Abstract
With the Omicron wave, SARS-CoV-2 infections improved, with less lung involvement and few cases of severe manifestations. In this pictorial review, there is a summary of the pathogenesis with particular focus on the interaction of the immune system and gut and lung axis in both pulmonary and extrapulmonary manifestations of COVID-19 and the computed tomography (CT) imaging features of COVID-19 pneumonia from the beginning of the pandemic, describing the typical features of COVID-19 pneumonia following the Delta variant and the atypical features appearing during the Omicron wave. There is also an outline of the typical features of COVID-19 pneumonia in cases of breakthrough infection, including secondary lung complications such as acute respiratory distress disease (ARDS), pneumomediastinum, pneumothorax, and lung pulmonary thromboembolism, which were more frequent during the first waves of the pandemic. Finally, there is a description of vascular extrapulmonary complications, including both ischemic and hemorrhagic abdominal complications.
1. Introduction
At the end of 2019, a novel pneumonia of unknown origin was first detected in Wuhan, China. The virus responsible for this “unknown pneumonia” was called SARS-CoV-2, and the disease was subsequently named COVID-19. The first cases of this novel disease appeared in China. However, several authors documented the presence of antibodies for SARS-CoV-2 in the European population before its discovery in China [1,2,3]. COVID-19 shocked health and economic systems worldwide. Industrialized countries experienced more damage in terms of deaths and economic stress caused by applying measures of closures and isolation, mainly based on political decisions. COVID-19 attracted the attention of many researchers, and many publications were also produced by non-academics with important contributions. COVID-19′s origin is considered natural [4,5,6,7]. Nonetheless, several authors have not excluded the hypothesis of a laboratory origin, posing the question of biological risks [8,9,10,11]. The first phase of the pandemic was characterized by severe cases of interstitial pneumonia and lung thromboembolisms and a rapid evolution of acute respiratory distress disease (ARDS). Initially, autopsies on deceased COVID-19 patients were scarce due to significant concerns, such as the risk of contagion, a lack of healthcare specialists, and the abrupt outbreak of the disease [12]. However, with increased clinical experience and the first COVID-19 autopsies, the crucial role of vasculature damage and the hyperinflammatory state in COVID-19 pathology became clear [12,13]. Therefore, COVID-19 evidently involves not only the lungs but also the gastrointestinal and central nervous systems [14,15]. The gastrointestinal system’s role remains under investigation, with the gut microbiome possibly implicated in disease severity [16,17]. Radiologists played an important role during the emergence of the pandemic. At the beginning of the pandemic, Chinese radiologists reported in detail the characteristics of COVID-19 pneumonia, and the main features of this disease were easily available worldwide [18,19]. Chest CT was used as the main imaging modality to score interstitial pneumonia and, along with clinical parameters such as age, D-dimer level, and albumin. It was an important predictive and prognostic indicator for disease severity and progression [20,21,22]. CT also depicted the systemic complications of the disease caused by an exaggerated inflammatory response, which involved severe alveolar damage in the lungs and exacerbated the hypercoagulation that led to venous thrombosis, ischemic attack, vascular dysfunction, and the infarction of the visceral abdominal organs [23,24]. Some hemorrhagic complications in the first waves of the pandemic were also a consequence of anticoagulant therapy [25,26]. However, the disease presentations improved with the Omicron variant, with less lung involvement and few cases of severe manifestations [27]. Vaccination and acquired immunity together with the lower virulence of the Omicron variant decreased the rate of severe infections [28]. Nevertheless, the current COVID-19 vaccines are limited, presenting a short duration of protection and risk of breakthrough infection, i.e., COVID-19 infection in a fully vaccinated person [29,30,31,32,33]. Risk factors for pneumonia severity in fully vaccinated people include old age, an immunosuppressed status, and the presence of comorbidities [29,30,31,32,33,34]. This paper presents a pictorial review on the pathogenesis, also focusing on the role of the gut and lung axis, the main features of radiological presentations of COVID-19 throughout the pandemic, with a focus on secondary lung complications, lung features in breakthrough infections, and abdominal vascular complications.
2. SARS-CoV-2 Pathogenesis
The Important Role of the Immune System and of Gut–Lung Axis in Both Pulmonary and Extrapulmonary Manifestations
SARS-CoV-2 was a previously unknown coronavirus, and the first viral genome was sequenced using high-throughput sequencing (HTS) from a sample collected in Wuhan, China [35]. SARS-CoV-2 shows high genetic similarities with SARS-CoV, the causative agent of SARS, and as SARS-CoV, it binds through the spike protein to the angiotensin-converting enzyme 2 (ACE2) receptors, which are largely expressed in the lung, heart, vascular, and gastrointestinal systems [36,37]. ACE2 expression usually increases with age, and this feature could be a possible explanation for the aggressive form of COVID-19 disease that was more commonly found in the elderly [37].
COVID-19 manifestations can vary from an asymptomatic to a mild symptomatic disease, with a prevalence of respiratory symptoms such as cough and dyspnea to moderate/severe disease with lung interstitial pneumonia to acute distress respiratory syndromes to multisystemic involvement [38,39]. However, COVID-19 can manifest itself with extrapulmonary symptoms as well, together with gastrointestinal and neurological manifestations [38,39].
Throughout the pandemic, it became clear that a dysregulation of the immune response, also known as the “cytokine storms”, played a key role in disease severity, both in pulmonary and in extrapulmonary manifestations [40,41]. In results from some recent studies regarding disease severity, there seems to be an involvement of the intricate complex of both the innate and adaptive immune response, also evidencing an imbalance between the hyperinflammatory and the immunosuppressive phenomena [40,41,42,43]. On the other hand, the disease severity seems to be directly correlated with early humoral immune response. It also seems inversely related to early cellular immune response [40,41,42]. Moreover, dysregulated innate and adaptive immune responses usually occur in parallel with activation of coagulation, and these features could explain both the vascular pulmonary and extrapulmonary manifestations of COVID-19 [40,41,42,43,44].
However, the interaction between the immunity and inflammations of the lung and gut microbiome and the role of gut–lung axis in the immune response in the context of COVID-19 continues to be studied [45,46,47]. Observing from a different perspective, it is evident that an alteration of the gut microbiome can be associated with many respiratory diseases. Furthermore, dysbiosis of gut microbiota was found to be implicated in various alterations of the immune system [46,47]. The intestinal microbiota affects the expression of the type I interferon receptors in respiratory epithelial cells [47].
During the pandemic, cases of SARS-CoV-2 positivity in the stool also emerged, and this feature posed some questions on SARS-CoV-2 epidemiology [48,49,50].
Recently, researchers have found a close interaction between SARS-CoV-2 and intestinal bacteria, reporting some toxicological interactions between the bacteria of the microbiome and SARS-CoV-2. This discovery could explain the lung involvement severity and could have a role in the long-COVID symptoms [16,50,51,52,53]. In particular, some “toxin-like” peptides were observed in bacteria stools samples of COVID-19 patients, and the toxins found are similar to conotoxin-like peptides, phospholipase A2, and neurotoxins. These findings could also explain the atypical features of ARDS in COVID-19 patients [13].
With this consideration, this scenario could open new therapeutic approaches also for the development of new vaccines.
3. Lung Complications
3.1. COVID-19 Pneumonia: Role of Chest CT and Chest Features from the Wild-Type to the Omicron Variant
The lungs were frequently involved in SARS-CoV-2 infection during the first pandemic’s waves; about 80% of patients with COVID-19 developed pneumonia up to and including the Delta variant [54].
Chest CT was widely used in China and globally in the early phases of the pandemic before the results of molecular SARS-CoV-2 nasal swabs were rapid and available. Chest CT was used due to its rapidity and to limit the contact between the radiographers and the infected patients [21,37,55,56].
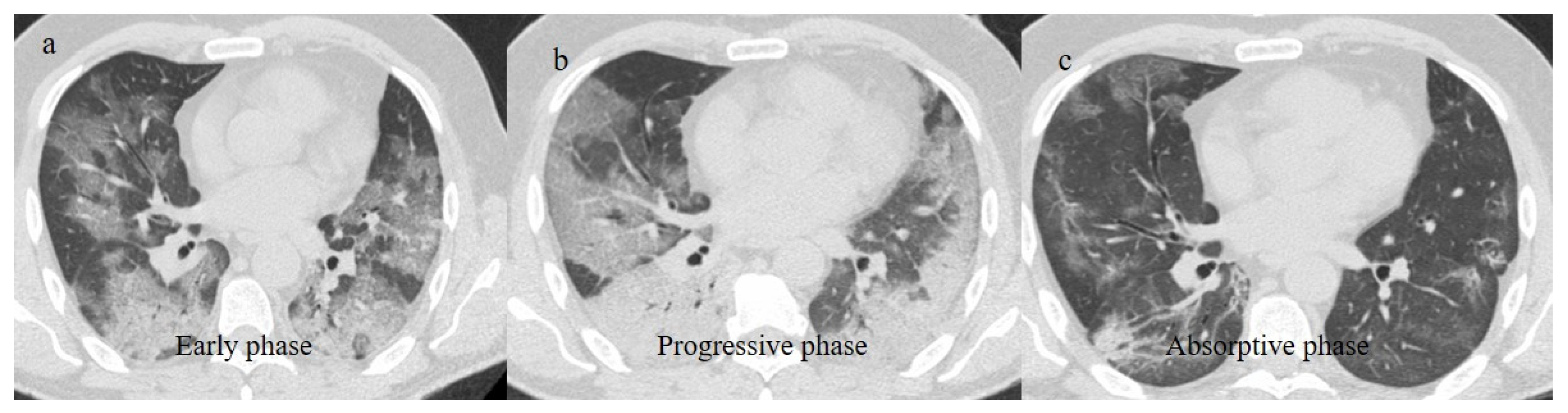
Chest CT was also important in the staging and follow-up of COVID-19 interstitial pneumonia [21,55,56,57] (Figure 1).
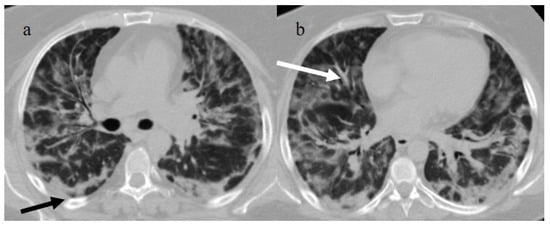
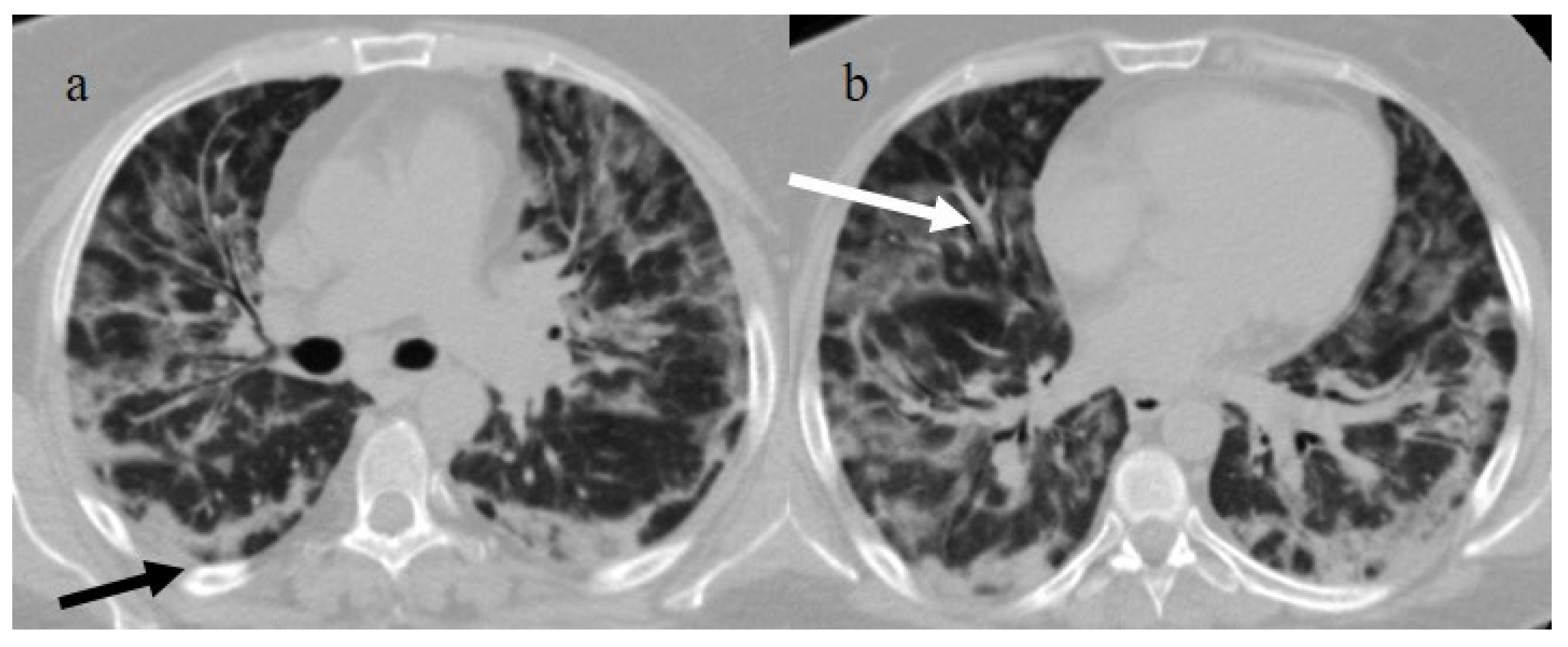
Figure 1.
Typical central and peripheral distribution in COVID-19 at the beginning of the pandemic in Italy (March 2020) in a 50-year-old patient with a history of arterial hypertension. The CT was carried out in an emergency, with an initial diagnosis suspected COVID-19 pneumonia made by the radiologist before the RT-PCR results, and a suspicion of COVID-19 pneumonia was raised by radiologists. In the image, (a) the subpleural bands (black arrow) and (b) the vascular enlargement (white arrow) are visible.
However, the Fleishner Society recommended the use of chest CT in emergency settings only for the triage of symptomatic COVID-19 patients presenting respiratory symptoms with a decline in O2 saturation level and patients at major risk of complications; it is not indicated to screen patients with COVID-19 [55]. At the beginning of the pandemic, Chinese radiologists described in detail the typical distribution of COVID-19 pneumonia, i.e., peripheral or peripheral and central. Ground glass opacities (GGOs) were visible in the early phase; as the pathology progressed, GGOs with a crazy-paving appearance and consolidations were evident along with subpleural and parenchymal bands. There was also a predominance of architectural distortion; in the peak stage, these findings had progressed, while the later stage demonstrated their resolution [55,56,57,58,59,60] (Figure 2).
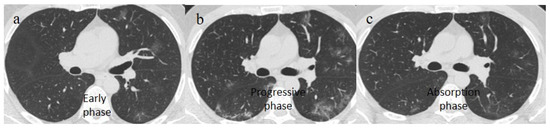
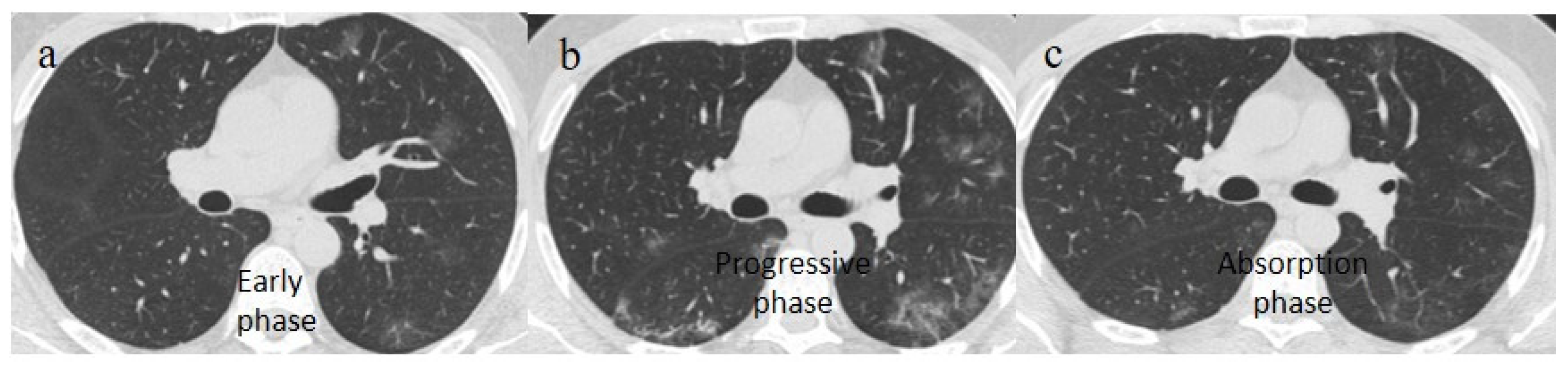
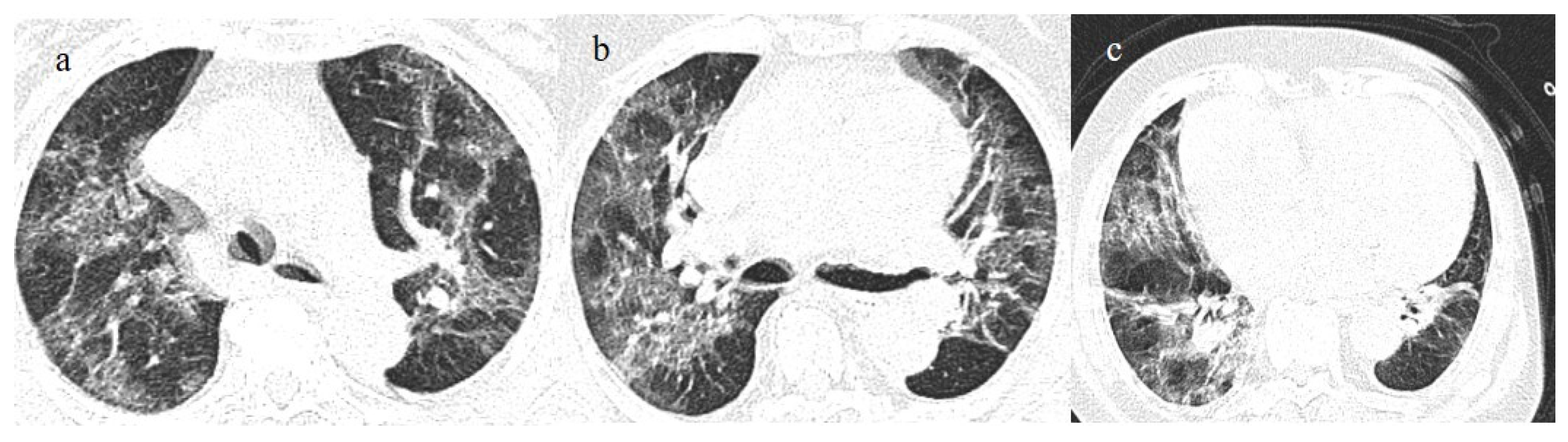
Figure 2.
Chest CT COVID-19 pneumonia evolution during serial follow-up; (a) early phase with some GGO areas in a typical central and peripheral distribution; (b) the progressive phase with evolution in crazy paving with initial consolidation areas; (c) absorptive phase with reduction in previous inflammatory areas.
Other typical signs were vessel enlargements, the reversed halo sign, and subpleural curvilinear lines (Figure 1 and Table 1).

Table 1.
This table summarizes the typical, indeterminate, and atypical appearances of COVID-19 pneumonia; ground glass opacities (GGOs).
Atypical appearances included lobar consolidation, lung nodules or masses, miliary patterns, cavitation, and pleural effusion [61].
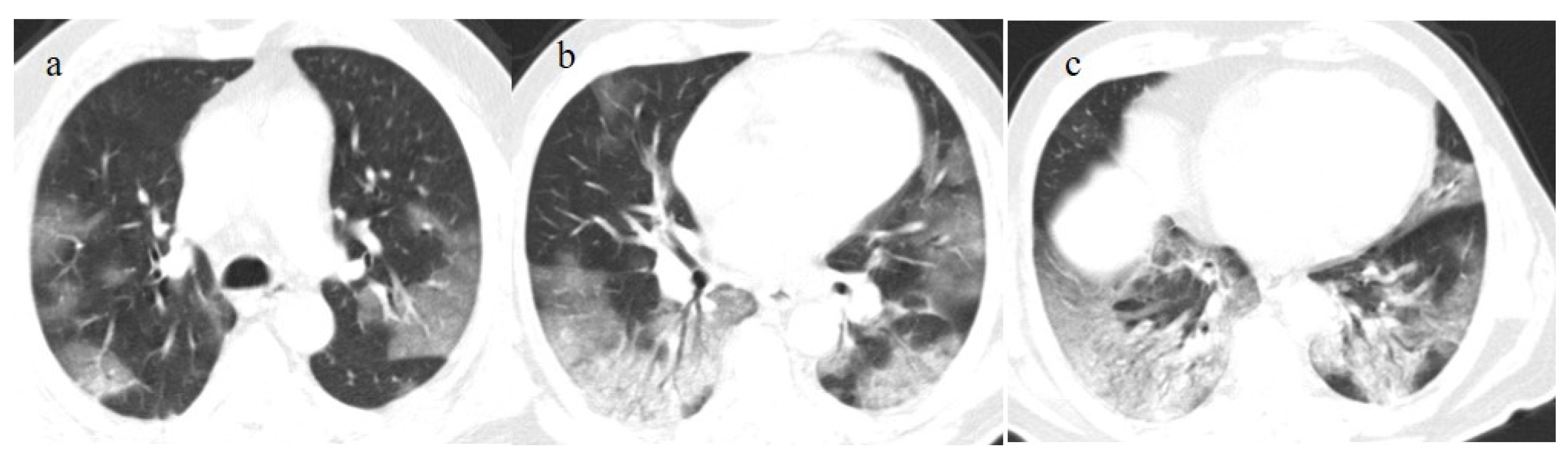
The differential diagnosis of COVID-19 pneumonia includes other infective forms of pneumonia such as bacterial pneumonia (usually lobar consolidation) and atypical pneumonia (multifocal distribution with centrilobular nodules), other viral forms of pneumonia, and cardiovascular pathologies such as pulmonary edema [62] (Figure 3 and Table 1).
