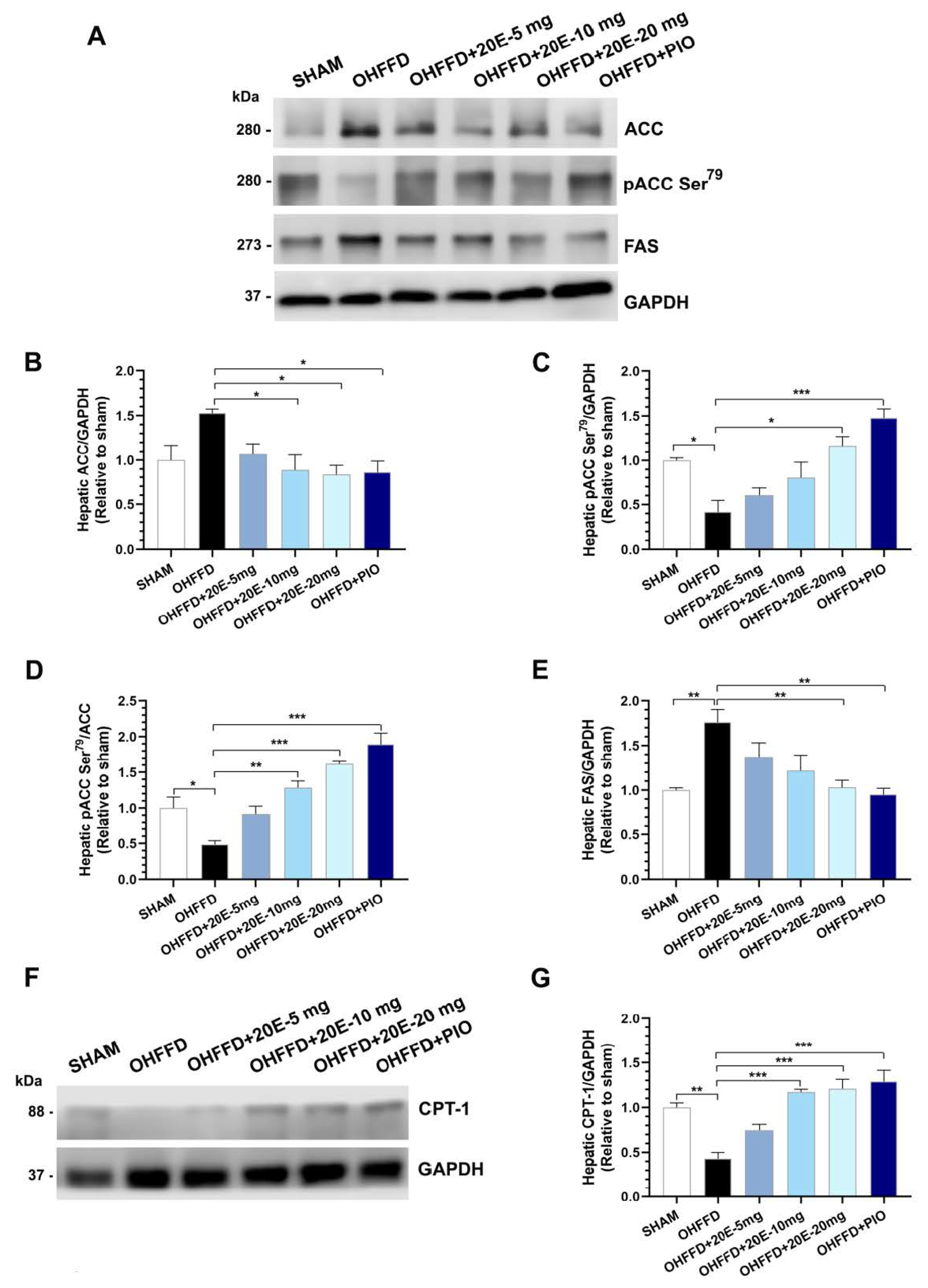
2.5. Immunoblotting Analysis
2.6. Statistical Analysis
3. Results
3.1. 20-Hydroxyecdysone Reduces Body Mass Gain and Visceral Adipose Mass
3.2. 20-Hydroxyecdysone Reduces Hepatic Lipid Accumulation
3.3. 20-Hydroxyecdysone Ameliorates Hepatic Steatosis by Activating AMPK Phosphorylation and Reducing the β-Oxidation of Fatty Acids
3.4. 20-Hydroxyecdysone Reduces Fat Accumulation by Reducing Lipogenesis in White Adipose Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Bombelli, M.; Mancia, G. Metabolic syndrome and cardiometabolic risk: An update. Blood Press 2009, 18, 7–16. [Google Scholar] [CrossRef]
- Farahmand, M.; Ramezani Tehrani, F.; Bahri Khomami, M.; Noroozzadeh, M.; Azizi, F. Surgical menopause versus natural menopause and cardio-metabolic disturbances: A 12-year population-based cohort study. J. Endocrinol. Investig. 2015, 38, 761–767. [Google Scholar] [CrossRef]
- Volzke, H.; Schwarz, S.; Baumeister, S.E.; Wallaschofski, H.; Schwahn, C.; Grabe, H.J.; Kohlmann, T.; John, U.; Doren, M. Menopausal status and hepatic steatosis in a general female population. Gut 2007, 56, 594–595. [Google Scholar] [CrossRef] [Green Version]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Watanabe, N.; Numata, M.; Ogawa, T.; Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: Analysis of clinical background. J. Gastroenterol. 2003, 38, 954–961. [Google Scholar] [CrossRef]
- Buniam, J.; Chukijrungroat, N.; Khamphaya, T.; Weerachayaphorn, J.; Saengsirisuwan, V. Estrogen and voluntary exercise attenuate cardiometabolic syndrome and hepatic steatosis in ovariectomized rats fed a high-fat high-fructose diet. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E908–E921. [Google Scholar] [CrossRef]
- Chukijrungroat, N.; Khamphaya, T.; Weerachayaphorn, J.; Songserm, T.; Saengsirisuwan, V. Hepatic FGF21 mediates sex differences in high-fat high-fructose diet-induced fatty liver. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E203–E212. [Google Scholar] [CrossRef] [Green Version]
- Buniam, J.; Chukijrungroat, N.; Rattanavichit, Y.; Surapongchai, J.; Weerachayaphorn, J.; Bupha-Intr, T.; Saengsirisuwan, V. 20-Hydroxyecdysone ameliorates metabolic and cardiovascular dysfunction in high-fat-high-fructose-fed ovariectomized rats. BMC Complement Med. Ther. 2020, 20, 140. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Mazzotti, A.; Poluzzi, E.; De Ponti, F.; Marchesini, G. Pharmacotherapy of type 2 diabetes in patients with chronic liver disease: Focus on nonalcoholic fatty liver disease. Expert Opin. Pharmacother. 