The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep
Abstract
1. Introduction
2. Memory, Aging, and Sleep Interaction
3. Neurofluids and Memory
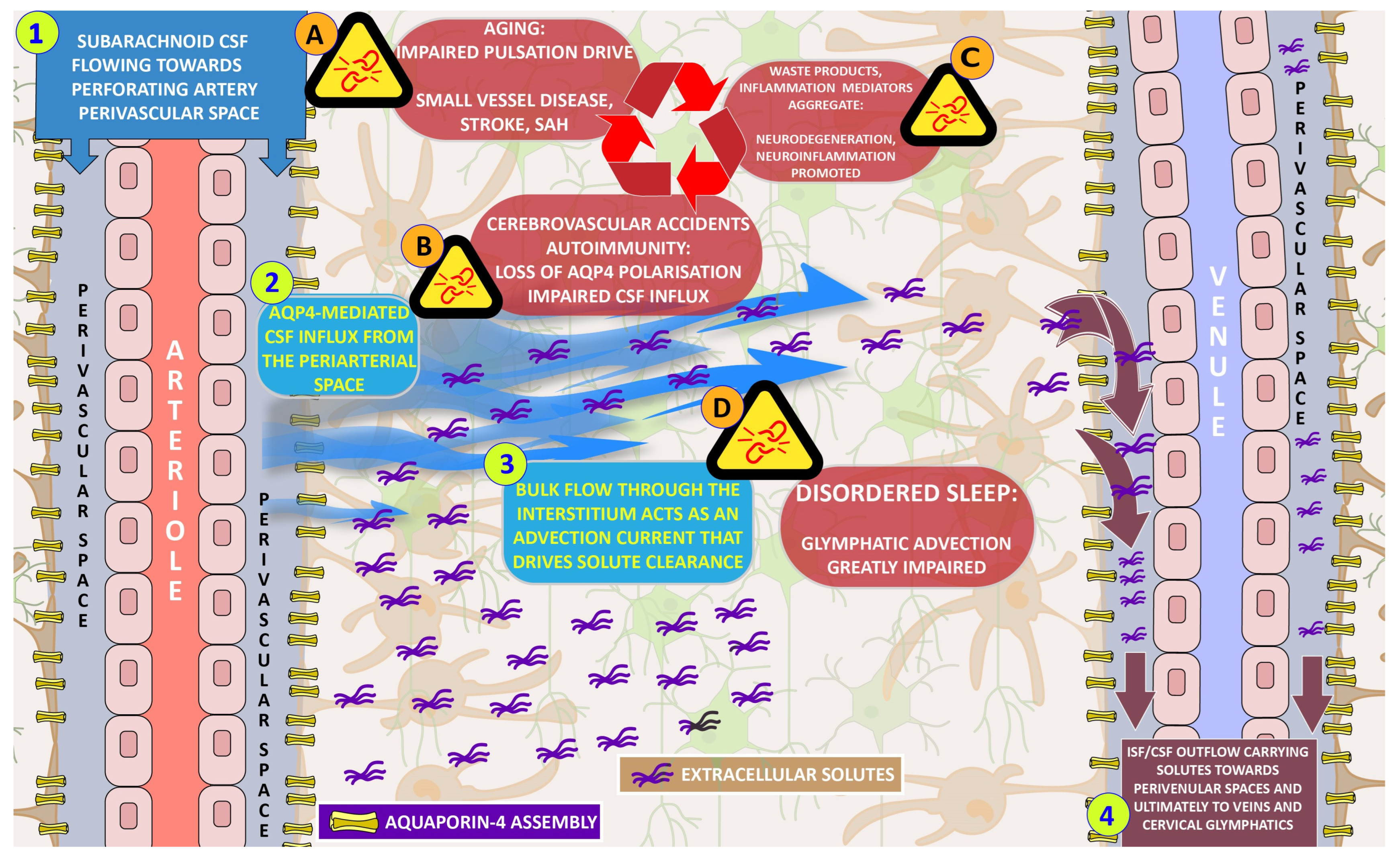
3.1. The Glymphatic System and Its Wider Scope
3.2. Glymphatic System and Brain Functionality
3.3. Glymphatic System, Aging, and Memory
3.4. Sleep and Glymphatic Interactions
3.5. Imaging and Intervention
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Oliveira, E.M.; Kissaki, P.T.; Ordonez, T.N.; Lima-Silva, T.B. A systematic review of the neurobiological aspects of memory in the aging process. Dement. Neuropsychol. 2011, 5, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.-J. Normal aging induces changes in the brain and neurodegeneration progress: Review of the structural, biochemical, metabolic, cellular, and molecular changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Taillard, J.; Gronfier, C.; Bioulac, S.; Philip, P.; Sagaspe, P. Sleep in normal aging, homeostatic and circadian regulation and vulnerability to sleep deprivation. Brain Sci. 2021, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Harand, C.; Bertran, F.; Doidy, F.; Guénolé, F.; Desgranges, B.; Eustache, F.; Rauchs, G. How aging affects sleep-dependent memory consolidation? Front. Neurol. 2012, 3, 8. [Google Scholar] [CrossRef]
- Bah, T.M.; Goodman, J.; Iliff, J.J. Sleep as a therapeutic target in the aging brain. Neurotherapeutics 2019, 16, 554–568. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Wafford, K.A. Aberrant waste disposal in neurodegeneration: Why improved sleep could be the solution. Cereb. Circ. Cogn. Behav. 2021, 2, 100025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in normal aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Nedergaard, M. The glymphatic system: A novel component of fundamental neurobiology. J. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The glymphatic system and waste clearance with brain aging: A review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Naganawa, S.; Taoka, T. The glymphatic system: A review of the challenges in visualizing its structure and function with MR imaging. Magn. Reson. Med. Sci. 2022, 21, 182–194. [Google Scholar] [CrossRef]
- Banks, W.A.; Reed, M.J.; Logsdon, A.F.; Rhea, E.M.; Erickson, M.A. Healthy aging and the blood-brain barrier. Nat. Aging 2021, 1, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Gao, P.; Zhu, T.; Yin, H.; Jin, S. Glymphatic system dysfunction: A novel mediator of sleep disorders and headaches. Front. Neurol. 2022, 13, 885020. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef]
- Christophel, T.B.; Klink, P.C.; Spitzer, B.; Roelfsema, P.R.; Haynes, J.-D. The distributed nature of working memory. Trends Cogn. Sci. 2017, 21, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. Long-Term Synaptic Potentiation; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 2019, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J.; Salinas, S.; Huang, H.-Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar] [CrossRef]