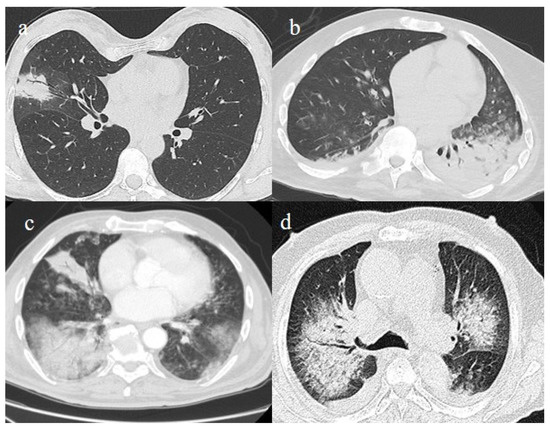
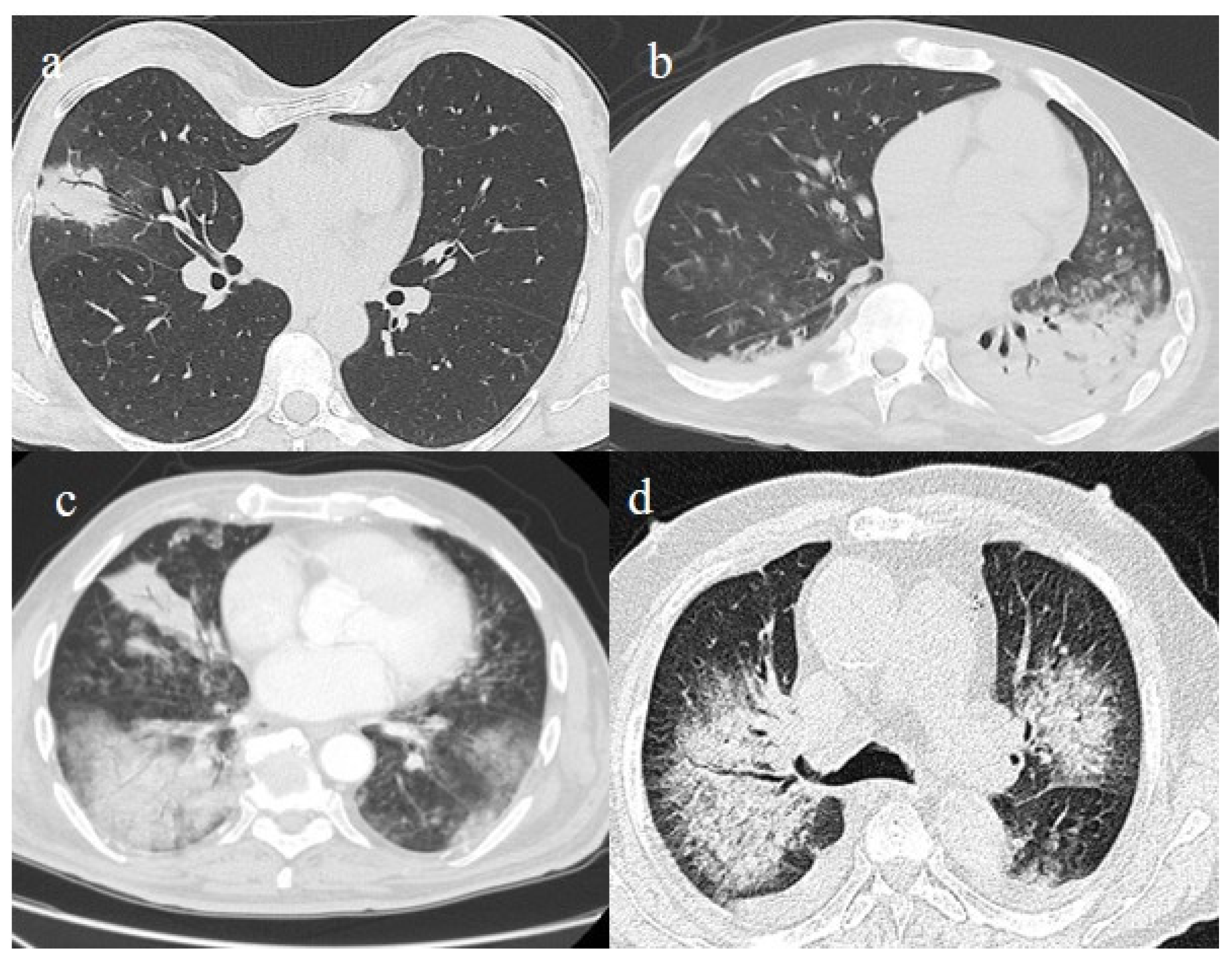
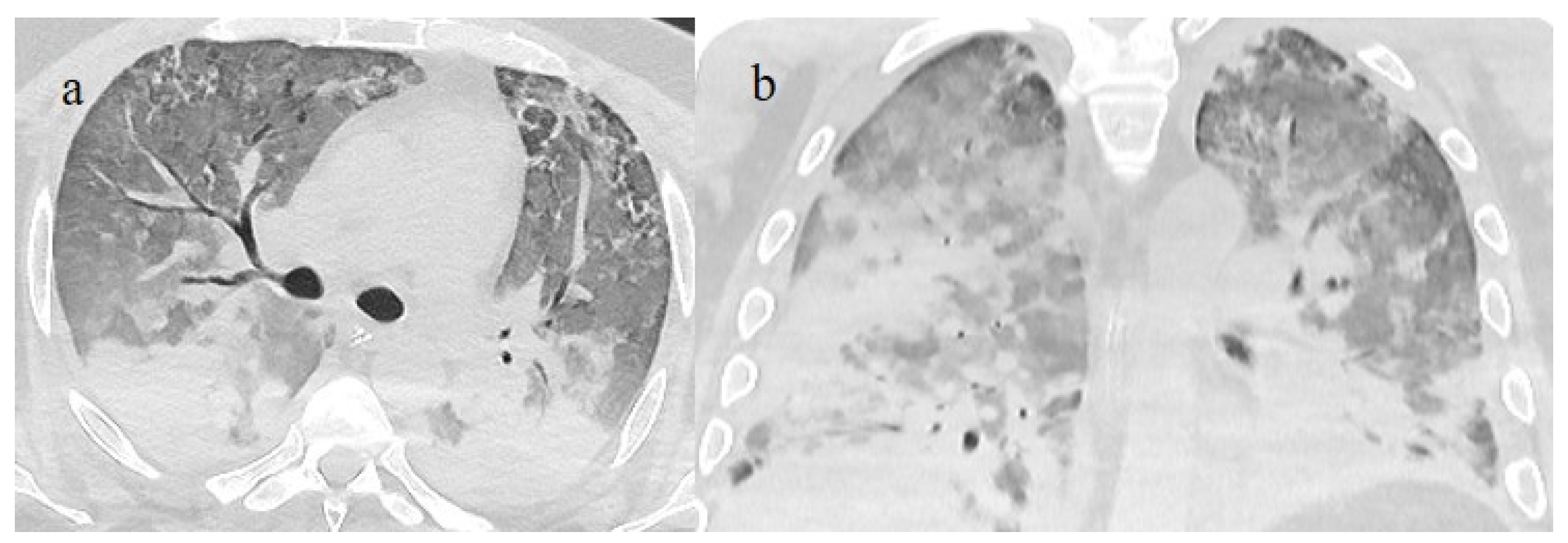
Figure 3.
In this figure some differential diagnosis of COVID-19 pneumonia with atypical features are shown; in image (a), a solitary segmental bronchopneumonia is seen; image (b) shows an atypical bacteria pneumonia with consolidation areas and centrolobular nodules in a patient with immunosuppression state with positivity on BAL of Escherichia coli; image (c) illustrates an atypical pneumonia with multiple consolidations and centrolobular nodules in a patients with syncytial respiratory virus infection; image (d) shows consolidations with central hilar distribution ad bilateral pleural effusion with pulmonary edema.
It is usually possible to differentiate COVID-19 pneumonia from other viral pneumonia, such as that of influenza or syncytial and parainfluenza viruses and other coronaviruses, with a CT scan [63,64,65]. The diagnostic accuracy usually increases with the use of deep learning [66]. The resulting CT features that have been reported as the most strongly associated with COVID-19 pneumonia are the subpleural band of GGOs, where thickening of the bronchial walls and micronodules are usually more frequently associated with other viral pneumonia [63,64,65] (Figure 3c). However, some CT findings may overlap between COVID-19 pneumonia in influenza pneumonia [65]. Both COVID-19 and influenza pneumonia could show a diffused distribution; however, a lower-lobe-predominant distribution was reported to be more common in COVID-19, and an upper-lung-predominant distribution is slightly more common in influenza [65].
The radiology reporting system during the pandemic included the typical, atypical, and indeterminate features of COVID-19 pneumonia on the basis of RSNA classification; the grading of suspicion (using the CO-RADS classification); and the staging of pneumonia extension based on chest CT severity score (CT-SS) with semiquantitative or quantitative methods [37]. Typical features of COVID-19 pneumonia have been reported since the Delta variant [54,67,68,69,70,71]. Blanca et al. [70] found no significant differences in terms of mortality between the first and second waves of the pandemic. However, a worse long-term prognosis was found for the second wave [72] (Figure 4). These findings could be explained by the fact that the patients infected in the first wave were significantly younger than those in the second wave [72,73,74].
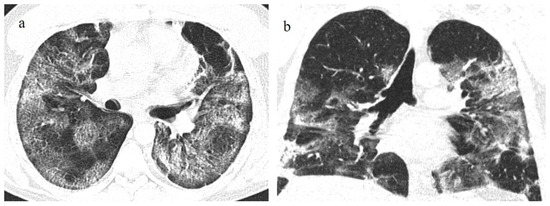
Figure 4.
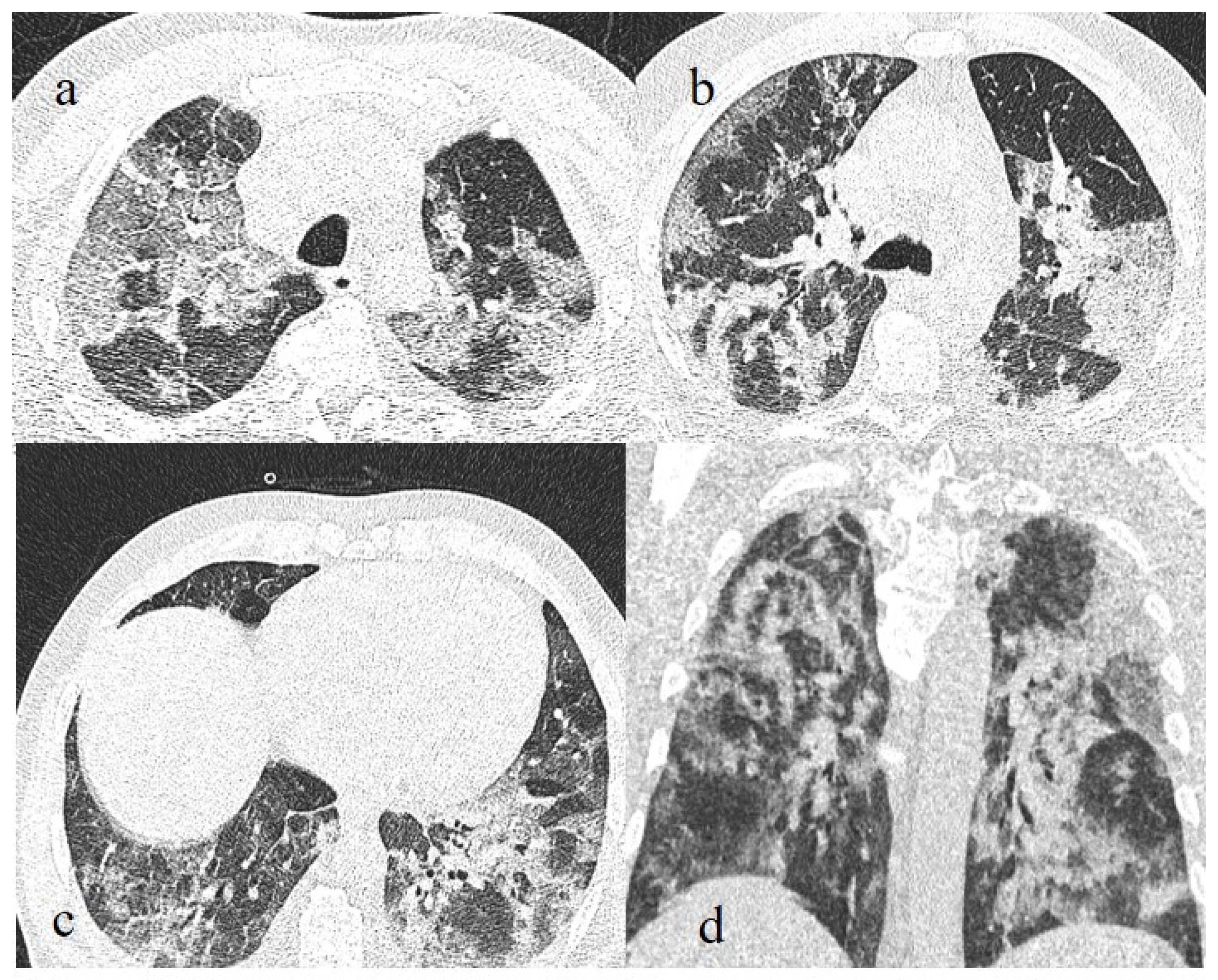
A 41-year-old patient during the Alpha wave (November 2020). The chest CT showed diffuse interstitial thickness with GGOs in a typical distribution on the axial plane (a) and coronal plane (b) and a high score. The patient had a history of bronchial asthma with any other known comorbidities. He was treated at home with paracetamol and azithromycin. He was hospitalized and died.
Inui et al. [68] compared chest CT features and severity scores between patients infected with wild-type, Alpha, and Delta variants of SARS-CoV-2 and found that pneumonia severity was highest for the Delta variant. COVID-19 pneumonia caused by the Delta variant also showed GGOs with reticulation or consolidation and more rapid repair capabilities than the wild-type and Alpha variants (Figure 5 and Figure 6). In contrast, no differences were found in the CT results between the wild-type and Alpha variants [67].
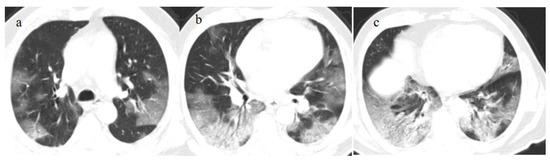
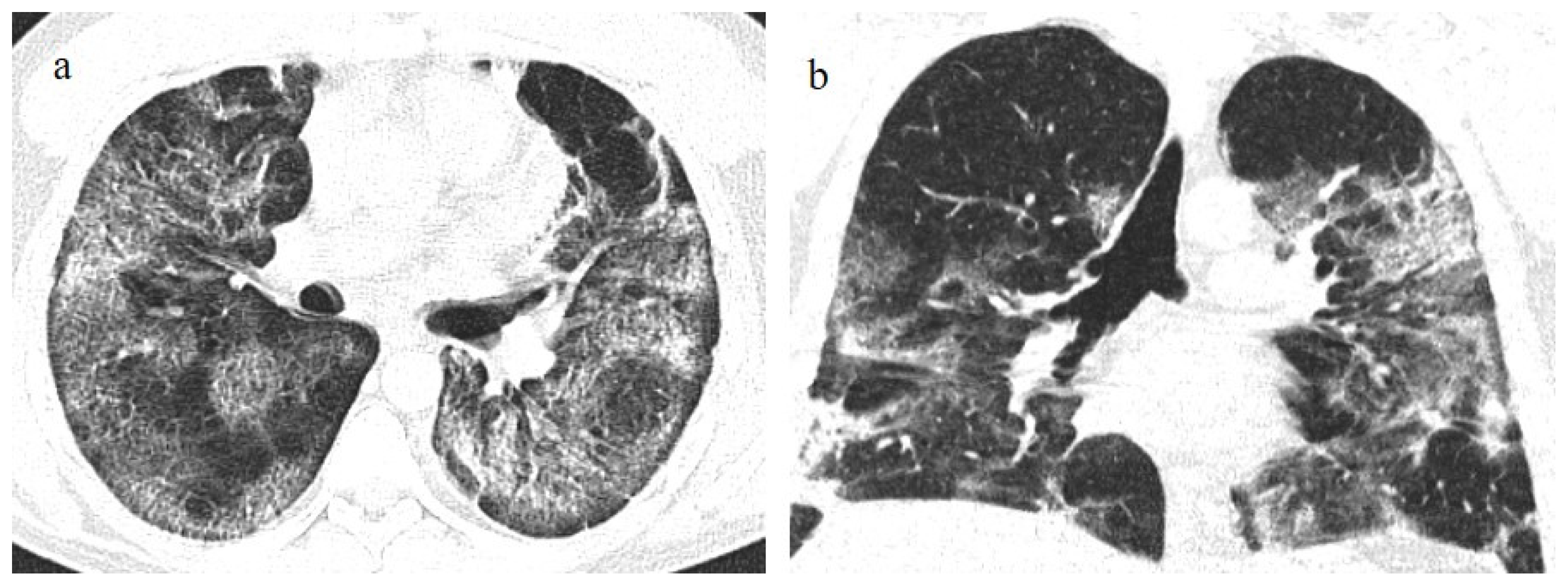
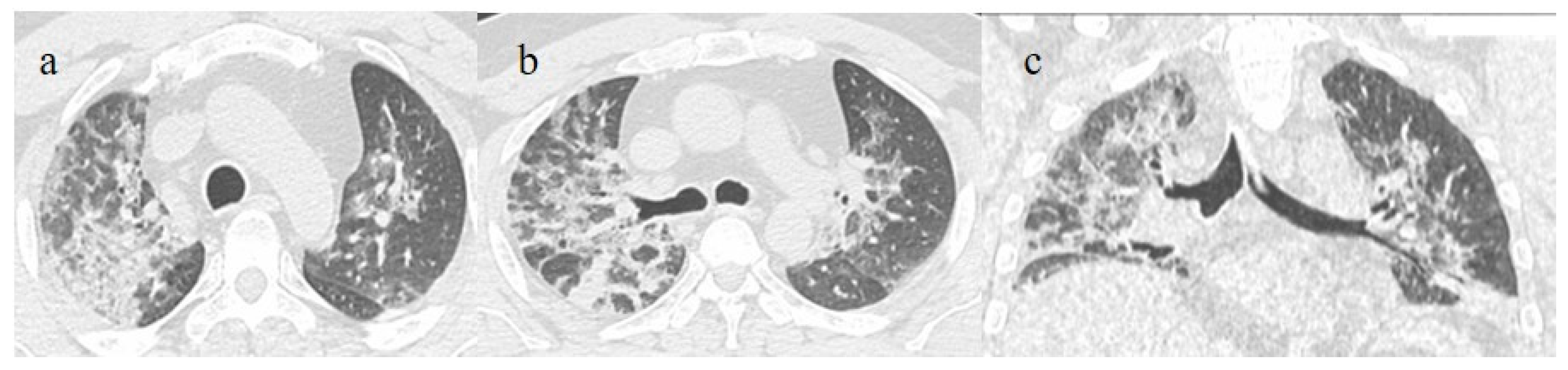
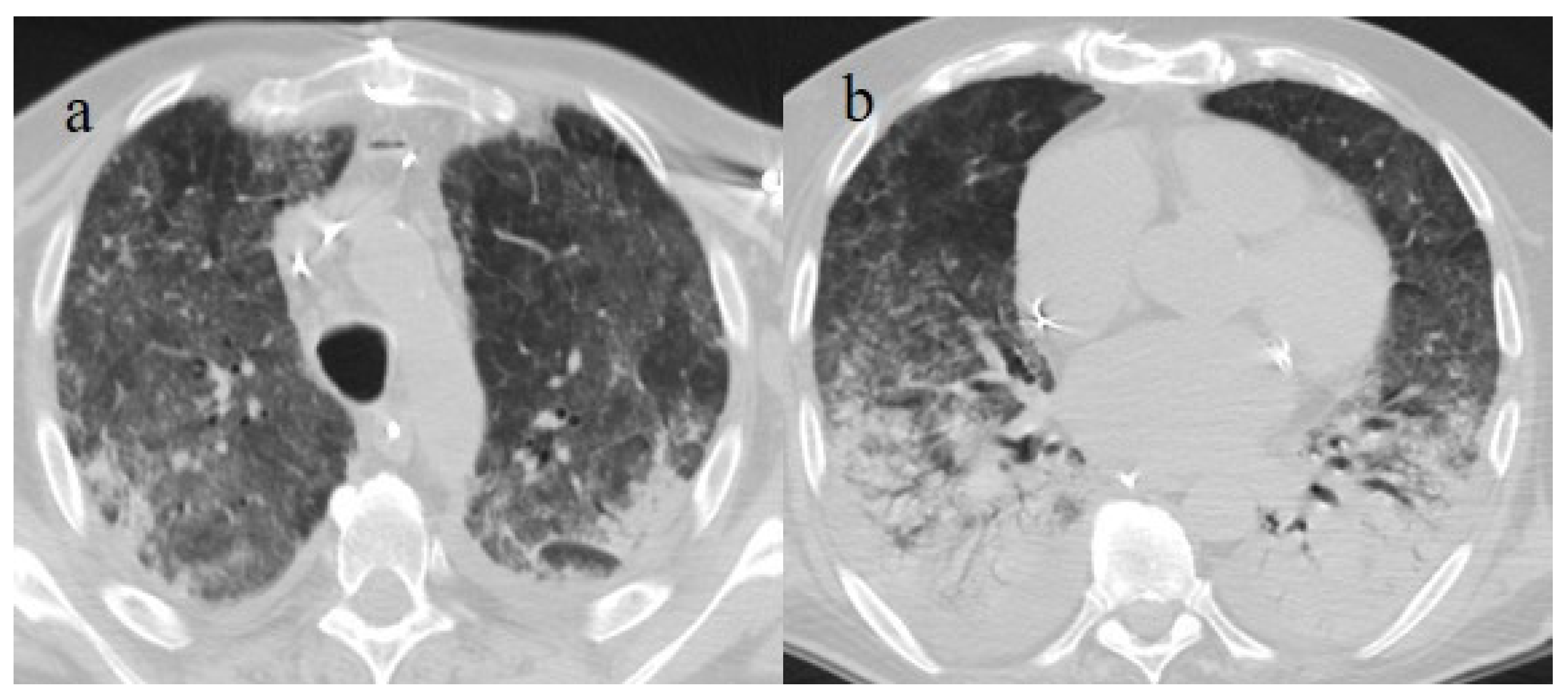
Figure 5.
A 57-year-old unvaccinated patient during the Delta waves (in November 2021). On chest CT, he presented diffuse interstitial thickness with GGOs in a typical distribution in the superior and inferior lobes as visualized in image (a), in the middle and inferior lobes, in image (b) and at the lung base in image (c) and high score (CT-SS 14/25). The patient was hospitalized and died.
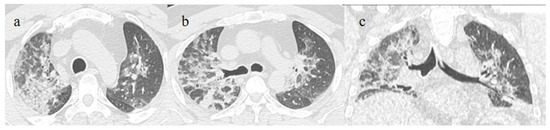
Figure 6.
A 51-year-old man unvaccinated patient without known comorbidities during the Delta waves (in November 2021). On CT he presented diffuse interstitial thickness with consolidation areas in a typical distribution in the superior lobes and superior and inferior lobes, on axial plane, respectively in image (a), and in image (b); diffuse interstitial thickness and consolidations on coronal plane in image (c) and high score. The patient was hospitalized and survived.
These features could be caused by the pathogenicity of the Delta variant, which shows mutations in the spike protein that enhance fusogenicity and facilitate more efficient expansion through the human body via cell–cell fusion than the wild-type and Alpha variants [67]. However, Han et al. [75] showed a decrease in the severity of pneumonia from the original strain to the Delta and Omicron variants.