2018, 19, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Bala, M.; Gupta, S.; Dua, A.; Dabur, R.; Injeti, E.; Mittal, A. Efficacy and risk profile of anti-diabetic therapies: Conventional vs traditional drugs-A mechanistic revisit to understand their mode of action. Pharmacol. Res. 2016, 113, 636–674. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L. The Karlson Lecture. Phytoecdysteroids: What use are they? Arch. Insect. Biochem. Physiol. 2009, 72, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Dioh, W.; Veillet, S.; Lafont, R. 20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases. Biomedicines 2021, 9, 492. [Google Scholar] [CrossRef]
- Dinan, L.; Lafont, R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Foucault, A.S.; Mathe, V.; Lafont, R.; Even, P.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulange, A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity 2012, 20, 270–277. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, Y.; Qiu, Z. Effect of ecdysterone on glucose metabolism in vitro. Life Sci. 2006, 78, 1108–1113. [Google Scholar] [CrossRef]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E433–E439. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Otaka, T.; Uchiyama, M.; Ogawa, S. Effect of ecdysterone on hyperglycemia in experimental animals. Biochem. Pharmacol. 1971, 20, 3263–3268. [Google Scholar] [CrossRef]
- Rattanavichit, Y.; Chukijrungroat, N.; Saengsirisuwan, V. Sex differences in the metabolic dysfunction and insulin resistance of skeletal muscle glucose transport following high fructose ingestion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1200–R1212. [Google Scholar] [CrossRef]
- Werawattanametin, K.; Podimuang, V.; Suksamrarn, A. Ecdysteroids from Vitex glabrata. J. Nat. Prod. 1986, 49, 365–366. [Google Scholar] [CrossRef]
- Hirunsai, M.; Yimlamai, T.; Suksamrarn, A. Effect of 20-Hydroxyecdysone on Proteolytic Regulation in Skeletal Muscle Atrophy. Vivo 2016, 30, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.H.; Peddu, D.; Amin, S.; Elsaid, M.I.; Minacapelli, C.D.; Chandler, T.M.; Catalano, C.; Rustgi, V.K. Nonalcoholic Fatty Liver Disease in Lean/Nonobese and Obese Individuals: A Comprehensive Review on Prevalence, Pathogenesis, Clinical Outcomes, and Treatment. J. Clin. Transl. Hepatol. 2023, 11, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rao, H.; Liu, F.; Wei, L.; Li, H.; Wu, C. Recent Advances in Adipose Tissue Dysfunction and Its Role in the Pathogenesis of Non-Alcoholic Fatty Liver Disease. Cells 2021, 10, 3300. [Google Scholar] [CrossRef]
- Arner, P.; Bernard, S.; Salehpour, M.; Possnert, G.; Liebl, J.; Steier, P.; Buchholz, B.A.; Eriksson, M.; Arner, E.; Hauner, H.; et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 2011, 478, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones 2018, 17, 299–313. [Google Scholar] [CrossRef]