- Schreurs, A.; Sabanov, V.; Balschun, D. Distinct properties of long-term potentiation in the dentate gyrus along the dorsoventral axis: Influence of age and inhibition. Sci. Rep. 2017, 7, 5157. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef]
- Reyes-Resina, I.; Samer, S.; Kreutz, M.R.; Oelschlegel, A.M. Molecular mechanisms of memory consolidation that operate during sleep. Front. Mol. Neurosci. 2021, 14, 767384. [Google Scholar] [CrossRef] [PubMed]
- Bisaz, R.; Travaglia, A.; Alberini, C.M. The neurobiological bases of memory formation: From physiological conditions to psychopathology. Psychopathology 2014, 47, 347–356. [Google Scholar] [CrossRef]
- Kukushkin, N.V.; Carew, T.J. Memory takes time. Neuron 2017, 95, 259–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Busquets-Garcia, A.; Gomis-González, M.; Salgado-Mendialdúa, V.; Galera-López, L.; Puighermanal, E.; Martín-García, E.; Maldonado, R.; Ozaita, A. Hippocampal protein kinase C signaling mediates the short-term memory impairment induced by Delta9-tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 1021–1031. [Google Scholar] [CrossRef]
- Griffith, L.C. Calcium/calmodulin-dependent protein kinase II: An unforgettable kinase. J. Neurosci. 2004, 24, 8391–8393. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Laroche, S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: A review. Genes Brain Behav. 2006, 5 (Suppl. S2), 61–72. [Google Scholar] [CrossRef]
- Chai, W.J.; Abd Hamid, A.I.; Abdullah, J.M. Working memory from the psychological and neurosciences perspectives: A review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Dash, P.K.; Moore, A.N.; Kobori, N.; Runyan, J.D. Molecular activity underlying working memory. Learn. Mem. 2007, 14, 554–563. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular senescence in brain aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef]
- Olesen, M.A.; Torres, A.K.; Jara, C.; Murphy, M.P.; Tapia-Rojas, C. Premature synaptic mitochondrial dysfunction in the hippocampus during aging contributes to memory loss. Redox Biol. 2020, 34, 101558. [Google Scholar] [CrossRef]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. 2014, 13, 35–43. [Google Scholar]
- Finger, C.E.; Moreno-Gonzalez, I.; Gutierrez, A.; Moruno-Manchon, J.F.; McCullough, L.D. Age-related immune alterations and cerebrovascular inflammation. Mol. Psychiatry 2022, 27, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lim, H.-S.; Masliah, E.; Lee, H.-J. Protein aggregate spreading in neurodegenerative diseases: Problems and perspectives. Neurosci. Res. 2011, 70, 339–348. [Google Scholar] [CrossRef]