The most recent variant, Omicron, despite being the most highly mutated and presenting increased contagiousness and infectivity, tends to involve the lungs to a lesser extent and predominantly infects the upper respiratory tract [54,68,74,75,76,77,78]. These findings suggest that the genetic and pathogenic characteristics of SARS-CoV-2 have evolved over time. In fact, the Omicron variant replicates in the bronchial epithelium cells, whereas previous waves of SARS-CoV-2 replicated in the alveolar epithelium [54,77].
Patients with the Omicron variant presented a lower pneumonia prevalence than in the previous waves and a peribronchial distribution (rather than peripheral), as compared to patients infected with the Delta variant [27,28,54,75,76,77,78] (Table 2).

Table 2.
This table summarizes the main manuscripts that compared some chest CT features during different waves of the pandemic.
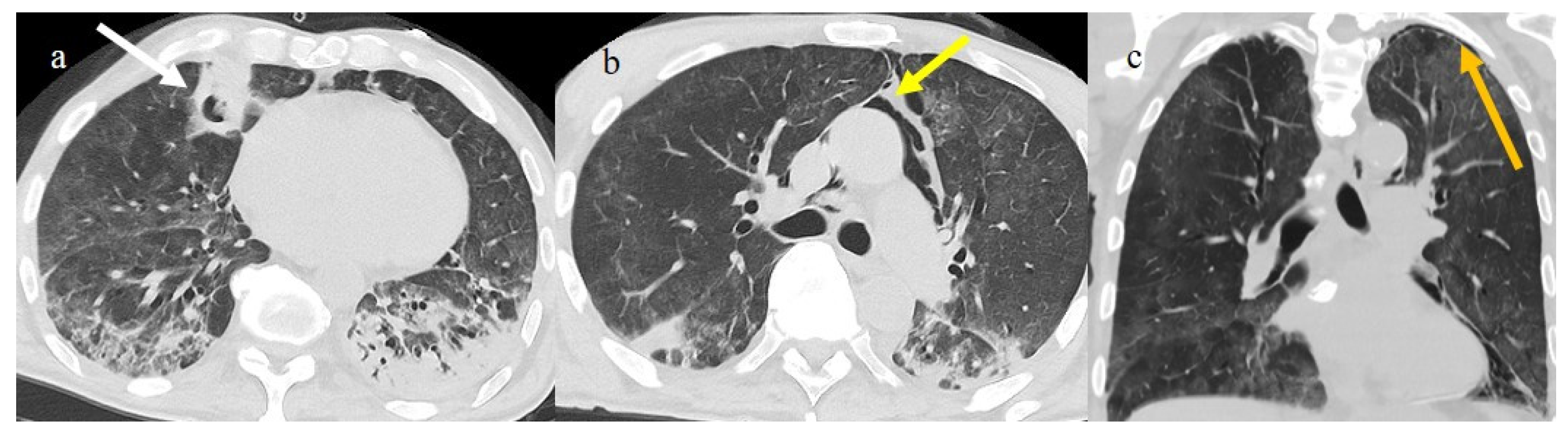
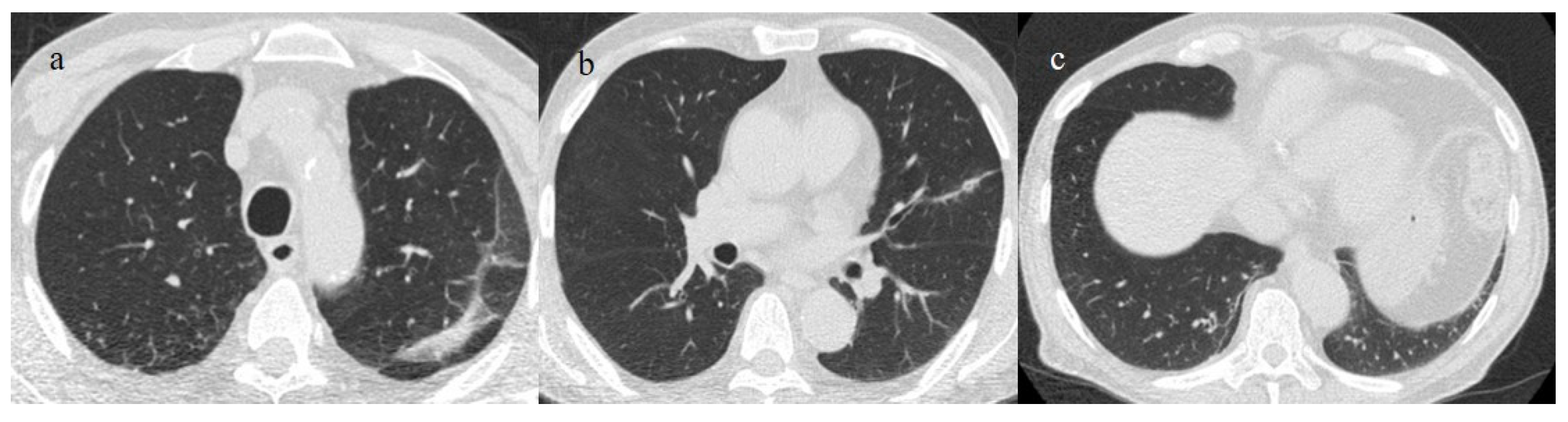
The Omicron variant has been reported to manifest itself with lung involvement in 34% of cases [54]. However, lung involvement in the Omicron variant has been more frequently characterized by atypical chest CT findings such as cluster-like GGOs, randomly distributed GGOs, or the absence of pneumonia [54,68,69,74,75,76,77,78] (Figure 7, Figure 8, Figure 9 and Figure 10). Other atypical findings in patients infected with the Omicron variant included centrilobular nodules or tree-in-bud opacities [75,76].
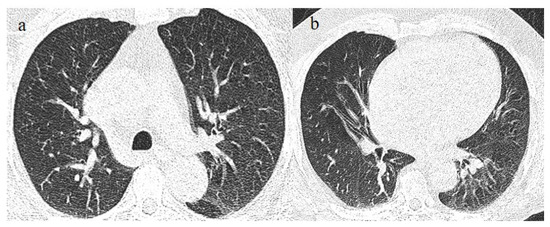
Figure 7.
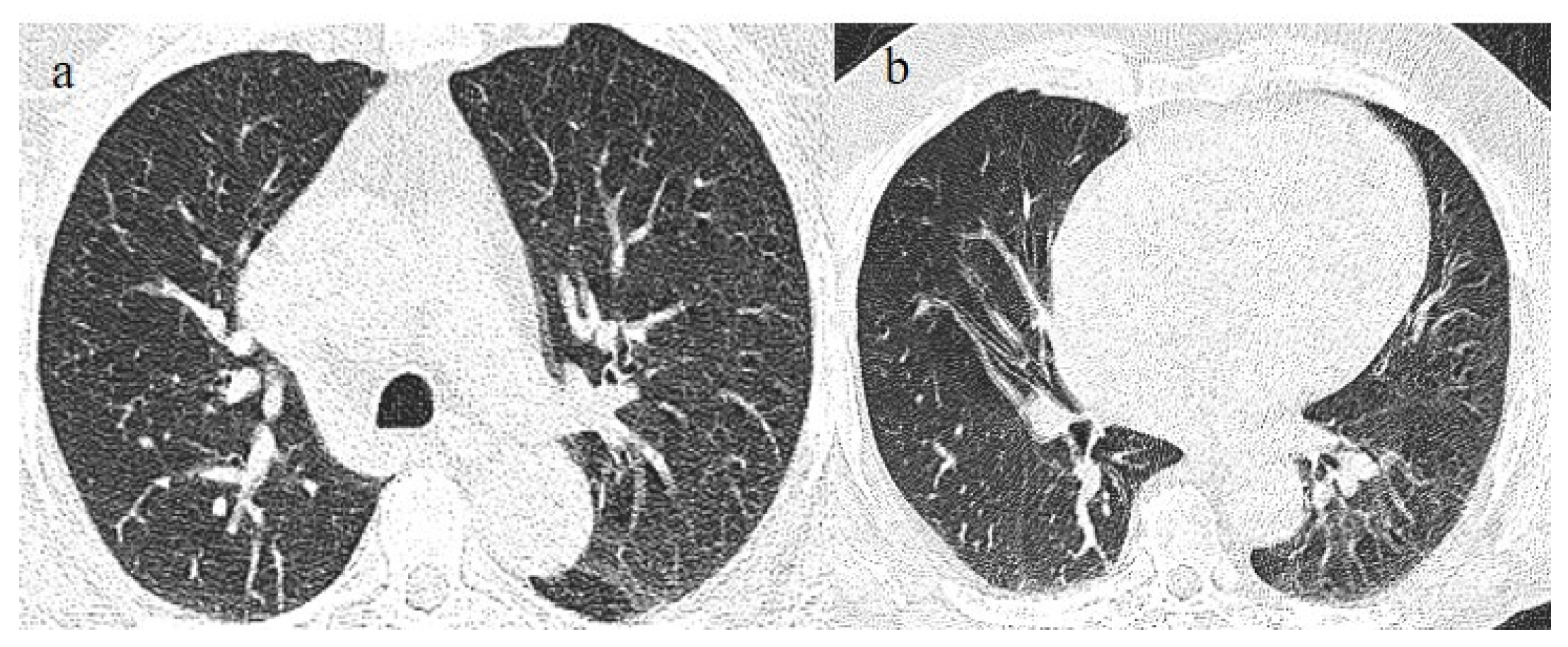
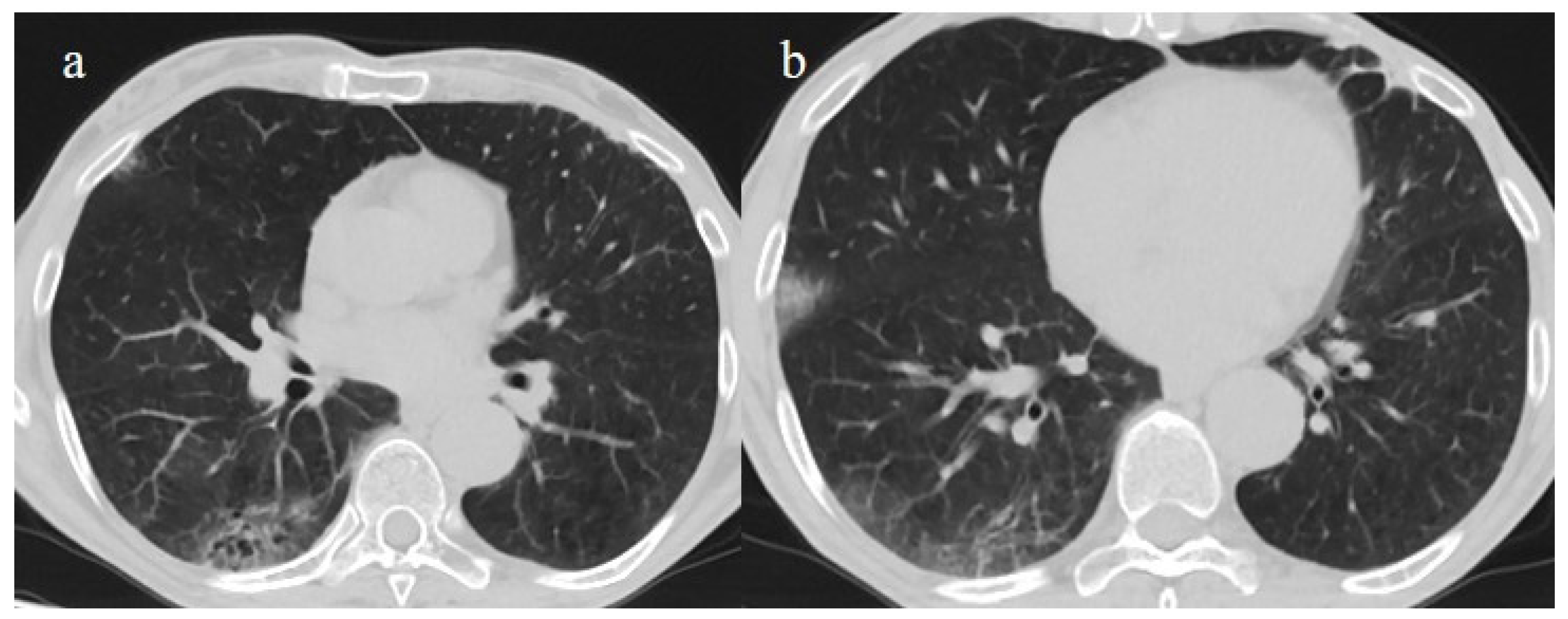
A 65-year-old patient in breakthrough infection with RT-PCR positivity for SARS-CoV-2 during the Omicron wave (in January 2023) with absence of pneumonia in chest CT as is depicted in image (a,b) on the axial plane.
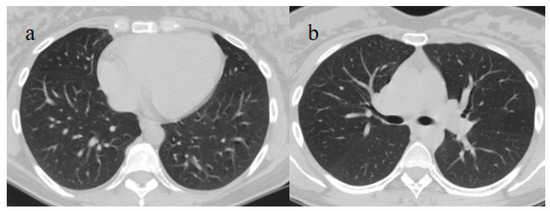
Figure 8.
Absence of pneumonia in chest CT in images (a,b) in an unvaccinated 40-year-old patient with some comorbidities during the Omicron wave (February 2023).
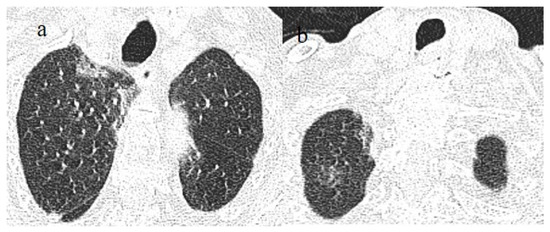
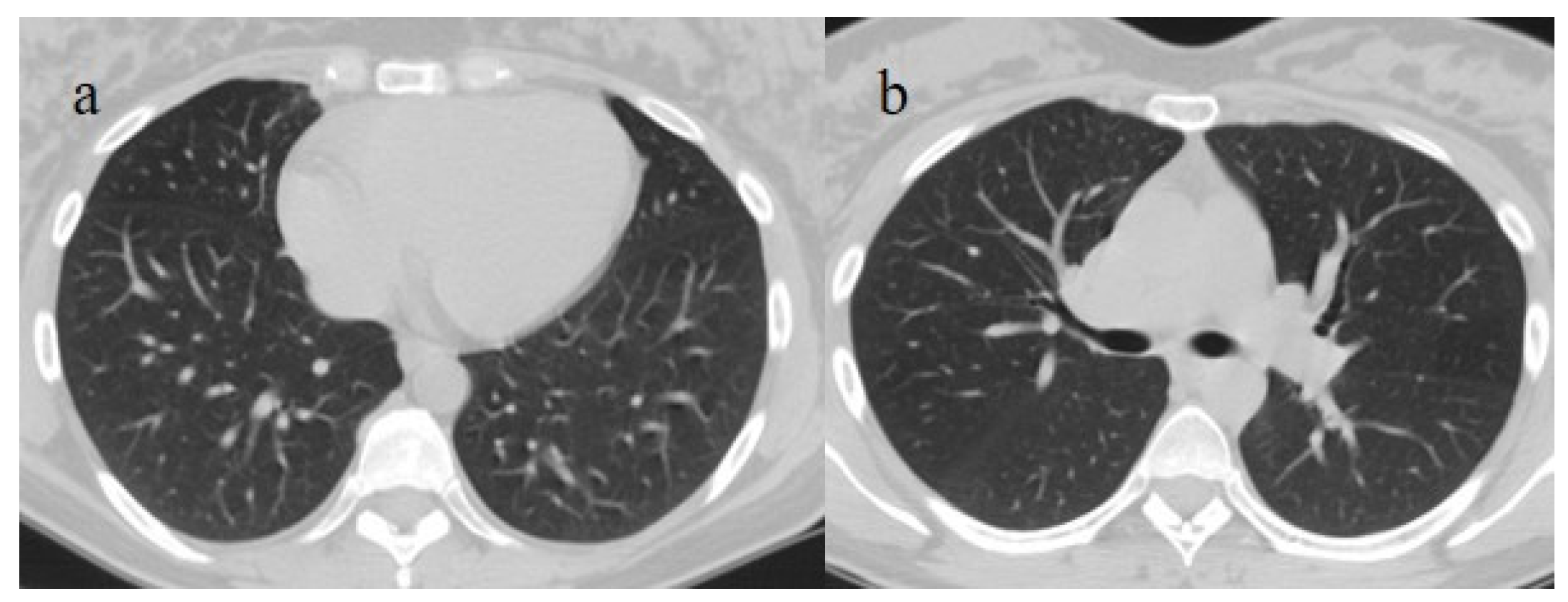
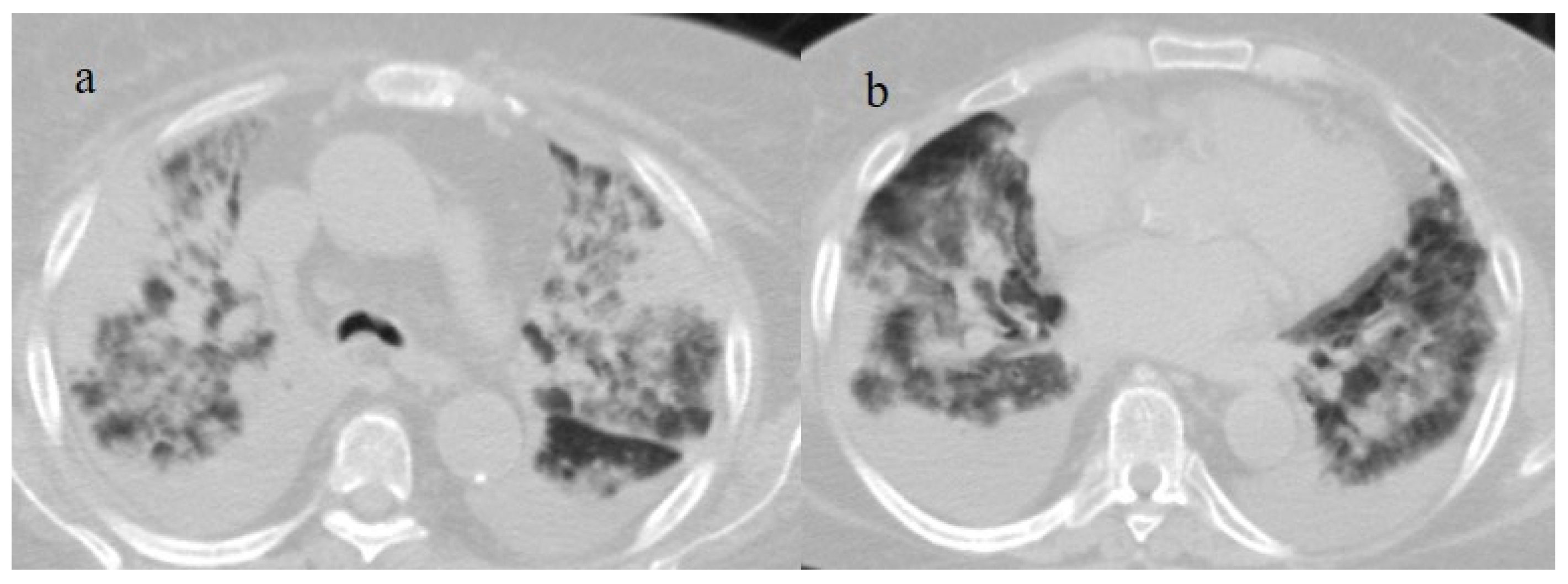
Figure 9.
An 83-year-old patient with RT-PCR positivity for SARS-CoV-2 during the Omicron wave in breakthrough infection (in May 2023) with some cluster GGOs on the superior right lung lobe in image (a) and image (b). The patient was hospitalized and survived.
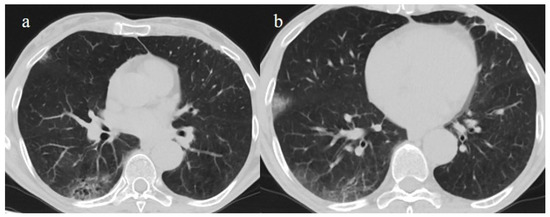
Figure 10.
A 67-year-old unvaccinated patient with a history of hypertension, emphysema, and breast cancer and RT-PCR positivity for SARS-CoV-2 during the Omicron wave in February 2023 with some peripheral GGOs in the middle lobe, in image (a), and right inferior lobe, in image (b), with a peripheral and typical distribution. The patient was not hospitalized and did not have other complications.
Askani et al. [67] found that the Delta variant was more commonly associated with the typical signs of COVID-19 pneumonia than the Omicron variant and showed vacuolar CT signs more frequently.
Patients with pneumonia during the Omicron wave tended to be older than those without COVID-19 pneumonia [27,28,54,77].
However, the persistence of fever during the Omicron wave could also be associated with lung involvement in young patients without comorbidities.
3.2. COVID-19 Pneumonia after Breakthrough Infections
COVID-19 vaccines were created using various technologies: mRNA, protein subunits, inactivated non-replicating viral vectors, and DNA [79].
These vaccines proved to be highly effective in reducing the rates of hospitalization and severe presentations in patients affected by SARS-CoV-2 [79,80,81].
However, the progressive waning of antibody levels over time, together with the emergence of new viral variants, led to an increased risk of breakthrough infections [37,82,83,84].
A breakthrough infection is defined as a COVID-19 infection occurring ≥14 days after a full dose of a COVID-19 vaccine. Breakthrough infections are common, even after a third dose, and effectiveness against symptomatic disease appears to decrease 12–16 weeks after vaccination [84,85,86].
Several studies have reported that COVID-19 breakthrough infections usually appear with mild symptoms, and vaccination reduces pneumonia severity [87,88,89]. The imaging findings for breakthrough infections with pneumonia are similar to those for unvaccinated patients, though usually less severe; nevertheless, the severe forms are also possible in fully vaccinated patients with risk factors such as old age, immunosuppressed status, comorbidities, and anti-IFN-α autoantibodies [30,31,32,33,34,89,90,91,92] (Figure 11).
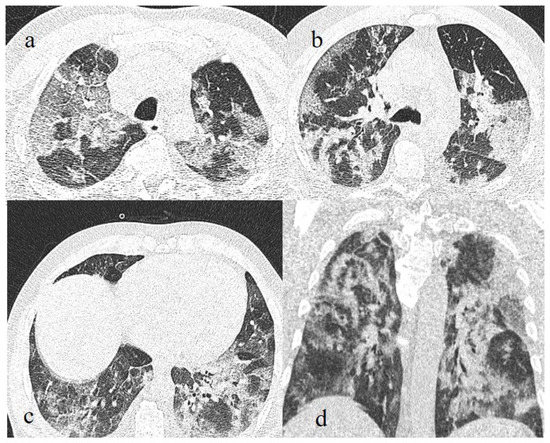
Figure 11.
Breakthrough infection with severe pneumonia in a fully 61-year-old vaccinated patient during the transition period of the Alpha-Delta variant (June 2021), with a history of hypertension, ischemic heart disease, and a previous kidney transplant. In images (a–c), there is evidence of interstitial thickness with GGOs in the superior, superior and inferior, and inferior lobes, respectively, on the axial plane; the image (d) shows the multilobar distribution on the coronal plane.