- Alisi, A.; Da Sacco, L.; Bruscalupi, G.; Piemonte, F.; Panera, N.; De Vito, R.; Leoni, S.; Bottazzo, G.F.; Masotti, A.; Nobili, V. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab. Investig. 2011, 91, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Jun, D.W.; Kim, E.K.; Jeon, H.J.; Nam, H.H.; Saeed, W.K. Histologic and Metabolic Derangement in High-Fat, High-Fructose, and Combination Diet Animal Models. Sci. World J. 2015, 2015, 306326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e716. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Roumans, K.H.M.; Basset Sagarminaga, J.; Peters, H.P.F.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Liver fat storage pathways: Methodologies and dietary effects. Curr. Opin. Lipidol. 2021, 32, 9–15. [Google Scholar] [CrossRef]
- Wei, J.; Tong, L. Crystal structure of the 500-kDa yeast acetyl-CoA carboxylase holoenzyme dimer. Nature 2015, 526, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Aguilar-Salinas, C.A.; Gomez-Perez, F.J. Metabolic actions of fibroblast growth factor 21. Curr. Opin. Pediatr. 2012, 24, 523–529. [Google Scholar] [CrossRef]
- Kralisch, S.; Fasshauer, M. Fibroblast growth factor 21: Effects on carbohydrate and lipid metabolism in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 354–359. [Google Scholar] [CrossRef]
- Chau, M.D.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Saengsirisuwan, V.; Pongseeda, S.; Prasannarong, M.; Vichaiwong, K.; Toskulkao, C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism 2009, 58, 38–47. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lopez, M. Central regulation of energy metabolism by estrogens. Mol. Metab. 2018, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D.; et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011, 14, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunn, E.R.; Shinde, A.B.; Zaganjor, E. Weighing in on Adipogenesis. Front. Physiol. 2022, 13, 821278. [Google Scholar] [CrossRef]
- Ye, R.Z.; Richard, G.; Gevry, N.; Tchernof, A.; Carpentier, A.C. Fat Cell Size: Measurement Methods, Pathophysiological Origins, and Relationships with Metabolic Dysregulations. Endocr. Rev. 2022, 43, 35–60. [Google Scholar] [CrossRef]
- Sutjarit, N.; Sueajai, J.; Boonmuen, N.; Sornkaew, N.; Suksamrarn, A.; Tuchinda, P.; Zhu, W.; Weerachayaphorn, J.; Piyachaturawat, P. Curcuma comosa reduces visceral adipose tissue and improves dyslipidemia in ovariectomized rats. J. Ethnopharmacol. 2018, 215, 167–175. [Google Scholar] [CrossRef]