- Wells, C.; Brennan, S.; Keon, M.; Ooi, L. The role of amyloid oligomers in neurodegenerative pathologies. Int. J. Biol. Macromol. 2021, 181, 582–604. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sang-Sun, Y.; Sangmee Ahn, J. Mechanisms of amyloid-β peptide clearance: Potential therapeutic targets for Alzheimer’s disease. Biomol. Ther. 2012, 20, 245–255. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Mauri, S.; Favaro, M.; Bernardo, G.; Mazzotta, G.M.; Ziviani, E. Mitochondrial autophagy in the sleeping brain. Front. Cell Dev. Biol. 2022, 10, 956394. [Google Scholar] [CrossRef]
- Sharma, S.; Kavuru, M. Sleep and metabolism: An overview. Int. J. Endocrinol. 2010, 2010, 270832. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Dworak, M.; McCarley, R.W.; Kim, T.; Kalinchuk, A.V.; Basheer, R. Sleep and brain energy levels: ATP changes during sleep. J. Neurosci. 2010, 30, 9007–9016. [Google Scholar] [CrossRef]
- Donlea, J.M. Neuronal and molecular mechanisms of sleep homeostasis. Curr. Opin. Insect Sci. 2017, 24, 51–57. [Google Scholar] [CrossRef]
- Nguyen, G.; Postnova, S. Progress in modelling of brain dynamics during anaesthesia and the role of sleep-wake circuitry. Biochem. Pharmacol. 2021, 191, 114388. [Google Scholar] [CrossRef]
- Brunetti, V.; Della Marca, G.; Servidei, S.; Primiano, G. Sleep disorders in mitochondrial diseases. Curr. Neurol. Neurosci. Rep. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Jeong, Y.Y. Mitophagy in Alzheimer’s disease and other age-related neurodegenerative diseases. Cells 2020, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Kokošová, V.; Filip, P.; Kec, D.; Baláž, M. Bidirectional association between sleep and brain atrophy in aging. Front. Aging Neurosci. 2021, 13, 726662. [Google Scholar] [CrossRef]
- Cordone, S.; Scarpelli, S.; Alfonsi, V.; De Gennaro, L.; Gorgoni, M. Sleep-based interventions in Alzheimer’s disease: Promising approaches from prevention to treatment along the disease trajectory. Pharmaceuticals 2021, 14, 383. [Google Scholar] [CrossRef]
- Torossian, M.; Fiske, S.M.; Jacelon, C.S. Sleep, mild cognitive impairment, and interventions for sleep improvement: An integrative review. West J. Nurs. Res. 2021, 43, 1051–1060. [Google Scholar] [CrossRef]
- Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 1981, 240, F319–F328. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Aquaporin-4, homeostasis, and neurologic disease. Neurology 2007, 69, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Subash, M.; Preston, S.D.; Mazanti, I.; Carare, R.O. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease: Perivascular drainage of Aβ peptides and cerebral amyloid angiopathy. Brain Pathol. 2008, 18, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-J.; Smith, A.J.; Verkman, A.S. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol. 2016, 148, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kedarasetti, R.T.; Drew, P.J.; Costanzo, F. Arterial pulsations drive oscillatory flow of CSF but not directional pumping. Sci. Rep. 2020, 10, 10102. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Contarino, C.; Toro, E.F. Neurofluids: A holistic approach to their physiology, interactive dynamics and clinical implications for neurological diseases. Veins Lymphat. 2019, 8. [Google Scholar] [CrossRef]