Old age can be considered an independent risk factor for pneumonia severity in both the unvaccinated population and breakthrough infections [31,32,77]. A lower antibody response after vaccination has been reported in older individuals [31,32]. Mirouse et al. [31] found that old age and disease severity were independently associated with high mortality. Tong et al. [32] determined that old age was the only independent risk factor for pneumonia development in patients infected with the Omicron variant (Figure 12 and Figure 13).
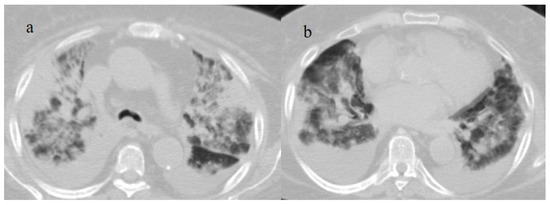
Figure 12.
Breakthrough infection with severe pneumonia in an 80-year-old patient with a high CT score during the Omicron wave. The patient had a history of immunosuppression for renal transplant; images (a,b) show interstitial thickness with consolidations at the level of superior lobes (a) and inferior lobes on the axial plane; pleural effusion was also present. The patient was hospitalized and died.
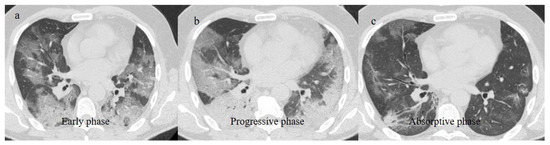
Figure 13.
Chest CT scan in images (a–c) show the pneumonia evolution in a breakthrough infection in a 67-year-old patient during the Omicron wave (March 2023) with multiple comorbidities as hypertension and chronic heart failure.
Ben et al. [91] reported the prevalence of severe or critical disease in vaccinated COVID-19 patients in 10.8% of cases. Granata et al. [92] found that the risk for severe COVID-19 outcomes after primary vaccination was higher among persons aged ≥65 years suffering from immunosuppression; diabetes; and chronic kidney, cardiac, pulmonary, neurologic, or liver disease.
The risk of developing pneumonia after breakthrough infection increase is higher in patients with cancer. The risk is heightened for those receiving active chemotherapy and anti-CD20 therapy [30,82,93].
Virus variants increased the risk of breakthrough infections [82,87,88]. However, severe cases of COVID-19 pneumonia after breakthrough infections were prevalent in the Delta variant wave [69,75,77,93,94,95]. Lamacchia et al. [90] found that vaccinated and hospitalized COVID-19 patients were most likely infected with the Delta variant. The Delta variant caused more severe disease and was less susceptible to vaccines than previous lineages [37] (Figure 14).
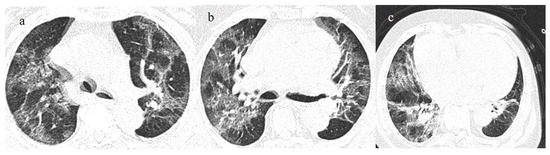
Figure 14.
Breakthrough infection with a higher score on chest CT with a typical distribution during the Delta wave (November 2021). The images (a,b) show diffuse interstitial thickness with GGOs at the level of the superior lobes and inferior lobes on the axial plane; image (c) shows the middle and inferior lobes.
Nevertheless, several studies showed that pneumonia severity was lower in vaccinated than in unvaccinated patients [37,87,88,89,90,91,96,97]. Lee et al. [96] reported that the proportion of patients with negative CT scans was significantly greater in the fully vaccinated group than in the unvaccinated group. Verma et al. [97] reported that the mean CT severity score was significantly lower in completely vaccinated patients in comparison to incompletely vaccinated and non-vaccinated patients. Wada et al. [89] found that vaccinated patients, with or without a booster/additional vaccination, presented milder COVID-19 pneumonia on CT scans than unvaccinated patients during the period of the Delta and Omicron variants. Nevertheless, Granata et al. [92] found no difference between vaccinated and unvaccinated patients admitted to the ICU. However, vaccinated patients with pneumonia were usually older than unvaccinated patients and had comorbidities [98].
Nevertheless, regardless of vaccination status, pneumonia prevalence progressively declined during the Omicron wave [77]. The CT features in breakthrough infections and the CT features of COVID-19 pneumonia in the different waves of pandemic are summarized in Table 3.

Table 3.
This table summarizes the main lung complications and extrapulmonary vascular abdominal complications during the COVID-19 pandemic (ARDS: Acute respiratory distress syndrome; ICU: intensity care unit; FU: follow-up; PMS: pneumomediastinum; PTX: pneumothorax).
3.3. Secondary Lung Complications
3.3.1. Acute Respiratory Distress Syndrome
An uncontrolled immune response to SARS-CoV-2 infection can lead to acute respiratory distress syndrome (ARDS), which was a relatively common complication of COVID-19 pneumonia in the first waves [99,100,101,102,103,104]. COVID-19 patients with ARDS were usually older and had comorbidities [100]. The majority of patients with ARDS required mechanical ventilation and were prone to complications including barotrauma with alveolar rupture and superimposed bacterial pneumonia [101,102,103].
COVID-19 ARDS is caused by both direct viral action and the indirect effect of the hyperactivation of the host immune system, which can cause the excessive production of proinflammatory cytokines, i.e., cytokine storms. COVID-19 ARDS differs from typical ARDS due to the existence of endothelial injury characterized by the presence of pulmonary capillary microthrombi, in addition to typical diffused alveolar injury [99,100,101,102,103,104,105]. This was revealed by histopathological examinations, although autopsies were rare in the first wave of the pandemic [103]. SARS-CoV-2 mainly attacks the endothelium through the ACE2 receptor. The injured pulmonary endothelium loses lung perfusion regulation with the activation of an intrapulmonary shunt. Thus, the lung volume and lung compliance are almost normal [99,100,101,102,103,104,105]. Therefore, COVID-19 ARDS is characterized by silent or happy hypoxemia. The imaging features of COVID-19 ARDS were similar to those of ARDS with other etiologies [103]. Chest CT findings were characterized by the anteroposterior gradient of the lung opacities. The rapid progression of lung opacities involving all five lobes in a patient with COVID-19 should also increase concern for ARDS. In the acute phase, a tendency towards dense consolidation involving the dependent posterior lower lobes while mostly sparing the anterior or non-dependent area has been observed [37,103] (Figure 15).
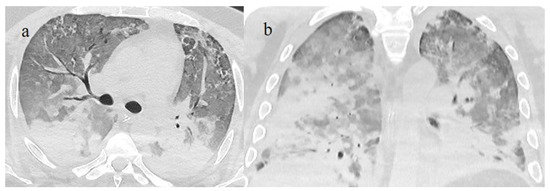
Figure 15.
Chest CT scan of a 51-year-old patient during the Alpha variant period (February 2021) showing in image (a) posterior consolidations with diffuse GGOs compatible as ARDS on the axial plane and in image (b) on the coronal plane.
ARDS in COVID-19 patients has been frequent since the Delta variant [37,99,104,105,106]. However, the risk of ARDS was lower during the Omicron variant [107]. Some rare cases of ARDS were reported after COVID-19 vaccinations as possible consequences of antibody-dependent enhancement (ADE) [108,109] (Figure 16).
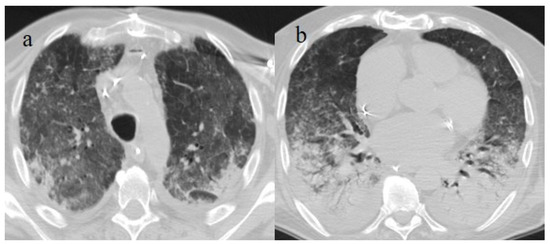
Figure 16.
Chest CT scan of a 70-year-old patient during the Alpha variant period (April 2021) with ARDS findings in the superior lobes as shown in image (a) and inferior lobes in image (b). The patient developed severe COVID-19 pneumonia, which evolved into ARDS a few days after the COVID-19 vaccination.
3.3.2. Pneumomediastinum and Pneumothorax
Pneumomediastinum (PMS) is the presence of air in the mediastinum. PMS has been identified as a complication in lung infections such as influenza and previous coronavirus infections such as SARS-CoV-1 and Middle East respiratory syndrome (MERS). It appears to be a marker of severe COVID-19 pneumonitis [110,111]. This condition can be spontaneous or secondary. The secondary form is the most common, with high incidence during the first three waves of the pandemic in critically ill patients (Figure 15) and may be related to mechanical ventilation in patients with ARDS receiving high positive pressure ventilation (PPV) [110,111,112,113,114,115,116]. Pneumothorax (PNX) was found to co-exist with PMS in 40.3% of cases [110]. Several studies have reported that the incidence of barotrauma in COVID-19 patients was 14% [114,115]. However, Martinelli et al. [117] reported a much higher incidence placing it at 25%. Subcutaneous emphysema is usually associated with PMS and PTX. The spontaneous forms of PMS and PNX have no apparent causes; however, several predisposing and precipitating factors have been identified, such as asthma, respiratory infections, lung cysts, inhaled drug use, corticosteroids, and the inhalation of irritants, as well as several anatomical predisposing alterations, including tracheomalacia [111,118,119,120,121,122]. Lung damage with alveolar rupture in COVID-19 infections can probably lead to interstitial pneumothorax, which can cause spontaneous PMS related to the Macklin effect [110,111,118,119,120,121,122]. In severe cases of COVID-19 pneumonia, parenchymal destruction can lead to the formation of cavitation and lung cysts, which can cause PTX and PMS after rupture [111,118,119,120,121,122].
The spontaneous forms of PTX and PMS occurred in 1–2% of all COVID-19 pneumonia patients [118,122]. In rare cases, spontaneous PMS was associated with PTX [123]. The prevalence of these conditions increased during the second wave of the pandemic with the increasing use of oxygen through non-invasive ventilation and steroid therapy [118,121,122,123,124,125,126]. The high airway pressures delivered by these modalities of respiration probably supported the spontaneous rupture of fragile small airways infected by the virus (Figure 17 and Figure 18).
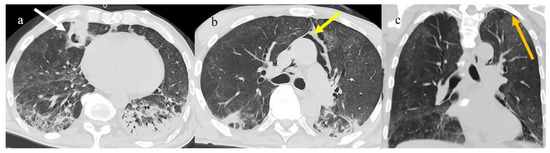
Figure 17.
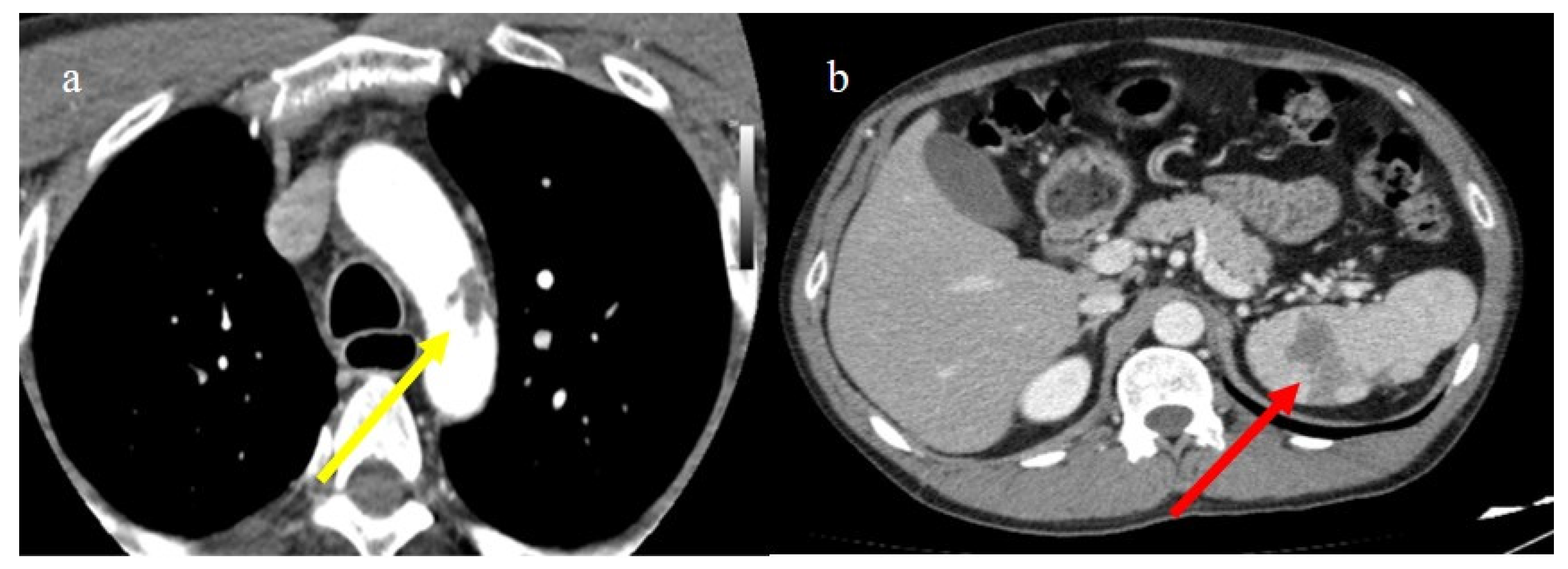
Chest CT during the second pandemic wave (November 2020) in a 71-year-old patient with a history of diabetes mellitus. The patient was under non-mechanical ventilation and developed a cavity lesion (white arrow) in image (a), pneumomediastinum (yellow arrow) in image (b), and a small pneumothorax (orange arrow) as visualized in image (c).
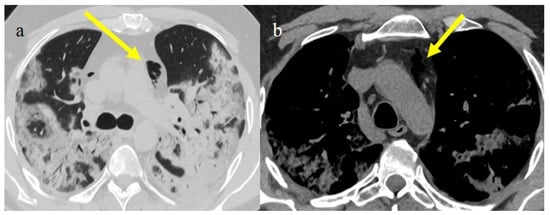
Figure 18.
Chest CT during the second pandemic wave (November 2020) in a 40-year-old patient without known comorbidities. The patient was under non-mechanical ventilation with CPAP and developed pneumomediastinum (yellow arrow), as visualized on axial plane in image (a) (on lung window) and in image (b) mediastinal window; severe pneumonia with consolidations is also visualized in image (a).
However, Palumbo et al. [126] hypothesized that the increased incidence of SMPS and SPTX during the second pandemic wave was caused by the increased use of dexamethasone, which might have induced lung frailty. Rare cases of SPMS were usually reported during the Omicron wave [127].
3.3.3. Pulmonary Fibrosis
Approximately one-third of patients hospitalized with COVID-19 pneumonia presented abnormalities on chest CT scans 1 year from infection [128,129]. In patients with severe disease, CT studies after hospital discharge reported fibrotic-like changes such as COVID-19 sequelae (from 3 to 12 months following COVID-19 pneumonia). Furthermore, parenchymal bands, interlobular septal thickening, coarse reticulations, and bronchiectasis are the most frequently reported CT findings (Figure 19). Air trapping has also been reported as a long-term finding in COVID-19 survivors in several studies [128]. However, the term “fibrosis” is problematic for CT because fibrosis tissues are not confirmed, and real fibrosis is usually an irreversible condition [128]. Therefore, it would be more correct when describing these observations as being fibrotic-like changes that could be caused by acute inflammatory damage, which usually resolves over time. Several studies have shown that in most patients, pulmonary post-COVID-19 fibrosis-like changes resolved over time [129,130,131]. Bocchino et al. [131] reported that at 1 year, lung abnormalities due to COVID-19 pneumonia were completely resolved in 93% of patients. However, only a minority of patients showed persistent fibrotic changes via CT, especially those with moderate-to-severed disease who required intensive care therapy or mechanical ventilation [128,129,130,131,132]. Older patients with severe comorbidities were also more prone to develop fibrotic changes [132].
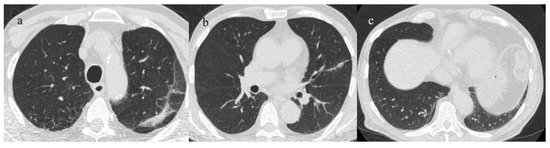
Figure 19.
A 73-year-old patient with a history of hypertension and with some fibrotic-like change on the CT during the follow-up after 3 months of COVID-19 pneumonia, during the first pandemic wave (May 2020). There are some subpleural fibrotic bands in the apicodorsal segment of the left upper lobe (a), at the level of lingual segment (b), and in the inferior lobes (c).
3.3.4. Pulmonary Thromboembolism
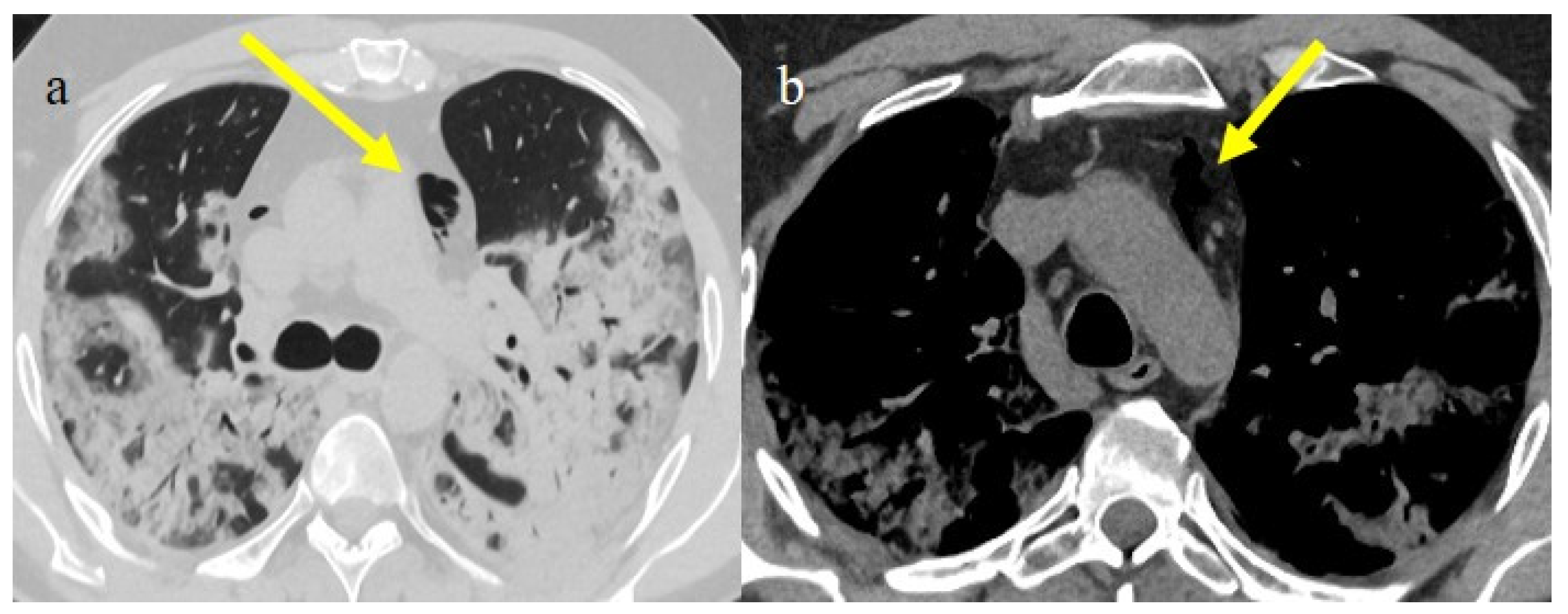
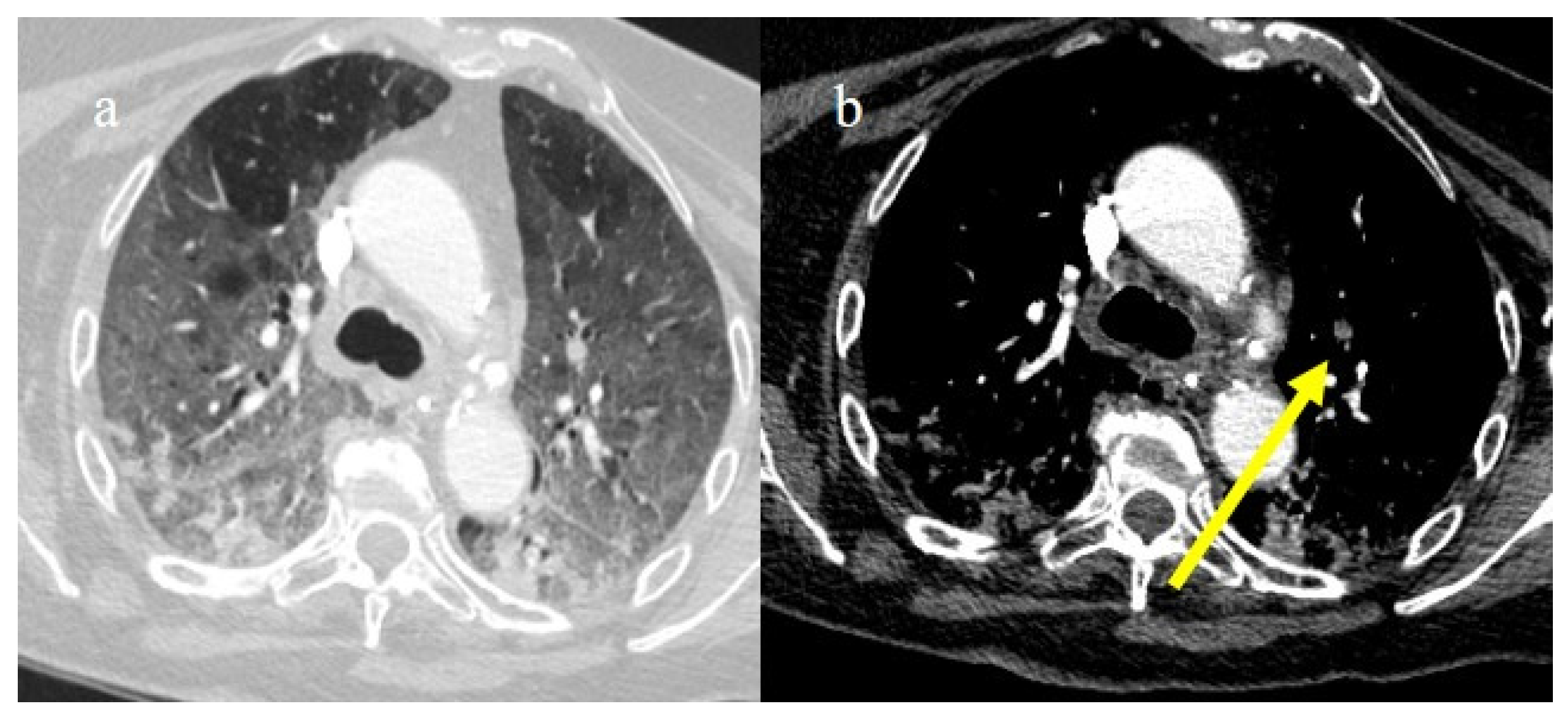
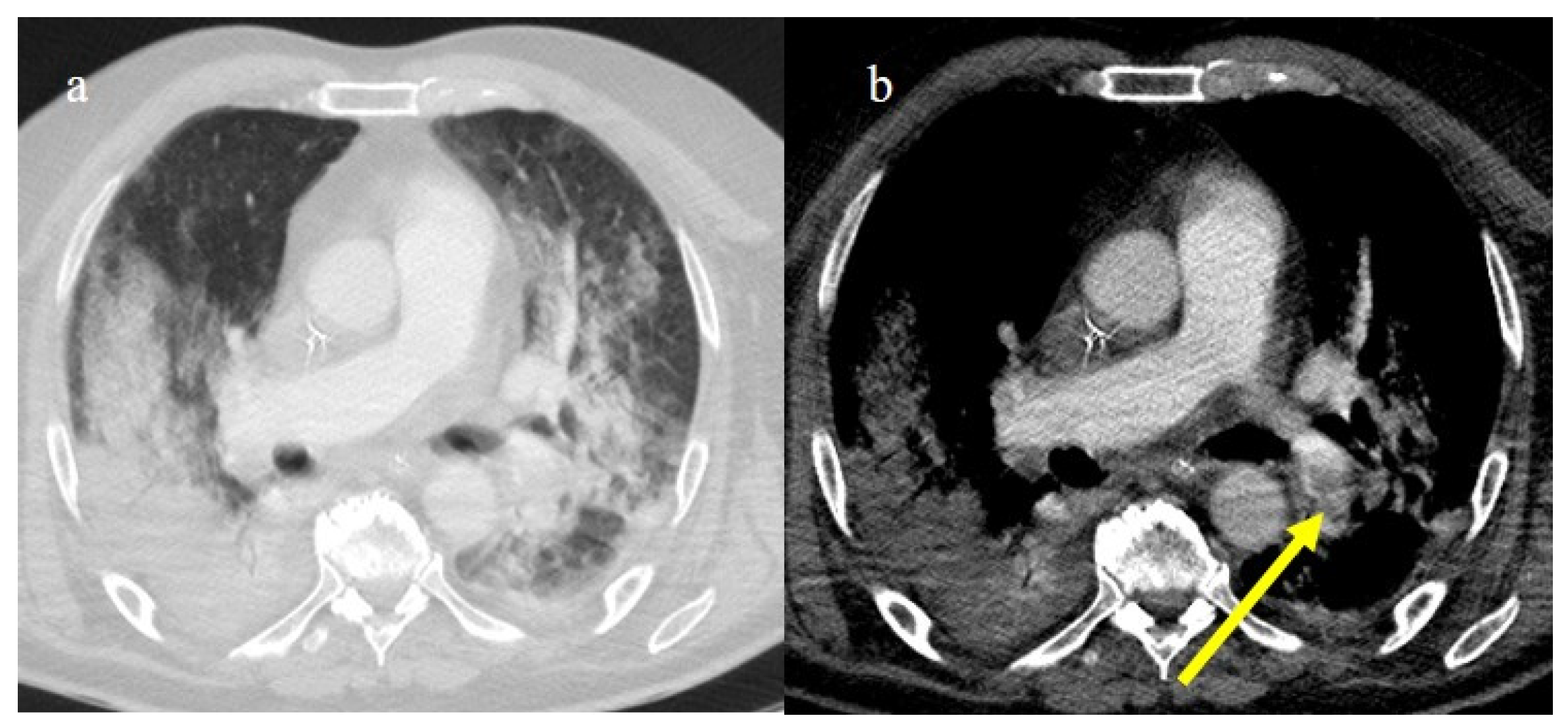
In the early phase of the pandemic, the important role of a prothrombotic state in the virus infection became rapidly clear, with evidence of dilated pulmonary vasculature in regions affected by pneumonia according to CT and a high incidence of pulmonary thromboembolism (PE), according to CT pulmonary angiography (CTPA) [12,133,134,135,136]. Nevertheless, during the first phase of the pandemic, complete autopsy studies were rarely performed because of the risk of infection, and PE could have been underestimated [133,134,135]. However, the first autopsies highlighted the importance of thromboembolic events in COVID-19, suggesting de novo coagulopathy in patients with COVID-19, and autopsy studies reported the presence of microscopic thrombi formation in the lung histology [12,133,134,135,136]. Several authors found a higher incidence of pulmonary embolism with or without deep venous thrombosis in COVID-19 patients with no history of venous thromboembolism (VTE). Thrombotic events in hospitalized COVID-19 patients were observed despite VTE prophylaxis [10,12,136,137,138,139]. A recent meta-analysis reported that autoptic findings of acute PE in COVID-19 patients were present in about 30% of the subjects [10]. Radiology case reviews reported high rates of PE, ranging from 17% and 50%, especially in patients who were admitted to the ICU [133] (Figure 20, Figure 21 and Figure 22).