- Hasan, A.U.; Ohmori, K.; Hashimoto, T.; Kamitori, K.; Yamaguchi, F.; Rahman, A.; Tokuda, M.; Kobori, H. PPARgamma activation mitigates glucocorticoid receptor-induced excessive lipolysis in adipocytes via homeostatic crosstalk. J. Cell Biochem. 2018, 119, 4627–4635. [Google Scholar] [CrossRef]
- Teixeira, C.; Sousa, A.P.; Santos, I.; Rocha, A.C.; Alencastre, I.; Pereira, A.C.; Martins-Mendes, D.; Barata, P.; Baylina, P.; Fernandes, R. Enhanced 3T3-L1 Differentiation into Adipocytes by Pioglitazone Pharmacological Activation of Peroxisome Proliferator Activated Receptor-Gamma (PPAR-gamma). Biology 2022, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Mahankali, A.; Matsuda, M.; Mahankali, S.; Hardies, J.; Cusi, K.; Mandarino, L.J.; DeFronzo, R.A. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2002, 87, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Smith, U. Adipose tissue distribution and risk of metabolic disease: Does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia 2007, 50, 1127–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA Carboxylases and Diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Nishimoto, N.; Matsuda, H. Pharmacology of Ecdysones in Vertebrates. In Invertebrate Endocrinology and Hormonal Heterophylly; Burdette, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 341–344. [Google Scholar]
- Seidlova-Wuttke, D.; Christel, D.; Kapur, P.; Nguyen, B.T.; Jarry, H.; Wuttke, W. Beta-ecdysone has bone protective but no estrogenic effects in ovariectomized rats. Phytomedicine 2010, 17, 884–889. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic effects of 20-OH-ecdysone in ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buniam, J.; Chansela, P.; Weerachayaphorn, J.; Saengsirisuwan, V. Dietary Supplementation with 20-Hydroxyecdysone Ameliorates Hepatic Steatosis and Reduces White Adipose Tissue Mass in Ovariectomized Rats Fed a High-Fat, High-Fructose Diet. Biomedicines 2023, 11, 2071. https://doi.org/10.3390/biomedicines11072071
Buniam J, Chansela P, Weerachayaphorn J, Saengsirisuwan V. Dietary Supplementation with 20-Hydroxyecdysone Ameliorates Hepatic Steatosis and Reduces White Adipose Tissue Mass in Ovariectomized Rats Fed a High-Fat, High-Fructose Diet. Biomedicines. 2023; 11(7):2071. https://doi.org/10.3390/biomedicines11072071
Chicago/Turabian StyleBuniam, Jariya, Piyachat Chansela, Jittima Weerachayaphorn, and Vitoon Saengsirisuwan. 2023. "Dietary Supplementation with 20-Hydroxyecdysone Ameliorates Hepatic Steatosis and Reduces White Adipose Tissue Mass in Ovariectomized Rats Fed a High-Fat, High-Fructose Diet" Biomedicines 11, no. 7: 2071. https://doi.org/10.3390/biomedicines11072071