- Agarwal, N.; Carare, R.O. Cerebral vessels: An overview of anatomy, physiology, and role in the drainage of fluids and solutes. Front. Neurol. 2020, 11, 611485. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-immune hemostasis: Homeostasis and diseases in the central nervous system. Front. Cell. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef]
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Shetty, A.K.; Zanirati, G. The interstitial system of the brain in health and disease. Aging Dis. 2020, 11, 200–211. [Google Scholar] [CrossRef]
- McKenzie, S.; Eichenbaum, H. New approach illuminates how memory systems switch. Trends Cogn. Sci. 2012, 16, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Kylkilahti, T.M.; Berends, E.; Ramos, M.; Shanbhag, N.C.; Töger, J.; Markenroth Bloch, K.; Lundgaard, I. Achieving brain clearance and preventing neurodegenerative diseases-A glymphatic perspective. J. Cereb. Blood Flow Metab. 2021, 41, 2137–2149. [Google Scholar] [CrossRef]
- Filiano, A.J.; Gadani, S.P.; Kipnis, J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci. 2017, 18, 375–384. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Chee, S.E.J.; Solito, E. The impact of ageing on the CNS immune response in Alzheimer’s disease. Front. Immunol. 2021, 12, 738511. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in aging and Alzheimer’s disease: A comparative species review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.; Park, E.J.; Kwon, S.; Kim, H.; Lee, J.Y.; Lee, D.S. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci. Rep. 2020, 10, 16144. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, S.; Lian, C.; Li, H.; Li, K.; Li, L.; Zhao, G. Dysfunction of the glymphatic system as a potential mechanism of perioperative neurocognitive disorders. Front. Aging Neurosci. 2021, 13, 659457. [Google Scholar] [CrossRef]
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.E.; Reddy, V.; Sharma, S. Physiology of Sleep; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Lundgaard, I.; Lu, M.L.; Yang, E.; Peng, W.; Mestre, H.; Hitomi, E.; Deane, R.; Nedergaard, M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 2017, 37, 2112–2124. [Google Scholar] [CrossRef]
- O’Donnell, J.; Zeppenfeld, D.; McConnell, E.; Pena, S.; Nedergaard, M. Norepinephrine: A neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res. 2012, 37, 2496–2512. [Google Scholar] [CrossRef] [PubMed]
- Maestú, F.; de Haan, W.; Busche, M.A.; DeFelipe, J. Neuronal excitation/inhibition imbalance: Core element of a translational perspective on Alzheimer pathophysiology. Ageing Res. Rev. 2021, 69, 101372. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Sleep Deprivation Increases Alzheimer’s Protein. 2018. Available online: https://www.nih.gov/news-events/nih-research-matters/sleep-deprivation-increases-alzheimers-protein (accessed on 5 April 2023).