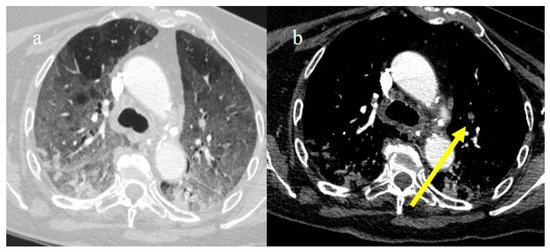
Figure 20.
Chest CT of a 79-year-old patient in ICU during the second pandemic wave (October 2020) with severe COVID-19 pneumonia with CT-SS OF 20/25 with diffuse GGOs and initial consolidations as shown in image (a). On the CT pulmonary angiogram, a thrombosis was found in the anterior pulmonary segmentary artery of the left superior lobe, as shown in the image (b) (yellow arrow).
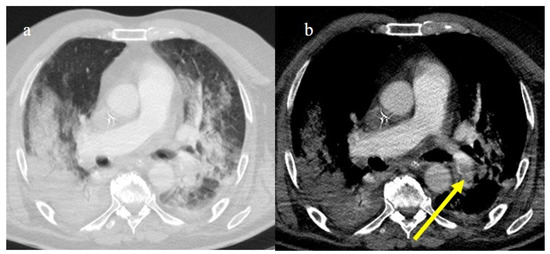
Figure 21.
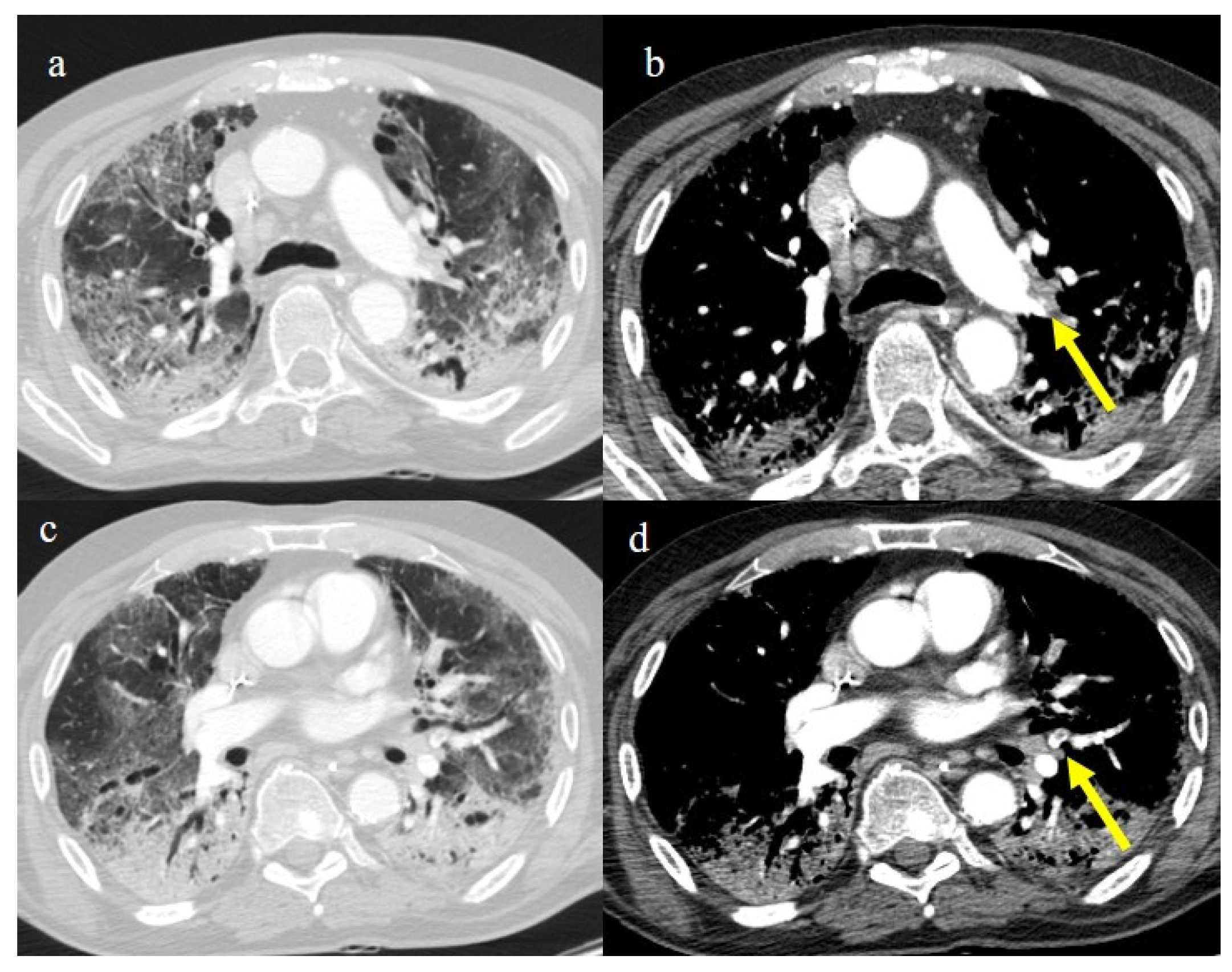
Chest CT of a 70-year-old patient in ICU in the second pandemic wave (November 2020) with severe COVID-19 pneumonia with CT-SS OF 23/25 with diffuse consolidations in the inferior lobe in image (a). The CT pulmonary angiogram showed that lung thromboembolism was present; in image (b), a thrombosis in the lobar artery branch for the left inferior lobe (yellow arrow) was described.
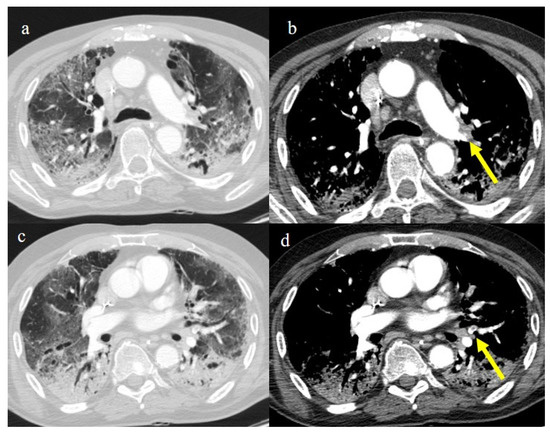
Figure 22.
Chest CT of an 81-year-old patient in ICU during the transition period of Delta-Omicron variant (January 2022) (vaccination status unknown, probably concerns a breakthrough infection) with severe COVID-19 pneumonia with CT-SS of 23/25 with diffuse interstitial thickness and consolidations in the superior lobes, as visualized in image (a). The CT pulmonary angiogram showed thrombosis in the distal portion of the left pulmonary artery (yellow arrow) in image (b); severe pneumonia with consolidations in the apical segment of inferior lobes in image (c) and thrombosis in a segmentary branch (yellow arrow) in image (d).
This increased prevalence occurred despite a simultaneous decrease in the overall number of CTPA exams performed during the pandemic. Recently, Wusmuller et al. [133] showed that the prevalence of PE increased significantly as reported by CTPA exams during the first wave of the COVID-19 pandemic, despite the reduction in the number of radiological examinations carried out due to lockdown measures. Segmental arteries were generally the most common location for PE [24,140] (Figure 23).
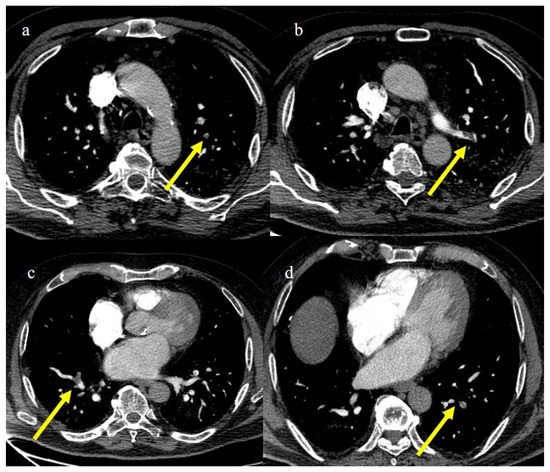
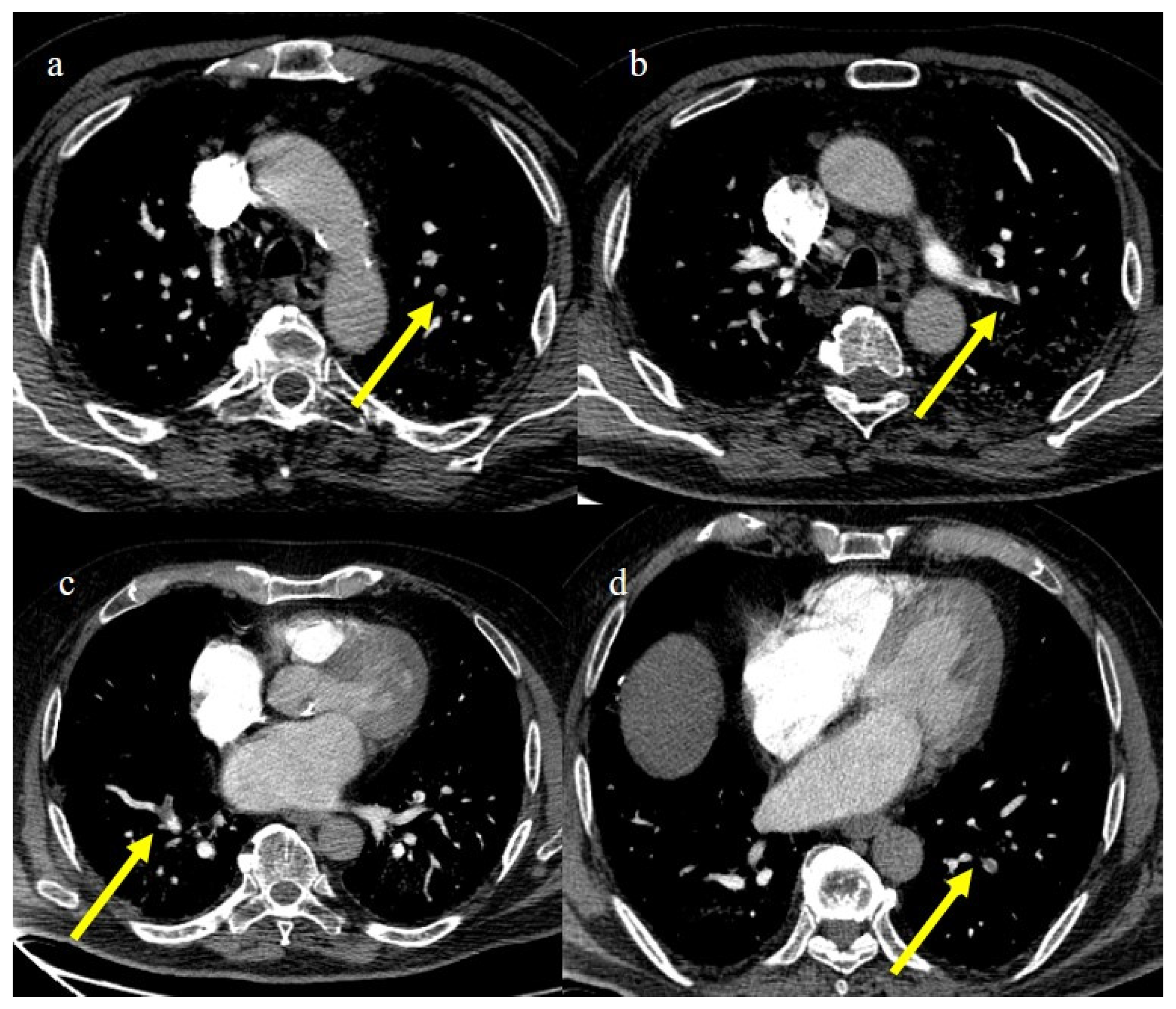
Figure 23.
CT pulmonary angiogram of an 80-year-old patient during the second wave (December 2020) with multiple lung thromboembolism mainly located in the segmentary pulmonary artery branches; in image (a) some defect of opacification compatible with thrombi in some subsegmental branches (yellow arrow) of left superior lobes and in the segmentary branch for the apicodorsal segment (yellow arrow) in image (b); in image (c) lung thromboembolism in the lobar branch for the middle lobe (yellow arrow); and in image (d) in a subsegmental branch in the left inferior lobe (yellow arrow).
Katsoularis et al. [141] found that the risk of a first pulmonary embolism event was highest during the first pandemic wave when compared to the second and third waves. However, Manzur-Pineda et al. [142] determined that 28% of patients showed PE during the Delta variant peak.
Nevertheless, the rate of thrombotic complications reduced during the Omicron wave [140,141,142,143,144]. The incidence of PE also showed a progressive reduction during the Delta variant wave compared to the ancestral variants [140,141].
However, a prothrombotic state associated with COVID-19 has been reported even in subclinical infections [142,143,144].
An increased risk of thrombosis was also found in the early and late sub-acute phases [143,144]. Patients with moderate and severe disease are reportedly at an increased risk of developing chronic thromboembolic pulmonary hypertension [145,146]. All the lung complications are summarized in Table 3.
4. Vascular Abdominal Extrapulmonary Complications
SARS-CoV-2 infection can cause a variety of extrapulmonary complications that are usually secondary to the state of hypercoagulability caused directly by the viral invasion of the endothelial cells and indirectly by the activation of the immune system with a high concentration of circulating proinflammatory cytokines [147,148,149,150]. Ischemic and hemorrhagic abdominal complications were mainly reported in the first two waves of the pandemic [147,149,150,151,152,153,154]. Some case series were also reported during the Delta and Omicron waves [155,156]. Both thrombotic and hemorrhagic abdominal complications were particularly associated with extended lung disease, especially in ICU patients and the elderly [147,149,150,157]. Other reported risk factors for developing these complications were arterial hypertension, diabetes, and obesity [147,150,157]. Hemorrhagic complications were also reported as a possible complication of the anticoagulant treatment usually applied for the management and prevention of PE [147,152,158]. Corticosteroid therapy was also associated with a slight increase in the incidence of gastrointestinal bleeding [158]. However, hemorrhagic abdominal complications were found to be a consequence of COVID-19-associated coagulopathies [153]. Contrast-enhanced CT (CECT) with multiphasic acquisition played a key role in the diagnosis of these complications and provided clinicians with important information for patient management. CECT is usually considered the imaging method of choice for evaluating patients with a clinical suspicion of arterial and venous thrombosis because it allows the direct visualization of the thrombus [159]. Additionally, CECT with angiographic acquisition is very useful for locating the source of active bleeding. Ischemic abdominal complications in COVID-19 patients affected the small and large bowel, the liver, the spleen, and the renal parenchyma [24,157,159]. ACE2 receptors are highly expressed on endothelial cells in the intestine and kidney [24,148,156,159].
4.1. Small Bowel Ischemia and Ischemic Colitis
Small bowel ischemia was the most frequent abdominal CT finding in COVID-19 patients with intestinal ischemia, followed by ischemic colitis. These complications were found more frequently in patients with severe disease [147,149,150,159,160,161,162,163]. Non-occlusive mesenteric ischemia (NOMI) was also a common pattern of bowel involvement, indicating microvascular involvement [161]. Small bowel ischemia had a higher mortality rate, and radiologists played a key role in diagnosis because a prompt diagnosis could prevent mortality in these patients [150,159,160,161,162,163,164]. Most COVID-19 patients with small bowel ischemia had a fatal outcome [147,149,159,160,161,162,163,164]. The CT pattern of small bowel ischemia varied based on vascular involvement (arterial or venous occlusion and hypoperfusion) and the timing of occlusion or hypoperfusion [159,160,161,162,163,164]. The early phase of arterial occlusion is characterized by a spastic reflex ileus with a diminished bowel mural enhancement [162,164]. CECT can show the presence of emboli or thrombi as a filling defect in the lumen of the artery. However, in some cases, if they are small and peripherally localized, their identification can be difficult. The mesentery is bloodless. In the later phase, the ischemia rapidly evolves into infarction with progression from a hypotonic reflex ileus to a paralytic ileus due to wall necrosis with perforation [159,160,161,162,163,164]. Small bowel venous ischemia is usually characterized in CT by the target appearance of the ischemic bowel with an inner hyperdense ring due to mucosal hypervascularity, hemorrhage, and ulceration; a middle hypodense edematous submucosa; and a normal or slightly thickened muscularis propria. If the vascular impairment persists, the condition progresses to intestinal infarction. In the early phase of NOMI, the intestinal wall injuries are usually similar to those in the ischemic phase [160,162]. If no reperfusion occurs or the collateral circulation is ineffective, no enhancement is observed, with wall thinning due to the necrosis. The colon, not only the intestinal wall, is also involved in hypoperfusion injury. However, if reperfusion occurs, it is possible to visualize via CT the target appearance of the intestinal and colic wall [162]. Ischemic colitis is more frequently non-occlusive in nature with NOMI CT patterns [159,160,161,162,163,164].
Bonaffini et al. [147] reported 10 cases of ischemic abdominal complications in the first wave of the pandemic, and seven of these received anticoagulant therapy. Six cases were related to small bowel ischemia and four to ischemic colitis. Sardeck et al. [156] reported 21 cases of macroscopic intestinal ischemia, which appeared about 7 days after COVID-19 diagnosis with a range of 2–21 days. Most of the patients were male and had a mean age of 61.5 years. In this case series, no patients received anticoagulant treatment before hospital admission, and only three patients had received a booster dose of COVID-19 vaccines, with one patient having received two doses. Almost half of the patients with small bowel ischemia in this study died. However, in most of the published cases of small bowel and colic ischemia, no evidence of arterial or venous acute thrombosis was found, suggesting the important role of microvascular obstruction in these patients [147,149,157,160,165,166]. In the recent review of Oja et al. [160], the thrombosis of the SMA was found in 24.9% of cases. Ischemic colitis in COVID-19 patients is relatively rare and occurs as a result of colonic hypoperfusion secondary to the hemodynamic compromise in severe cases of COVID-19 infection [165,166,167] (Figure 24). The left colon is most commonly affected and includes the watershed areas from the splenic flexure to the sigmoid colon. Rare cases have also been reported in the right colon, probably due to microvascular thrombosis [168]. Additionally, rare cases of ischemic colitis have been reported after COVID-19 vaccination [169,170].
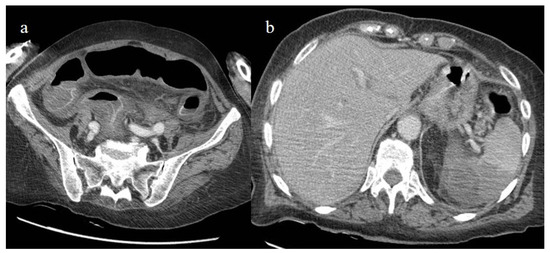
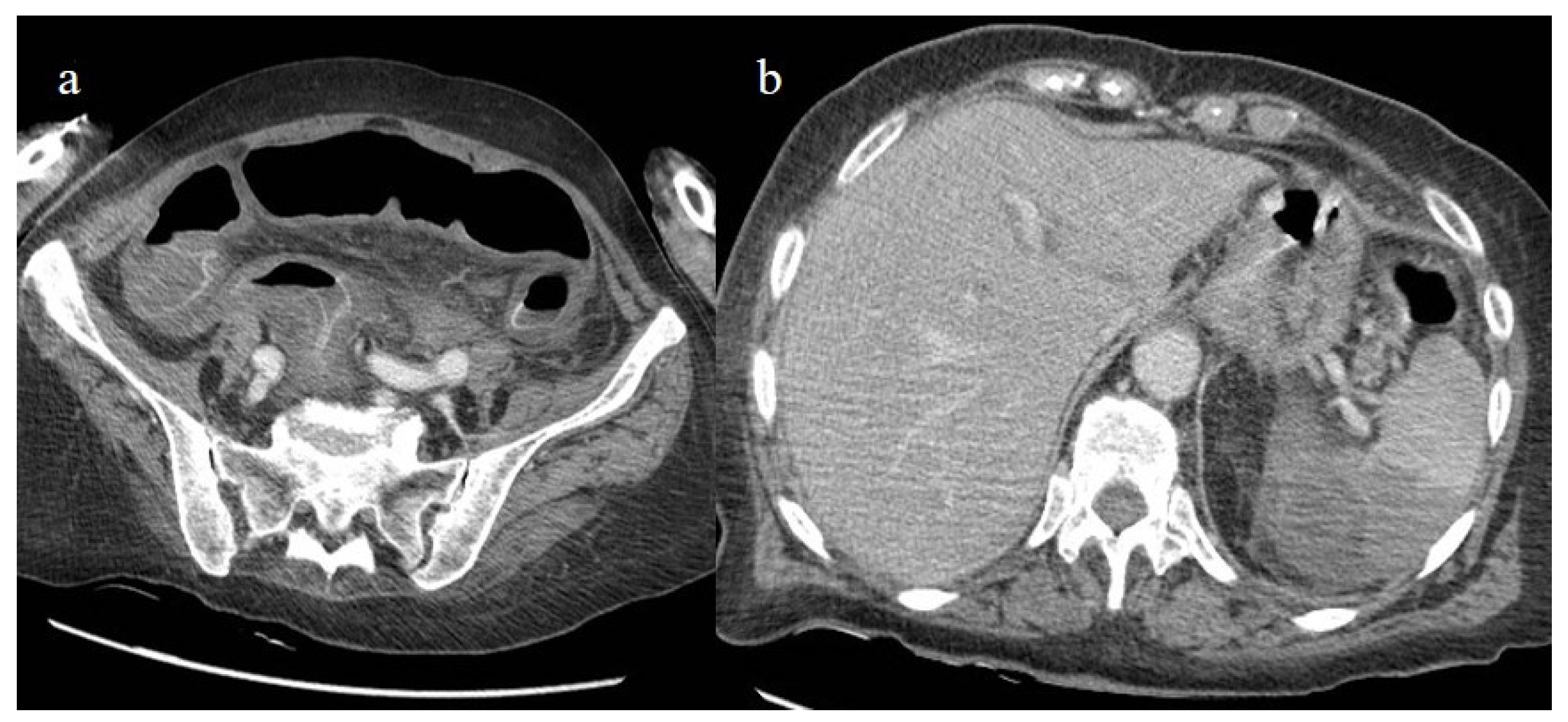
Figure 24.
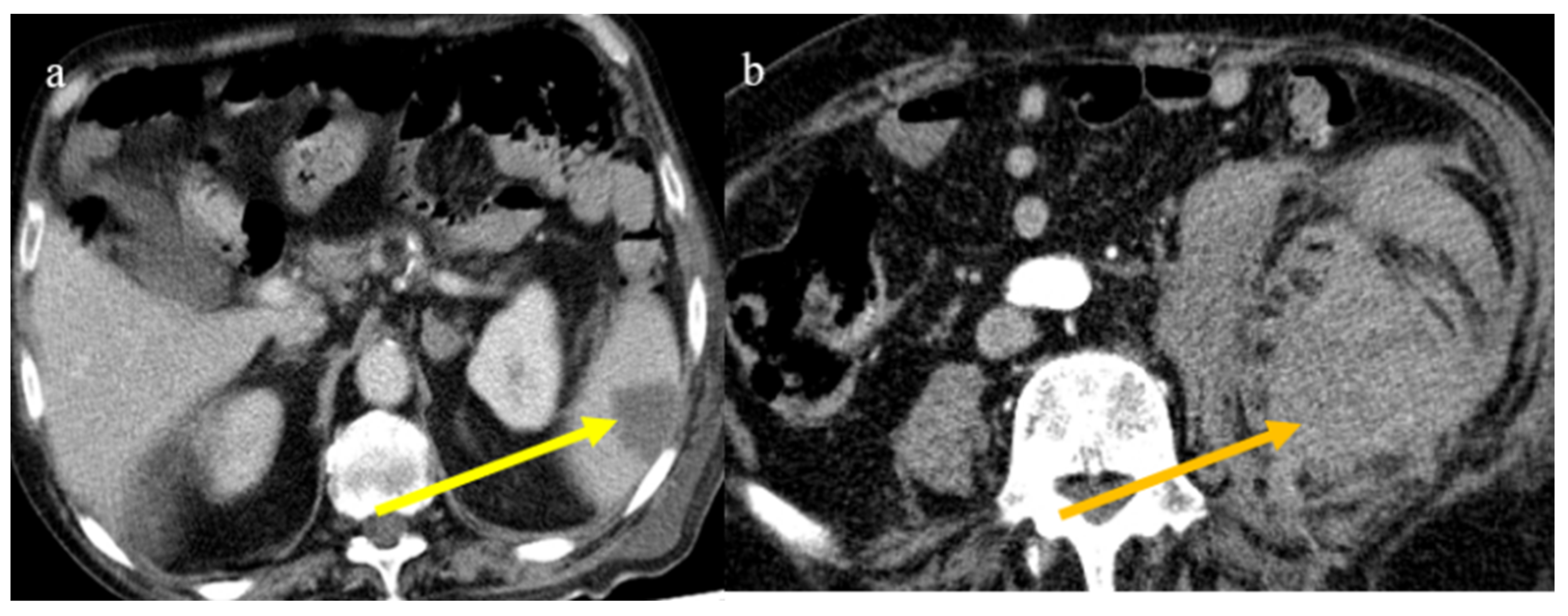
The enhanced computed tomography of an 80-year-old patient during the second pandemic wave (December 2020) with the findings in image (a) of diffuse colic thickness with target appearance and some hemorrhagic changes compatible with ischemic colitis; in image (b), a large ischemic area of the spleen is also present.
4.2. Splenic and Renal Infarction
Splenic and renal infarction are rare ischemic abdominal complications in COVID-19 patients and have only been presented in case reports and a few case series [147,149,171,172,173,174,175,176,177,178,179]. Splenic and renal infarctions generally have a minor clinical impact compared to intestinal infarction. In very few cases, associations between these complications have been reported [147,180,181,182,183], and in some rare cases these were the first manifestations of a COVID-19 infection [171,177]. These complications are usually secondary to the state of hypercoagulability in COVID-19 infection that leads to an increased risk of arterial and venous thrombosis and thromboembolic events (Figure 24 and Figure 25).
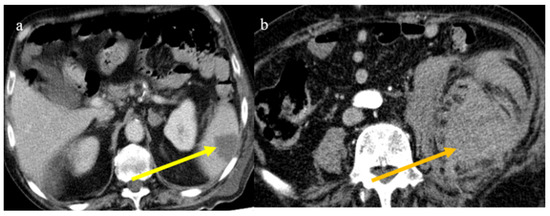
Figure 25.
Enhanced computed tomography of an 80-year-old patient during the second pandemic wave showing an ischemic lesion of the spleen in image (a) (yellow arrow); the patient also had a large hematoma in the left psoas muscle, depicted in image (b) (orange arrow).
Splenic infarctions follow arterial or venous occlusions, causing tissue ischemia and necrosis. Some rare cases have involved splenic infarction in COVID-19 patients without pulmonary involvement [171,173]. Childer et al. [174] described a case of splenic infarction in a COVID-19 patient associated with a floating thrombus in the aorta, suggesting a thromboembolic origin. Splenic infarction is also the most common finding associated with mesenteric ischemia in COVID-19 patients, followed by renal infarction [147,149]. In CECT, splenic infarction appears as a peripheral, wedge-shaped, hypo-enhancing region.
The management of splenic infarction is usually conservative. However, Dimitriou et al. [184] described a case of splenic infarction in an unvaccinated COVID-19 patient who required surgical treatment. A case of splenic infarction was also reported during the peak of the Delta variant wave in an unvaccinated male [185]. Rare cases of splenic infarction have been described after COVID-19 m-RNA vaccines [186,187,188,189,190] (Figure 26).
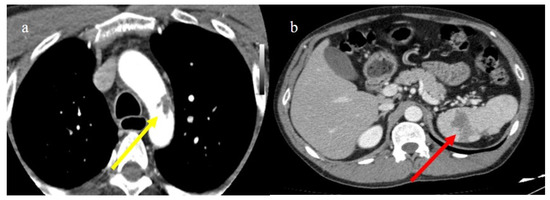
Figure 26.
Enhanced computed tomography of a 61-year-old patient with a floating thrombus in the thoracic aorta at the level of the arch image (a) (yellow arrow) and with multiple vascular thromboembolism and ischemic areas in the spleen as depicted in the image (b) (red arrow). The patient had no known thromboembolic risk and had the second dose of m-RNA vaccine a few days earlier.
The pathogenesis of renal infarction in COVID-19 is multifactorial and caused directly by the virus acting on the renal endothelium and indirectly by the cytokine storm, state of hypercoagulability, hemodynamic alterations, and glomerulopathy collapse [189]. Because the manifestations of a renal infarction can be subclinical, its diagnosis can be incidental and underestimated [189]. Renal infarction appears in CECT as a wedge-shaped focal hypodensity. Vasquez Espinosa et al. [190] reported cases of renal infarction in a young woman who was fully vaccinated. Rare cases of renal infarction have been reported in asymptomatic COVID-19 infections [177].
4.3. Hemorrhagic Abdominal Complications
Hemorrhagic abdominal complications in COVID-19 patients can occur due to predisposing factors such as the use of anticoagulants, thrombocytopenia, and the consumption of coagulation factors [152,153,154].
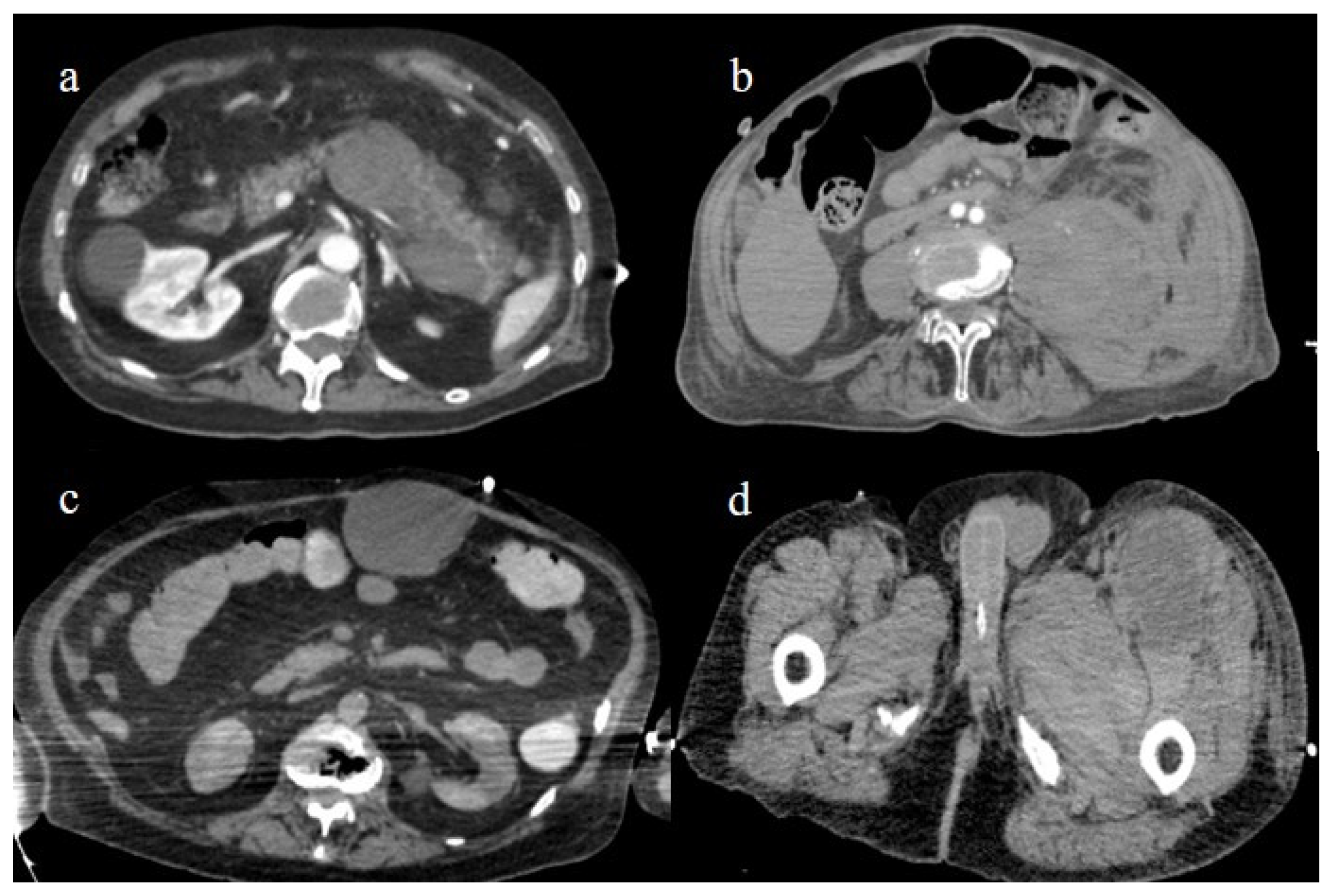
Several case reports and case series have described abdominal spontaneous hematoma in COVID-19 patients [152,153,154]. Spontaneous bleeding can theoretically involve all anatomic abdominal structures such as muscles, parenchymas, the retroperitoneum, and the peritoneum. Spontaneous bleeding is the dominant adverse event of anticoagulant treatment and was more frequent during the first two the pandemic waves. The most frequent locations were the retroperitoneum and the muscular abdominal walls [152,153,154,191,192,193,194,195] (Figure 27). The muscles most commonly affected were the iliopsoas and the rectus abdominis [152,153,154,191,192,193,194,195].
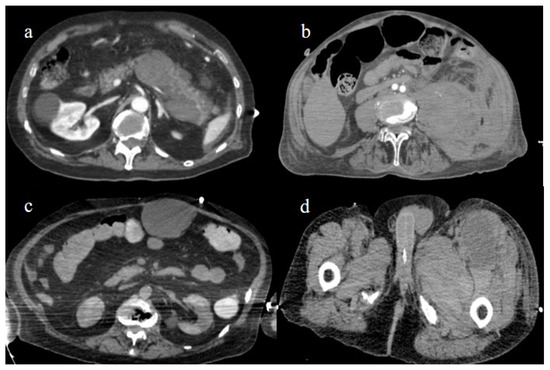
Figure 27.
Enhanced computed tomography during the first and second pandemic waves, showing in image (a) a retroperitoneal hemorrhage involving the pancreas; in image (b) a large hematoma of the left psoas muscle; in image (c) hematoma of the rectus abdomins; and in image (d) a depiction of a hematoma in the muscles in the left thigh.
In most cases, patients were older, receiving a prophylactic dose of subcutaneous enoxaparin, and showed moderate-to-severe COVID-19 pneumonia. Evrev et al. [152] reported 11 cases of spontaneous abdominal and gastrointestinal bleeding in hospitalized COVID-19 patients, with a male predominance and an average age of 74 years. In nine patients, the retroperitoneal and gastrointestinal bleeding complications followed anticoagulant treatment and were associated with pneumonia severity. Rare cases of gastrointestinal bleeding were also reported during the Omicron wave and were related to the colon [156]. All vascular abdominal complications are summarized in Table 3.
5. Conclusions
In this pictorial review, the main features of COVID-19 pneumonia throughout the pandemic waves of the pandemic are summarized, together with the secondary lung complications and abdominal vascular complications. Radiologists played a key role in the first two pandemic waves when the ancestral variants showed higher pneumonia scores according to CT. Radiologists also played an important role in the diagnosis of COVID-19 pneumonia after breakthrough infections. CECT had a crucial role in the diagnosis of vascular abdominal complications, which were more frequent during the first two pandemic waves. The role of the interaction of the gut/lung axis microbiome with both innate and adaptive immune response remains under investigations with a possible important role in the disease development and severity. This scenario could open the way for the development of new therapeutic strategies.
Author Contributions
B.B. and E.B., conceptualization, methodology, review and editing, validation; B.B. and E.B., original draft preparation, data curation, investigation; M.V. and L.A.M., supervision; B.B., A.M., A.L. and A.L.R., formal analysis, software, investigation, data curation, visualization; A.M., B.B., A.M., A.L. and M.V., investigation, visualization, data curation, formal analysis; A.M. and A.L., investigation, data curation, software; A.L., software; L.A.M., visualization, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The manuscript is a pictorial review and does not contain clinical studies. The authors comply with international and national ethical standards.