- Wang, Y.; Huang, C.; Guo, Q.; Chu, H. Aquaporin-4 and cognitive disorders. Aging Dis. 2022, 13, 61–72. [Google Scholar] [CrossRef]
- Chen, D.-W.; Wang, J.; Zhang, L.-L.; Wang, Y.-J.; Gao, C.-Y. Cerebrospinal fluid amyloid-β levels are increased in patients with insomnia. J. Alzheimer’s Dis. 2017, 61, 645–651. [Google Scholar] [CrossRef]
- Tzschätzsch, H.; Kreft, B.; Schrank, F.; Bergs, J.; Braun, J.; Sack, I. In vivo time-harmonic ultrasound elas tography of the human brain detects acute cerebral stiffness changes induced by intracranial pressure variations. Sci. Rep. 2018, 8, 17888. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Taoka, T.; Ito, R.; Kawamura, M. The glymphatic system in humans: Investigations with magnetic resonance imaging. Investig. Radiol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Joo, B.; Won, S.Y.; Sinkus, R.; Lee, S.-K. Viscoelastic property of the brain assessed with magnetic resonance elastography and its association with glymphatic system in neurologically normal individuals. Korean J. Radiol. 2023, 24, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabětová, S.; Kiviniemi, V.; Lilius, T. The glymphatic system: Current understanding and modeling. iScience 2022, 25, 104987. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Solomonia, R.O.; McCabe, B.J. Molecular mechanisms of memory in imprinting. Neurosci. Biobehav. Rev. 2015, 50, 56–69. [Google Scholar] [CrossRef]
| Long-Term Memory (LTM) | Short-Term Memory (STM) | Working Memory (WM) |
|---|---|---|
| Storage and Retrieval of information over an extended period of time | Temporary storage and manipulation of informationLimited Capacity | Responsible for temporarily holding and actively manipulating information |
| Unlimited Capacity Two main types: Explicit/Declarative Memory (Facts, concepts, general knowledge) Implicit/Non-declarative Memory (motor skills, practices) Main location: Hippocampus, amygdala | A small amount of information for a brief duration of data (20–30 s) Processing of incoming sensory information Main location: The lower part of the temporal lobe | Limited Capacity Interact with attentional control, information updating, and mental manipulation of stored information Main location: Frontoparietal brain regions and prefrontal, cingulate, and parietal cortices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voumvourakis, K.I.; Sideri, E.; Papadimitropoulos, G.N.; Tsantzali, I.; Hewlett, P.; Kitsos, D.; Stefanou, M.; Bonakis, A.; Giannopoulos, S.; Tsivgoulis, G.; et al. The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep. Biomedicines 2023, 11, 2092. https://doi.org/10.3390/biomedicines11082092
Voumvourakis KI, Sideri E, Papadimitropoulos GN, Tsantzali I, Hewlett P, Kitsos D, Stefanou M, Bonakis A, Giannopoulos S, Tsivgoulis G, et al. The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep. Biomedicines. 2023; 11(8):2092. https://doi.org/10.3390/biomedicines11082092
Chicago/Turabian StyleVoumvourakis, Konstantinos I., Eleni Sideri, Georgios N. Papadimitropoulos, Ioanna Tsantzali, Paul Hewlett, Dimitrios Kitsos, Marianna Stefanou, Anastasios Bonakis, Sotirios Giannopoulos, Georgios Tsivgoulis, and et al. 2023. "The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep" Biomedicines 11, no. 8: 2092. https://doi.org/10.3390/biomedicines11082092
APA StyleVoumvourakis, K. I., Sideri, E., Papadimitropoulos, G. N., Tsantzali, I., Hewlett, P., Kitsos, D., Stefanou, M., Bonakis, A., Giannopoulos, S., Tsivgoulis, G., & Paraskevas, G. P. (2023). The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep. Biomedicines, 11(8), 2092. https://doi.org/10.3390/biomedicines11082092