Informed Consent Statement
Not applicable because this study is a pictorial review and does not contain clinical studies.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors would like to thank all the doctors and nurses that were engaged in the fight against COVID-19.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lai, A.; Tambuzzi, S.; Bergna, A.; Battistini, A.; Della Ventura, C.; Galli, M.; Zoja, R.; Zehender, G.; Cattaneo, C. Evidence of SARS-CoV-2 Antibodies and RNA on Autopsy Cases in the Pre-Pandemic Period in Milan (Italy). Front. Microbiol. 2022, 13, 886317. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Figoni, J.; Henny, J.; Desenclos, J.C.; Kab, S.; de Lamballerie, X.; Zins, M. MEvidence of early circulation of SARS-CoV-2 in France: Findings from the population-based “CONSTANCES” cohort. Eur. J. Epidemiol. 2021, 36, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Monti, M.; Santini, S.A.; Marri, S.; Madia, F.; Lorini, S.; Petraccia, L.; Stasi, C.; Basile, U.; Luti, V.; et al. SARS-CoV-2 was already circulating in Italy, in early December 2019. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3342–3349. [Google Scholar] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Morens, D.M.; Breman, J.G.; Calisher, C.H.; Doherty, P.C.; Hahn, B.H.; Keusch, G.T.; Kramer, L.D.; LeDuc, J.W.; Monath, T.P.; Taubenberger, J.K. The Origin of COVID-19 and Why It Matters. Am. J. Trop. Med. Hyg. 2020, 103, 955–959. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Bloom, J.D.; Chan, Y.A.; Baric, R.S.; Bjorkman, P.J.; Cobey, S.; Deverman, B.E.; Fisman, D.N.; Gupta, R.; Iwasaki, A.; Lipsitch, M.; et al. Investigate the origins of COVID-19. Science 2021, 372, 694. [Google Scholar] [CrossRef]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef]
- Zuin, M.; Engelen, M.M.; Bilato, C.; Vanassche, T.; Rigatelli, G.; Verhamme, P.; Vandenbriele, C.; Zuliani, G.; Roncon, L. Prevalence of Acute Pulmonary Embolism at Autopsy in Patients with COVID-19. Am. J. Cardiol. 2022, 171, 159–164. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Y.; Shen, W.; Zhao, R.; Jiang, B.; Song, H.; Yan, M.; Ma, H. Surveillance of emerging infectious diseases for biosecurity. Sci. China Life Sci. 2022, 65, 1504–1516. [Google Scholar] [CrossRef]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Brogna, C.; Martino, A.; Minichiello, S.; Romeo, D.M.; Romano, P.; Bignardi, E.; Mazza, E.M.; Musto, L. SARS-CoV-2 Infection with Different Radiological Insights. Diagnostics 2020, 10, 283. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Zhang, M.; Liu, S.; Zhou, L.; Yang, C.; Liu, C. The Role of the Gastrointestinal System in Neuroinvasion by SARS-CoV-2. Front. Neurosci. 2021, 15, 694446. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Jahromy, M.; Ashrafi, P.; Vatani, K.; Nemati, M.H.; Moghadam, P.; Rostamian, F.; Jahromi, M. Multi-organ system involvement in coronavirus disease 2019 (COVID-19): A mega review. J. Fam. Med. Prim. Care 2022, 11, 5014–5023. [Google Scholar] [CrossRef]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Dutta, S. Role of the Microbiome in the Pathogenesis of COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 736397. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gong, T.; Wang, G.; Wang, J.; Guo, X.; Cai, E.; Li, S.; Li, X.; Yu, Y.; Lin, L. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores from Sequential Chest CT. Am. J. Roentgenol. 2020, 215, 367–373. [Google Scholar] [CrossRef]
- Guillo, E.; Gomez, I.B.; Dangeard, S.; Bennani, S.; Saab, I.; Tordjman, M.; Jilet, L.; Chassagnon, G.; Revel, M.-P. COVID-19 pneumonia: Diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur. J. Radiol. 2020, 131, 109209. [Google Scholar] [CrossRef]
- Brogna, B.; Bignardi, E.; Brogna, C.; Volpe, M.; Lombardi, G.; Rosa, A.; Gagliardi, G.; Capasso, P.F.M.; Gravino, E.; Maio, F.; et al. A Pictorial Review of the Role of Imaging in the Detection, Management, Histopathological Correlations, and Complications of COVID-19 Pneumonia. Diagnostics 2021, 11, 437. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Vestri, A.R.; Spagnoli, A.; Cipollone, F.; Ceccarelli, G.; Oliva, A.; Amitrano, M.; Pirro, M.; Taliani, G.; et al. ADA (Age-D-Dimer-Albumin) Score to Predict Thrombosis in SARS-CoV-2. Thromb. Haemost. 2022, 122, 1567–1572. [Google Scholar] [CrossRef]
- Acharya, Y.; Alameer, A.; Calpin, G.; Alkhattab, M.; Sultan, S. A comprehensive review of vascular complications in COVID-19. J. Thromb. Thrombolysis 2021, 53, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Gong, A.J.; Gawande, R.S.; Fishman, E.K.; Vadvala, H.V. Vascular findings in CTA body and extremity of critically ill COVID-19 patients: Commonly encountered vascular complications with review of literature. Emerg. Radiol. 2022, 29, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.R.; Dev, H.; Lane, E.G.; Margolis, D.J.; DeSancho, M.T. Major hemorrhage and mortality in COVID-19 patients on therapeutic anticoagulation for venous thromboembolism. J. Thromb. Thrombolysis 2022, 54, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Romano, A.; Tibullo, L.; Montuori, M.; Nunziata, M.; Russo, G.; Musto, L.A. Rare findings of spontaneous hemothorax and small subpleural lung hematoma in a COVID-19 patient: A case report. Acta Radiol. Open 2021, 10, 1–6. [Google Scholar] [CrossRef]
- Kirca, F.; Aydoğan, S.; Gözalan, A.; Kayipmaz, A.E.; Özdemir, F.A.E.; Tekçe, Y.T.; Beşer, İ.O.; Gün, P.; Ökten, R.S.; Dinç, B. Comparison of clinical characteristics of wild-type SARS-CoV-2 and Omicron. Rev. Assoc. Med. Bras. 2022, 68, 1476–1480. [Google Scholar] [CrossRef]
- Lee, J.E.; Hwang, M.; Kim, Y.H.; Chung, M.J.; Sim, B.H.; Jeong, W.G.; Jeong, Y.J. SARS-CoV-2 Variants Infection in Relationship to Imaging-based Pneumonia and Clinical Outcomes. Radiology 2023, 306, e221795. [Google Scholar] [CrossRef]
- Addo, I.Y.; Dadzie, F.A.; Okeke, S.R.; Boadi, C.; Boadu, E.F. Duration of immunity following full vaccination against SARS-CoV-2: A systematic review. Arch. Public Health 2022, 80, 200. [Google Scholar] [CrossRef]
- Gong, I.Y.; Vijenthira, A.; Powis, M.; Calzavara, A.; Patrikar, A.; Sutradhar, R.; Hicks, L.K.; Wilton, D.; Singh, S.; Krzyzanowska, M.K.; et al. Association of COVID-19 Vaccination with Breakthrough Infections and Complications in Patients with Cancer. JAMA Oncol. 2023, 9, 386. [Google Scholar] [CrossRef]
- Mirouse, A.; Friol, A.; Moreau, A.-S.; Jung, B.; Jullien, E.; Bureau, C.; Djibré, M.; de Prost, N.; Zafrani, L.; Argaud, L.; et al. Severe SARS-Cov2 pneumonia in vaccinated patients: A multicenter cohort study. Sci. Rep. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Tong, X.; Huang, Z.; Zhang, X.; Si, G.; Lu, H.; Zhang, W.; Xue, Y.; Xie, W. Old Age is an Independent Risk Factor for Pneumonia Development in Patients with SARS-CoV-2 Omicron Variant Infection and a History of Inactivated Vaccine Injection. Infect. Drug Resist. 2022, 15, 5567–5573. [Google Scholar] [CrossRef]
- Kim, A.H.; Sparks, J.A. Immunosuppression and SARS-CoV-2 breakthrough infections. Lancet Rheumatol. 2022, 4, e379–e380. [Google Scholar] [CrossRef]
- Brogna, B.; Bignardi, E.; Brogna, C.; Capasso, C.; Gagliardi, G.; Martino, A.; Musto, L.A. COVID-19 Pneumonia in Vaccinated Population: A Six Clinical and Radiological Case Series. Medicina 2021, 57, 891. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Zhou, C.; Chen, B.; Fang, H.; Chen, S.; Zhang, X.; Wang, L.; Zhang, L. A review of SARS-CoV2: Compared with SARS-CoV and MERS-CoV. Front. Med. 2021, 8, 628370. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Wi, Y.M.; Park, H.; Lee, J.E.; Kim, S.-H.; Lee, K.S. Current and Emerging Knowledge in COVID-19. Radiology 2023, 306, e222462. [Google Scholar] [CrossRef]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Sarkesh, A.; Sorkhabi, A.D.; Sheykhsaran, E.; Alinezhad, F.; Mohammadzadeh, N.; Hemmat, N.; Baghi, H.B. Extrapulmonary Clinical Manifestations in COVID-19 Patients. Am. J. Trop. Med. Hyg. 2020, 103, 1783–1796. [Google Scholar] [CrossRef]
- Pozdnyakova, V.; Weber, B.; Cheng, S.; Ebinger, J.E. Review of Immunologic Manifestations of COVID-19 Infection and Vaccination. Cardiol. Clin. 2022, 40, 301–308. [Google Scholar] [CrossRef]
- Davitt, E.; Davitt, C.; Mazer, M.B.; Areti, S.S.; Hotchkiss, R.S.; Remy, K.E. COVID-19 disease and immune dysregulation. Best Pract. Res. Clin. Haematol. 2022, 35, 101401. [Google Scholar] [CrossRef] [PubMed]
- Arish, M.; Qian, W.; Narasimhan, H.; Sun, J. COVID-19 immunopathology: From acute diseases to chronic sequelae. J. Med. Virol. 2022, 95, e28122. [Google Scholar] [CrossRef] [PubMed]
- Klavina, P.A.; Leon, G.; Curtis, A.M.; Preston, R.J. Dysregulated haemostasis in thrombo-inflammatory disease. Clin. Sci. 2022, 136, 1809–1829. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Q.; Liu, H. Coronavirus disease 2019 and the gut–lung axis. Int. J. Infect. Dis. 2021, 113, 300–307. [Google Scholar] [CrossRef]
- Allali, I.; Bakri, Y.; Amzazi, S.; Ghazal, H. Gut-Lung Axis in COVID-19. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.D.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387. [Google Scholar] [CrossRef]
- Brogna, B.; Brogna, C.; Petrillo, M.; Conte, A.M.; Benincasa, G.; Montano, L.; Piscopo, M. SARS-CoV-2 detection in fecal sample from a patient with typical findings of COVID-19 pneumonia on CT but negative to multiple SARS-CoV-2 RT-PCR tests on oropharyngeal and nasopharyngeal swab samples. Medicina 2021, 57, 290. [Google Scholar] [CrossRef]
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Van den Eede, G. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Brogna, B.; Bisaccia, D.R.; Marino, G.; Viduto, V.; Montano, L.; Piscopo, M. Toxin-like Peptides from the Bacterial Cultures Derived from Gut Microbiome Infected by SARS-CoV-2—New Data for a Possible Role in the Long COVID Pattern. Biomedicines 2022, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Petrillo, M.; Clerbaux, L.A.; Leoni, G.; Ponti, J.; Bogni, A.; Brogna, C.; Cristoni, S.; Sanges, R.; Mendoza-de Gyves, E.; et al. Effects of spike protein and toxin-like peptides found in COVID-19 patients on human 3D neuronal/glial model undergoing differentiation: Possible implications for SARS-CoV-2 impact on brain development. Reprod. Toxicol. 2022, 111, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kitahara, Y.; Miwata, K.; Okimoto, M.; Takafuta, T. Comparison of COVID-19 pneumonia during the SARS-CoV-2 Omicron wave and the previous non-Omicron wave in a single facility. Respir. Investig. 2022, 60, 772–778. [Google Scholar] [CrossRef]
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the Radiologist Needs to Know. Radiographics 2022, 42, E32. [Google Scholar] [CrossRef]
- Kim, H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur. Radiol. 2020, 30, 3266–3267. [Google Scholar] [CrossRef]
- Inui, S.; Gonoi, W.; Kurokawa, R.; Nakai, Y.; Watanabe, Y.; Sakurai, K.; Ishida, M.; Fujikawa, A.; Abe, O. The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19). Insights Imaging 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef]
- Hu, Q.; Guan, H.; Sun, Z.; Huang, L.; Chen, C.; Ai, T.; Pan, Y.; Xia, L. CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur. J. Radiol. 2020, 128, 109017. [Google Scholar] [CrossRef]
- Li, X.; Zeng, W.; Li, X.; Chen, H.; Shi, L.; Li, X.; Xiang, H.; Cao, Y.; Chen, H.; Liu, C.; et al. CT imaging changes of corona virus disease 2019(COVID-19): A multi-center study in Southwest China. J. Transl. Med. 2020, 18, 154. [Google Scholar] [CrossRef]
- Caruso, D.; Polidori, T.; Guido, G.; Nicolai, M.; Bracci, B.; Cremona, A.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Rucci, C.; et al. Typical and atypical COVID-19 computed tomography findings. World J. Clin. Cases 2020, 8, 3177–3187. [Google Scholar] [CrossRef]
- Guarnera, A.; Podda, P.; Santini, E.; Paolantonio, P.; Laghi, A. Differential diagnoses of COVID-19 pneumonia: The current challenge for the radiologist—A pictorial essay. Insights Imaging 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Jalaber, C.; Chassagnon, G.; Hani, C.; Dangeard, S.; Babin, M.; Launay, O.; Revel, M.P. Is COVID-19 pneumonia differentiable from other viral pneumonia on CT scan? Respir. Med. Res. 2021, 79, 100824. [Google Scholar]
- Duzgun, S.A.; Durhan, G.; Demirkazik, F.B.; Akpinar, M.G.; Ariyurek, O.M. COVID-19 pneumonia: The great radiological mimicker. Insights Imaging 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Garrana, S.H.; Som, A.; Ndakwah, G.S.; Yeung, T.; Febbo, J.; Heeger, A.P.; Lang, M.; McDermott, S.; Mendoza, D.P.; Zhang, E.W.; et al. Comparison of Chest CT Findings of COVID-19, Influenza, and Organizing Pneumonia: A Multireader Study. Am. J. Roentgenol. 2021, 217, 1093–1102. [Google Scholar] [CrossRef]
- Zheng, F.; Li, L.; Zhang, X.; Song, Y.; Huang, Z.; Chong, Y.; Chen, Z.; Zhu, H.; Wu, J.; Chen, W.; et al. Accurately Discriminating COVID-19 from Viral and Bacterial Pneumonia According to CT Images Via Deep Learning. Interdiscip. Sci. Comput. Life Sci. 2021, 13, 273–285. [Google Scholar] [CrossRef]
- Askani, E.; Mueller-Peltzer, K.; Madrid, J.; Knoke, M.; Hasic, D.; Bamberg, F.; Schlett, C.L.; Agarwal, P. Computed Tomographic Imaging Features of COVID-19 Pneumonia Caused by the Delta (B.1.617.2) and Omicron (B.1.1.529) Variant in a German Nested Cohort Pilot Study Group. Tomography 2022, 8, 2435–2449. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Fujikawa, A.; Gonoi, W.; Kawano, S.; Sakurai, K.; Uchida, Y.; Ishida, M.; Abe, O. Comparison of CT findings of coronavirus disease 2019 (COVID-19) pneumonia caused by different major variants. Jpn. J. Radiol. 2022, 40, 1246–1256. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.H.; Kim, B.N. Chest CT Findings in Hospitalized Patients with SARS-CoV-2: Delta versus Omicron Variants. Radiology 2023, 305, E66. [Google Scholar] [CrossRef]
- Blanca, D.; Nicolosi, S.; Bandera, A.; Blasi, F.; Mantero, M.; Hu, C.; de Amicis, M.M.; Lucchi, T.; Schinco, G.; Peyvandi, F.; et al. Comparison between the first and second COVID-19 waves in Internal Medicine wards in Milan, Italy: A retrospective observational study. Intern. Emerg. Med. 2022, 17, 2219–2228. [Google Scholar] [CrossRef]
- González-Castro, A.; Fito, E.C.; Fernandez, A.; Acha, P.E.; Borregán, J.R.; Peñasco, Y. First and second wave of coronavirus-19 disease: A comparative study in patients hospitalized in an ICU of a third-level university hospital. Med. Intensiv. 2022, 46, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Portacci, A.; Carpagnano, G.E.; Tummolo, M.G.; Santomasi, C.; Palma, L.; Fasano, D.; Resta, E.; Lozupone, M.; Solfrizzi, V.; Panza, F.; et al. COVID-19 clinical phenotypes and short-term outcomes: Differences between the first and the second wave of pandemic in Italy. Expert Rev. Respir. Med. 2021, 15, 1619–1625. [Google Scholar] [CrossRef]
- Bajči, M.P.; Lendak, D.F.; Ristić, M.; Drljača, M.M.; Brkić, S.; Turkulov, V.; Petrović, V. COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview. Vaccines 2022, 10, 1818. [Google Scholar] [CrossRef]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, J.; Chen, L.; Jia, X.; Fan, Y.; Zheng, Y.; Alwalid, O.; Liu, J.; Li, Y.; Li, N.; et al. Comparative Analysis of Clinical and CT Findings in Patients with SARS-CoV-2 Original Strain, Delta and Omicron Variants. Biomedicines 2023, 11, 901. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Kakizaki, Y.; Saito, A.; Tsutsui, T.; Hanawa, S.; Yamaki, H.; Ide, S.; Kawaguchi, M.; Kobayashi, H.; Miyashita, Y.; et al. Lung tropism in hospitalized patients following infection with SARS-CoV-2 variants from D614G to Omicron BA. 2. Commun. Med. 2023, 3, 32. [Google Scholar] [CrossRef]
- Tsakok, M.T.; Watson, R.A.; Saujani, S.J.; Kong, M.; Xie, C.; Peschl, H.; Wing, L.; MacLeod, F.K.; Shine, B.; Talbot, N.P.; et al. Reduction in Chest CT Severity and Improved Hospital Outcomes in SARS-CoV-2 Omicron Compared with Delta Variant Infection. Radiology 2023, 306, 261–269. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.; Huang, J.; Dong, J.; Ye, J.; Fu, Y.; Huang, J.; Xu, D.; Cao, G.; Qian, G. Clinical and Pulmonary CT Characteristics of Patients Infected with the SARS-CoV-2 Omicron Variant Compared with Those of Patients Infected with the Alpha Viral Strain. Front. Public Health 2022, 10, 931480. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 1–32. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A.; Tjhin, Y. COVID-19 Vaccination Efficacy and Safety Literature Review. J. Clin. Med. Res. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- León, T.M.; Dorabawila, V.; Nelson, L.; Lutterloh, E.; Bauer, U.E.; Backenson, B.; Bassett, M.T.; Henry, H.; Bregman, B.; Midgley, C.M.; et al. COVID-19 Case and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis—California and New York, May–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hewins, B.; Rahman, M.; Bermejo-Martin, J.F.; Kelvin, A.A.; Richardson, C.D.; Rubino, S.; Kumar, A.; Ndishimye, P.; Toloue Ostadgavahi, A.; Mahmud-Al-Rafat, A.; et al. Alpha, Beta, Delta, Omicron, and SARS-CoV-2 Breakthrough Cases: Defining Immunological Mechanisms for Vaccine Waning and Vaccine-Variant Mismatch. Front. Virol. 2022, 2, 849936. [Google Scholar] [CrossRef]
- Piñana, J.L.; Vazquez, L.; Calabuig, M.; López-Corral, L.; Martin-Martin, G.; Villalon, L.; Sanz-Linares, G.; Conesa-Garcia, V.; Sanchez-Salinas, A.; Gago, B.; et al. One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients. Blood Cancer J. 2023, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance-VISION Network, 10 States, August 2021–January MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 255–263. [Google Scholar]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat. Commun. 2022, 13, 1237. [Google Scholar] [CrossRef]
- Bardosh, K.; de Figueiredo, A.; Gur-Arie, R.; Jamrozik, E.; Doidge, J.; Lemmens, T.; Keshavjee, S.; E Graham, J.; Baral, S. The unintended consequences of COVID-19 vaccine policy: Why mandates, passports and restrictions may cause more harm than good. BMJ Glob. Health 2022, 7, e008684. [Google Scholar] [CrossRef]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metab. Open 2022, 14, e100180. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Wu, D.; Tang, L.; Wang, X.; Yan, T.; An, Z.; Yin, Z.; Gao, G.F.; Wang, F.; et al. Association of COVID-19 Vaccination and Clinical Severity of Patients Infected with Delta or Omicron Variants-China, May 21, 2021–February 28, 2022. China CDC Wkly. 2022, 4, 293–297. [Google Scholar] [CrossRef]
- Wada, N.; Li, Y.; Hino, T.; Gagne, S.; Valtchinov, V.I.; Gay, E.; Nishino, M.; Madore, B.; Guttmann, C.R.; Bond, S.; et al. COVID-19 Vaccination reduced pneumonia severity. Eur. J. Radiol. Open 2022, 9, e100456. [Google Scholar] [CrossRef]
- Lamacchia, G.; Mazzoni, A.; Spinicci, M.; Vanni, A.; Salvati, L.; Peruzzi, B.; Bencini, S.; Capone, M.; Carnasciali, A.; Farahvachi, P.; et al. Clinical and Immunological Features of SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals Requiring Hospitalization. J. Clin. Immunol. 2022, 42, 1379–1391. [Google Scholar] [CrossRef]
- Ben, F.S.; Ghammem, R.; Zammit, N.; Maatouk, A.; Haddad, N.; Haddad, N.; Kachroudi, M.; Rebai, S.; Laadhari, H.; Ghodhbani, M.M.; et al. Risk factors for severe COVID-19 breakthrough infections: An observational longitudinal study. BMC Infect. Dis. 2022, 22, 894. [Google Scholar]
- Granata, V.; Fusco, R.; Villanacci, A.; Magliocchetti, S.; Urraro, F.; Tetaj, N.; Marchioni, L.; Albarello, F.; Campioni, P.; Cristofaro, M.; et al. Imaging Severity COVID-19 Assessment in Vaccinated and Unvaccinated Patients: Comparison of the Different Variants in a High Volume Italian Reference Center. J. Pers. Med. 2022, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, A.; Luna, A.; Micic, D. Serologic response following SARS-COV2 vaccination in patients with cancer: A systematic review and meta-analysis. J. Hematol. Oncol. 2022, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, Ł.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. [Google Scholar] [CrossRef]
- Lee, J.E.; Hwang, M.; Kim, Y.-H.; Chung, M.J.; Sim, B.H.; Chae, K.J.; Yoo, J.Y.; Jeong, Y.J. Imaging and Clinical Features of COVID-19 Breakthrough Infections: A Multicenter Study. Radiology 2022, 303, 682–692. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, I.; Singh, P.K.; Ansari, M.S.; Singh, H.A.; Sonkar, S.; Prakash, A.; Ojha, R.; Shukla, R.C. Initial comparative analysis of pulmonary involvement on HRCT between vaccinated and non-vaccinated subjects of COVID-19. Eur. Radiol. 2022, 32, 4275–4283. [Google Scholar] [CrossRef]
- Hughes, T.D.; Subramanian, A.; Chakraborty, R.; Cotton, S.A.; Herrera, M.D.P.G.; Huang, Y.; Lambert, N.; Pinto, M.D.; Rahmani, A.M.; Sierra, C.J.; et al. The effect of SARS-CoV-2 variant on respiratory features and mortality. Sci. Rep. 2023, 13, 4503. [Google Scholar] [CrossRef]
- Schultz, M.J.; van Meenen, D.M.; Bos, L.D. COVID-19-related acute respiratory distress syndrome: Lessons learned during the pandemic. Lancet Respir. Med. 2022, 10, 1108–1110. [Google Scholar] [CrossRef]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Liu, R.; Lan, Y.; Lei, Y.; Zeng, F.; Tang, X.; He, H. Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: Similarities and Differences. Front. Med. 2022, 9, 829771. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Spinetti, T.; Urman, R.D.; Luedi, M.M.; Schefold, J.C. COVID-19-associated acute respiratory distress syndrome (CARDS): Current knowledge on pathophysiology and ICU treatment—A narrative review. Best Pract. Res. Clin. Anaesthesiol. 2020, 35, 351–368. [Google Scholar] [CrossRef]
- Gosangi, B.; Rubinowitz, A.N.; Irugu, D.; Gange, C.; Bader, A.; Cortopassi, I. COVID-19 ARDS: A review of imaging features and overview of mechanical ventilation and its complications. Emerg. Radiol. 2022, 29, 23–34, Erratum in Emerg. Radiol. 2022, 29, 225. [Google Scholar] [CrossRef]
- Zhang, B. Gross Pathology in COVID-19. Encyclopedia 2022, 2, 1790–1802. [Google Scholar] [CrossRef]
- Van Do, T.; Manabe, T.; Van Vu, G.; Nong, V.M.; Fujikura, Y.; Phan, D.; Pham, T.T.; Do, C.D.; Doan, T.T.; Nguyen, N.T.; et al. Clinical characteristics and mortality risk among critically ill patients with COVID-19 owing to the B.1.617.2 (Delta) variant in Vietnam: A retrospective observational study. PLoS ONE 2023, 18, e0279713. [Google Scholar] [CrossRef]
- Jeican, I.I.; Inișca, P.; Gheban, D.; Anton, V.; Lazăr, M.; Vică, M.L.; Mironescu, D.; Rebeleanu, C.; Crivii, C.B.; Aluaș, M.; et al. Histopathological Lung Findings in COVID-19 B.1.617.2 SARS-CoV-2 Delta Variant. J. Pers. Med. 2023, 13, 279. [Google Scholar] [CrossRef]
- Van Goethem, N.; Chung, P.Y.J.; Meurisse, M.; Vandromme, M.; De Mot, L.; Brondeel, R.; Stouten, V.; Klamer, S.; Cuypers, L.; Braeye, T.; et al. Clinical Severity of SARS-CoV-2 Omicron Variant Compared with Delta among Hospitalized COVID-19 Patients in Belgium during Autumn and Winter Season 2021–2022. Viruses 2022, 14, 1297. [Google Scholar] [CrossRef]
- Sridhar, P.; Singh, A.; Salomon, N.; Steiger, D.J. Vaccine-Induced Antibody Dependent Enhancement in COVID-19. Chest 2022, 162, A646–A647. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Sasaki, H.; Miyata, N.; Miyazaki, K.; Okudela, K.; Tateishi, Y.; Hayashi, H.; Kawana-Tachikawa, A.; Iwashita, H.; Maeda, K.; et al. An autopsy case of COVID-19-like acute respiratory distress syndrome after mRNA-1273 SARS-CoV-2 vaccination. Int. J. Infect. Dis. 2022, 121, 98–101. [Google Scholar] [CrossRef]
- Melhorn, J.; Achaiah, A.; Conway, F.M.; Thompson, E.M.; Skyllberg, E.W.; Durrant, J.; Hasan, N.A.; Madani, Y.; Naran, P.; Vijayakumar, B.; et al. Pneumomediastinum in COVID-19: A phenotype of severe COVID-19 pneumonitis? The results of the United Kingdom (POETIC) survey. Eur. Respir. J. 2022, 60, 2102522. [Google Scholar] [CrossRef]
- Khaire, N.; Deshmukh, S.; Agarwal, E.; Mahale, N.; Khaladkar, S.; Desai, S.; Kulkarni, A. “Pneumomediastinum: A marker of severity in COVID-19 disease”. Heliyon 2023, 9, e12981. [Google Scholar] [CrossRef] [PubMed]
- Tetaj, N.; Garotto, G.; Albarello, F.; Mastrobattista, A.; Maritti, M.; Stazi, G.V.; Marini, M.C.; Caravella, I.; Macchione, M.; De Angelis, G.; et al. Incidence of Pneumothorax and Pneumomediastinum in 497 COVID-19 Patients with Moderate–Severe ARDS over a Year of the Pandemic: An Observational Study in an Italian Third Level COVID-19 Hospital. J. Clin. Med. 2021, 10, 5608. [Google Scholar] [CrossRef] [PubMed]
- Kecskes, G.; Szabo, A.; Sutori, D.; Maroti, P.; Marovics, G.; Molnar, T.F. Pneumothorax/pneumomediastinum and pre-existing lung pathology in ventilated COVID-19 patients: A cohort study. J. Thorac. Dis. 2022, 14, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Sen, K.K.; Mishra, A. Barotrauma and its complications in COVID-19 patients: A retrospective study at tertiary care hospital of Eastern India. Bull. Natl. Res. Cent. 2022, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Capaccione, K.M.; D’souza, B.; Leb, J.; Luk, L.; Duong, J.; Tsai, W.-Y.; Navot, B.; Dumeer, S.; Mohammed, A.; Salvatore, M.M. Pneumothorax rate in intubated patients with COVID-19. Acute Crit. Care 2021, 36, 81–84. [Google Scholar] [CrossRef]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. IncreaIncidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020, 297, E252–E262. [Google Scholar] [CrossRef]
- Martinelli, A.W.; Ingle, T.; Newman, J.; Nadeem, I.; Jackson, K.; Lane, N.D.; Melhorn, J.; Davies, H.E.; Rostron, A.J.; Adeni, A.; et al. COVID-19 and pneumothorax: A multicentre retrospective case series. Eur. Respir. J. 2020, 56, 2002697. [Google Scholar] [CrossRef]
- Rodriguez-Arciniega, T.G.; Sierra-Diaz, E.; Flores-Martinez, J.A.; Alvizo-Perez, M.E.; Lopez-Leal, I.N.; Corona-Nakamura, A.L.; Castellanos-Garcia, H.E.; Bravo-Cuellar, A. Frequency and Risk Factors for Spontaneous Pneumomediastinum in COVID-19 Patients. Front. Med. 2021, 8, 662358. [Google Scholar] [CrossRef]
- Zantah, M.; Castillo, E.D.; Townsend, R.; Dikengil, F.; Criner, G.J. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir. Res. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Shahsavarinia, K.; Rahvar, G.; Soleimanpour, H.; Saadati, M.; Vahedi, L.; Mahmoodpoor, A. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in critically ill COVID-19 patients: A systematic review. Pak. J. Med. Sci. 2022, 38, 730–735. [Google Scholar] [CrossRef]
- Hamouri, S.; AlQudah, M.; Albawaih, O.; Al-Zoubi, N.; Syaj, S. Spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema in non-ventilated COVID-19 patients. Futur. Sci. OA 2022, 8, FSO771. [Google Scholar] [CrossRef]
- Shaikh, N.; Al Ameri, G.; Shaheen, M.; Abdaljawad, W.I.; Al Wraidat, M.; Al Alawi, A.A.S.; Ali, H.S.; Mohamed, A.S.; Daeri, H.; Khatib, M.Y.; et al. Spontaneous pneumomediastinum and pneumothorax in COVID-19 patients: A tertiary care experience. Health Sci. Rep. 2021, 4, e339. [Google Scholar] [CrossRef]
- Gandolfo, C.; Bonfiglio, M.; Spinetto, G.; Ferraioli, G.; Barlascini, C.; Nicolini, A.; Solidoro, P. Pneumomediastinum associated with severe pneumonia related to COVID-19: Diagnosis and management. Minerva Medica 2022, 112, 779–785. [Google Scholar] [CrossRef]
- Espinosa, C.; Morente, L.M.; Mansour, E.H.; Yousefzadeh, M.L.; Muzaffarr, Z.M.; Salguero, D.; Vianna, S.D.; Quesada, L.D.; Poli, S.; Garcia, H. Predictors of Spontaneous Pneumomediastinum in Patients with COVID-19 and Ards on High-Flow Nasal Cannula. Chest 2022, 162, A1360–A1361. [Google Scholar] [CrossRef]
- Coppola, M.G.; Lugarà, M.; Tamburrini, S.; Madonna, P.; Panico, C.; Noschese, G.; Pone, E. Pneumomediastinum and Pneumothorax as Relevant Complications of Sub-Intensive Care of Patients with COVID-19: Description of a Case Series. Medicina 2021, 57, 919. [Google Scholar] [CrossRef]
- Palumbo, D.; Campochiaro, C.; Belletti, A.; Marinosci, A.; Dagna, L.; Zangrillo, A.; De Cobelli, F. Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: Differences between first and second Italian pandemic wave. Eur. J. Intern. Med. 2021, 88, 144–146. [Google Scholar] [CrossRef]
- Xiang, T.; Fang, J.; Cheng, T.; Li, Z.; Wu, D.; Zhang, S.; Ge, S.; Zhang, W. Case report: Severe pneumonia and pneumomediastinum in a previously robust adolescent caused by Omicron BA.5.2. Front. Med. 2023, 10, e1132630. [Google Scholar] [CrossRef]
- Kanne, J.P.; Little, B.P.; Schulte, J.J.; Haramati, A.; Haramati, L.B. Long-term Lung Abnormalities Associated with COVID-19 Pneumonia. Radiology 2023, 306, e221806. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Zhang, X.; Jia, X.; Zheng, Y.; Shi, H. Fibrotic Interstitial Lung Abnormalities at 1-year Follow-up CT after Severe COVID-19. Radiology 2021, 301, E438–E440. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef]
- Bocchino, M.; Lieto, R.; Romano, F.; Sica, G.; Bocchini, G.; Muto, E.; Capitelli, L.; Sequino, D.; Valente, T.; Fiorentino, G.; et al. Chest CT–based Assessment of 1-year Outcomes after Moderate COVID-19 Pneumonia. Radiology 2022, 305, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Cocconcelli, E.; Bernardinello, N.; Giraudo, C.; Castelli, G.; Giorgino, A.; Leoni, D.; Petrarulo, S.; Ferrari, A.; Saetta, M.; Cattelan, A.; et al. Characteristics and Prognostic Factors of Pulmonary Fibrosis After COVID-19 Pneumonia. Front. Med. 2022, 8, 823600. [Google Scholar] [CrossRef] [PubMed]
- Wismüller, A.; Dsouza, A.M.; Abidin, A.Z.; Vosoughi, M.A.; Gange, C.; Cortopassi, I.O.; Bozovic, G.; Bankier, A.A.; Batra, K.; Chodakiewitz, Y.; et al. Early-stage COVID-19 pandemic observations on pulmonary embolism using nationwide multi-institutional data harvesting. NPJ Digit. Med. 2022, 5, 1–9. [Google Scholar] [CrossRef]
- Mouzarou, A.; Ioannou, M.; Leonidou, E.; Chaziri, I. Pulmonary Embolism in Post-CoviD-19 Patients, a Literature Review: Red Flag for Increased Awareness? SN Compr. Clin. Med. 2022, 4, 190. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, C. Thromboembolic Findings in COVID-19 Autopsies: Pulmonary Thrombosis or Embolism? Ann. Intern. Med. 2020, 173, 394–395. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 with Fatal Outcome: Results from a Prospective, Single-Center, Clinicopathologic Case Series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef]
- Sakr, Y.; Giovini, M.; Leone, M.; Pizzilli, G.; Kortgen, A.; Bauer, M.; Tonetti, T.; Duclos, G.; Zieleskiewicz, L.; Buschbeck, S.; et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: A narrative review. Ann. Intensive Care 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Močibob, L.; Šušak, F.; Šitum, M.; Višković, K.; Papić, N.; Vince, A. COVID-19 and Pulmonary Thrombosis—An Unresolved Clinical Puzzle: A Single-Center Cohort Study. J. Clin. Med. 2022, 11, 7049. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Connolly, A.M.F. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: Nationwide self- controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef]
- Manzur-Pineda, K.; O’neil, C.F.; Bornak, A.; Lalama, M.J.; Shao, T.; Kang, N.; Kennel-Pierre, S.; Tabbara, M.; Velazquez, O.C.; Rey, J. COVID-19-related thrombotic complications experience before and during delta wave. J. Vasc. Surg. 2022, 76, 1374–1382.e1. [Google Scholar] [CrossRef]
- Law, N.; Chan, J.; Kelly, C.; Auffermann, W.F.; Dunn, D.P. Incidence of pulmonary embolism in COVID-19 infection in the ED: Ancestral, Delta, Omicron variants and vaccines. Emerg. Radiol. 2022, 29, 625–629. [Google Scholar] [CrossRef]
- Ho, F.K.; Pell, J.P. Thromboembolism and bleeding after COVID-19. BMJ 2022, 377, o817. [Google Scholar] [CrossRef]
- Murphy, M.C.; Little, B.P. Chronic Pulmonary Manifestations of COVID-19 Infection: Imaging Evaluation. Radiology 2023, 307, e222379. [Google Scholar] [CrossRef]
- Ravaglia, C.; Doglioni, C.; Chilosi, M.; Piciucchi, S.; Dubini, A.; Rossi, G.; Pedica, F.; Puglisi, S.; Donati, L.; Tomassetti, S.; et al. Clinical, radiological and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur. Respir. J. 2022, 60, 2102411. [Google Scholar] [CrossRef]
- Bonaffini, P.A.; Franco, P.N.; Bonanomi, A.; Giaccherini, C.; Valle, C.; Marra, P.; Norsa, L.; Marchetti, M.; Falanga, A.; Sironi, S. Ischemic and hemorrhagic abdominal complications in COVID-19 patients: Experience from the first Italian wave. Eur. J. Med. Res. 2022, 27, 1–9. [Google Scholar] [CrossRef]
- Peshevska-Sekulovska, M.; Boeva, I.; Sekulovski, M.; Zashev, M.; Peruhova, M. Gastrointestinal Ischemia—Stumbling Stone in COVID-19 Patients. Gastroenterol. Insights 2022, 13, 206–217. [Google Scholar] [CrossRef]
- Norsa, L.; Bonaffini, P.A.; Caldato, M.; Bonifacio, C.; Sonzogni, A.; Indriolo, A.; Valle, C.; Furfaro, F.; Bonanomi, A.; Franco, P.N.; et al. Intestinal ischemic manifestations of SARS-CoV-2: Results from the ABDOCOVID multicentre study. World J. Gastroenterol. 2021, 27, 5448–5459. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P. COVID-19 and acute mesenteric ischemia: A review of literature. Hematol. Transfus. Cell Ther. 2020, 43, 112–116. [Google Scholar] [CrossRef]
- Fathy, A.; Rizk, A.; Elnekeidy, A.; Gharraf, H.S.; Abdelgawad, M.S.; Samir, A. Imaging of COVID-19 vasculopathy from head to toe: Egyptian collective experience after 2 years of the pandemic. Egypt. J. Radiol. Nucl. Med. 2022, 53, 1–21. [Google Scholar] [CrossRef]
- Evrev, D.; Sekulovski, M.; Gulinac, M.; Dobrev, H.; Velikova, T.; Hadjidekov, G. Retroperitoneal and abdominal bleeding in anticoagulated COVID-19 hospitalized patients: Case series and brief literature abdominal bleeding in anticoagulated COVID-19 hospitalized patients: Case series and brief literature review. World J. Clin. Cases 2023, 11, 1528–1548. [Google Scholar] [CrossRef] [PubMed]
- Sposato, B.; Croci, L.; Di Tomassi, M.; Puttini, C.; Olivieri, C.; Alessandri, M.; Ronchi, M.C.; Donati, E.; Garcea, A.; Brazzi, A.; et al. Spontaneous abdominal bleeding associated with SARS-CoV-2 infection: Causality or coincidence? Acta Biomed. 2021, 92, e2021199. [Google Scholar] [PubMed]
- Mahmoudabadi, H.Z.; Hadadi, A.; Fattahi, M.R.; Kafan, S.; Ashouri, M.; Allahbeigi, R.; Hajebi, R. Rectus Sheath Hematoma in COVID-19 Patients as a Mortal Complication: A Retrospective Report. Int. J. Clin. Pract. 2022, 2022, 7436827. [Google Scholar] [CrossRef]
- Maruyama, S.; Wada, D.; Oishi, T.; Saito, F.; Yoshiya, K.; Nakamori, Y.; Kuwagata, Y. A descriptive study of abdominal complications in patients with mild COVID-19 presenting to the emergency department: A single-center experience in Japan during the omicron variant phase. BMC Gastroenterol. 2023, 23, 43. [Google Scholar] [CrossRef]
- Sarkardeh, M.; Meftah, E.; Mohammadzadeh, N.; Koushki, J.; Sadrzadeh, Z. COVID-19 and Intestinal Ischemia: A Multicenter Case Series. Front. Med. 2022, 9, 879996. [Google Scholar] [CrossRef]
- Bhayana, R.; Som, A.; Li, M.D.; Carey, D.E.; Anderson, M.A.; Blake, M.A.; Catalano, O.; Gee, M.S.; Hahn, P.F.; Harisinghani, M.; et al. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology 2020, 297, E207–E215. [Google Scholar] [CrossRef]
- Makker, J.; Mantri, N.; Patel, H.K.; Abbas, H.; Baiomi, A.; Sun, H.; Choi, Y.; Chilimuri, S.; Nayudu, S.K. The Incidence and Mortality Impact of Gastrointestinal Bleeding in Hospitalized COVID-19 Patients. Clin. Exp. Gastroenterol. 2021, 14, 405–411. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Pucciarelli, F.; Lucertini, E.; Bracci, B.; Polidori, T.; Guido, G.; Polici, M.; Rucci, C.; Iannicelli, E.; et al. Imaging of abdominal of abdominal complications of COVID-19 infection. BJR Open 2021, 2, 20200052. [Google Scholar] [CrossRef]
- Ojha, V.; Mani, A.; Mukherjee, A.; Kumar, S.; Jagia, P. Mesenteric ischemia in patients with COVID-19: An updated systematic review of abdominal CT findings in 75 patients. Abdom. Imaging 2021, 47, 1565–1602. [Google Scholar] [CrossRef]
- Boraschi, P.; Giugliano, L.; Mercogliano, G.; Donati, F.; Romano, S.; Neri, E. Abdominal and gastrointestinal manifestations in COVID-19 patients: Is imaging useful? World J. Gastroenterol. 2021, 27, 4143–4159. [Google Scholar] [CrossRef]
- Keshavarz, P.; Rafiee, F.; Kavandi, H.; Goudarzi, S.; Heidari, F.; Gholamrezanezhad, A. Ischemic gastrointestinal complications of COVID-19: A systematic review on imaging presentation. Clin. Imaging 2020, 73, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, O.; Srikanth, K.; Mishra, R.; Tandon, A.; Rajput, D. Review of Mesenteric Ischemia in COVID-19 Patients. Indian J. Surg. 2022, 85, 313–321. [Google Scholar] [CrossRef]
- Reginelli, A.; Genovese, E.; Cappabianca, S.; Iacobellis, F.; Berritto, D.; Fonio, P.; Coppolino, F.; Grassi, R. Intestinal Ischemia: US-CT findings correlations. Crit. Ultrasound J. 2013, 5, S7. [Google Scholar] [CrossRef]
- Paul, T.; Joy, A.R.; Alsoub, H.A.R.S.; Parambil, J.V. Case Report: Ischemic Colitis in Severe COVID-19 Pneumonia: An Unforeseen Gastrointestinal Complication. Am. J. Trop. Med. Hyg. 2021, 104, 63–65. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; Ramachandran, R.; Then, E.; Parvataneni, S.; Grantham, T.; Gaduputi, V. COVID-19-Associated Ischemic Colitis: A Rare Manifestation of COVID-19 Infection—Case Report and Review. J. Investig. Med. High Impact Case Rep. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Krejčová, I.; Berková, A.; Kvasnicová, L.; Vlček, P.; Veverková, L.; Penka, I.; Zoufalý, D.; Červeňák, V. Ischemic Colitis in a Patient with Severe COVID-19 Pneumonia. Case Rep. Gastroenterol. 2022, 16, 526–534. [Google Scholar] [CrossRef]
- Alratrout, H.; Debroux, E. Acute right-sided ischemic colitis in a COVID-19 patient: A case report and review of the literature. J. Med. Case Rep. 2022, 16, 1–5. [Google Scholar] [CrossRef]
- Cui, M.-H.; Hou, X.-L.; Liu, J.-Y. Ischemic colitis after receiving the second dose of a COVID-19 inactivated vaccine: A case report. World J. Clin. Cases 2022, 10, 3866–3871. [Google Scholar] [CrossRef]
- Mönkemüller, K.; Abdullayeva, E.; Manovski, K.; Cacho-Díaz, M. Ischemic colitis after COVID-19 mRNA vaccine. Endoscopy 2022, 54, E765–E766. [Google Scholar] [CrossRef]
- Castro, G.R.A.; Collaço, I.A.; Bosco, C.L.B.D.; Corrêa, G.G.; Bosco, G.B.D.; Corrêa, G.L. Splenic infarction as a complication of covid-19 in a patient without respiratory symptoms: A case report and literature review. IDCases 2021, 24, e01062. [Google Scholar] [CrossRef] [PubMed]
- Prentice, G.; Wilson, S.; Coupland, A.; Bicknell, S. Complete splenic infarction in association with COVID-19. BMJ Case Rep. 2021, 14, e246274. [Google Scholar] [CrossRef] [PubMed]
- Al Suwaidi, S.; Alakasheh, B.J.; Al-Ozaibi, L.S. Splenic Infarction in a COVID-19 Patient without Respiratory Symptoms. Dubai Med. J. 2022, 5, 74–77. [Google Scholar] [CrossRef]
- Childers, J.; Do, T.V.C.; Smith, F.; Vangara, A.; Ganti, S.S.; Akella, R. Incidental and Asymptomatic Splenic Infarction and Infrarenal Thrombus in a COVID-19 Patient. Cureus 2022, 14, e26555. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; den Deurwaarder, E.S.G.; Bakker, S.J.L.; de Haas, R.J.; van Meurs, M.; Gansevoort, R.T.; Berger, S.P. Kidney Infarction in Patients with COVID-19. Am. J. Kidney Dis. 2020, 76, 431–435. [Google Scholar] [CrossRef]
- Jana, K.; Janga, K.C.; Greenberg, S.; Kumar, K. Bilateral renal infarction with COVID-19 pneumonia: A case report. Oxf. Med. Case Rep. 2021, 2021, omab121. [Google Scholar] [CrossRef]
- Murray, N.P.; Fuentealba, C.; Reyes, E.; Salazar, A. Renal infarction associated with asymptomatic COVID-19 infection. Hematol. Transfus. Cell Ther. 2021, 43, 353–356. [Google Scholar] [CrossRef]
- Plouffe, B.; Van Hooren, T.; Barton, M.; Nashid, N.; Demirkaya, E.; Norozi, K.; Rachinsky, I.; Delport, J.; Knauer, M.; Tole, S.; et al. Renal Infarcts—A Perplexing Case in the Middle of the COVID-19 Pandemic. Front. Pediatr. 2021, 9, 669453. [Google Scholar] [CrossRef]
- Gentili, G.; Pérez, P.L.; Laplumé-Elizalde, E.; España, S. Kidney infarction in patient with covid-19: Clinical case. Am. J. Kidney Dis. 2022, 82, 788. [Google Scholar] [CrossRef]
- Al-Mashdali, A.F.; Alwarqi, A.F.; Elawad, S.M. Simultaneous renal infarction and splenic infarction as a possible initial manifestation of COVID-19: A case report. Clin. Case Rep. 2021, 9, 4819. [Google Scholar] [CrossRef]
- Brem, F.L.; Abu Al Tayef, T.; Rasras, H.; El Mahi, O.; El Ouafi, N.; Zakaria, B. Concomitant renal and splenic infarctions in a COVID-19-patient with a catastrophic thrombotic syndrome. Radiol. Case Rep. 2022, 17, 4030–4033. [Google Scholar] [CrossRef]
- Ramanathan, M.; Chueng, T.; Fernandez, E.; Gonzales-Zamora, J. Concomitant renal and splenic infarction as a complication of COVID-19: A case report and literature review. Infez. Med. 2020, 28, 611–615. [Google Scholar]
- Mavraganis, G.; Ioannou, S.; Kallianos, A.; Rentziou, G.; Trakada, G. A COVID-19 Patient with Simultaneous Renal Infarct, Splenic Infarct and Aortic Thrombosis during the Severe Disease. Healthcare 2022, 10, 150. [Google Scholar] [CrossRef]
- Dimitriou, I.; Christodoulou, N.; Chatzimargaritis, K.; Kaikis, A.; Kasti, E.; Triantos, G. Splenic Artery Infarct Requiring Surgery: A Rare Complication of COVID-19 Infection. Case Rep. Surg. 2022, 2022, e3391405. [Google Scholar] [CrossRef]
- Javaid, U.; Young, P.; Gill, G.; Bhargava, P. Acute complete splenic infarction secondary to COVID-19 infection. Radiol. Case Rep. 2022, 17, 1402–1406. [Google Scholar] [CrossRef]
- Graça, L.L.; Amaral, M.J.; Serôdio, M.; Costa, B. Extensive thrombosis after COVID-19 vaccine: Cause or coincidence? BMJ Case Rep. 2021, 14, e244878. [Google Scholar] [CrossRef]
- Anderson, A.; Seddon, M.; Shahzad, K.; Lunevicius, R. Post-COVID-19 vaccination occurrence of splenic infarction due to arterial thrombosis. BMJ Case Rep. 2021, 14, e243846. [Google Scholar] [CrossRef]
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination—United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensiv. Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Espinosa, W.A.V.; Argueta, A.S.; Tandazo, V.A.H.; Espinosa, C.F.V. A Case Report of a Young Female with Renal Infarction Secondary to Breakthrough COVID Infection. Cureus 2022, 14, 25527. [Google Scholar] [CrossRef]
- Mahboubi-Fooladi, Z.; Arabi, K.P.; Khazaei, M.; Nekooghadam, S.; Shadbakht, B.; Moharamzad, Y.; Taheri, M.S. Parenteral Anticoagulation and Retroperitoneal Hemorrhage in COVID-19: Case Report of Five Patients. SN ComPract. Clin. Med. 2021, 3, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Dubovský, M.; Hajská, M.; Panyko, A.; Vician, M. Severe Retroperitoneal Hemorrhage in a COVID-19 Patient on a Therapeutic Dose of Low Molecular Weight Heparin: A Case Report. Cureus 2022, 14, 26275. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, W.C.; Lee, K.T.; Zainul, N.H.; Alwi, S.B.S.; Low, L.L. Spontaneous retroperitoneal hematoma: A rare bleeding occurrence in COVID-19. Oxf. Med. Case Rep. 2021, 2021, omab081. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Colombo, J.P.; Chandna, S.; Rana, H. Life-Threatening Retroperitoneal Hematoma in a Patient with COVID-19. Case Rep. Hematol. 2021, 2021, e8774010. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Russo, G.M.; Gallo, L.; Atripaldi, U.; Tamburrini, S.; Caliendo, V.; Impieri, L.; Del Canto, M.T.; Ciani, G.; Parrella, P.; et al. Secondary Complications in COVID-19 Patients: A Case Series. Tomography 2022, 8, 1836–1850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).