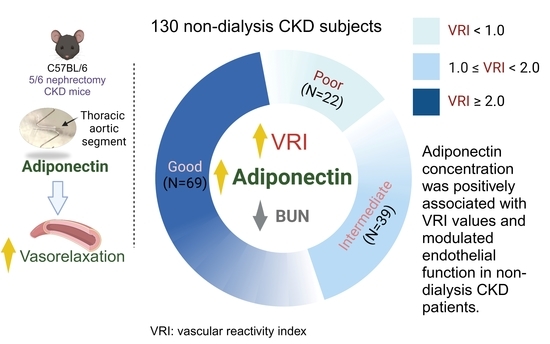
The Association between Serum Adiponectin Levels and Endothelial Function in Non-Dialysis-Dependent Chronic Kidney Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Analysis
2.3. Biochemical Investigations and CKD Stage
2.4. Endothelial Function Measurements
2.5. Animal Model of Chronic Kidney Disease by 5/6 Nephrectomy
2.6. Endothelial Function Measurements Assessment of Vascular Tension Reactivity Using In Vitro Blood-Vessel Myography
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerqueira, A.; Quelhas-Santos, J.; Sampaio, S.; Ferreira, I.; Relvas, M.; Marques, N.; Dias, C.C.; Pestana, M. Endothelial dysfunction is associated with cerebrovascular events in pre-dialysis CKD patients: A prospective study. Life 2021, 11, 128. [Google Scholar] [CrossRef]
- Nowak, K.L.; Jovanovich, A.; Farmer-Bailey, H.; Bispham, N.; Struemph, T.; Malaczewski, M.; Wang, W.; Chonchol, M. Vascular dysfunction, oxidative stress, and inflammation in chronic kidney disease. Kidney360 2020, 1, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Joannides, R.; Bakkali, E.H.; Le Roy, F.; Rivault, O.; Godin, M.; Moore, N.; Fillastre, J.P.; Thuillez, C. Altered flow-dependent vasodilatation of conduit arteries in maintenance haemodialysis. Nephrol. Dial. Transplant. 1997, 12, 2623–2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Guldener, C.; Janssen, M.J.; Lambert, J.; Steyn, M.; Donker, A.J.; Stehouwer, C.D. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol. Dial. Transplant. 1998, 13, 1782–1786. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Perry, H.M.; Okusa, M.D. Endothelial dysfunction in renal interstitial fibrosis. Nephron 2016, 134, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio-Mayoral, A.; Banerjee, D.; Streather, C.; Kaski, J.C. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease—A cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 2011, 216, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Tsai, J.P.; Hsu, B.G. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins 2019, 11, 589. [Google Scholar] [CrossRef] [Green Version]
- Liew, H.; Roberts, M.A.; Pope, A.; McMahon, L.P. Endothelial glycocalyx damage in kidney disease correlates with uraemic toxins and endothelial dysfunction. BMC Nephrol. 2021, 22, 21. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [Green Version]
- Ouedraogo, R.; Gong, Y.; Berzins, B.; Wu, X.; Mahadev, K.; Hough, K.; Chan, L.; Goldstein, B.J.; Scalia, R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Investig. 2007, 117, 1718–1726. [Google Scholar] [CrossRef]
- Tan, K.C.; Xu, A.; Chow, W.S.; Lam, M.C.; Ai, V.H.; Tam, S.C.; Lam, K.S. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J. Clin. Endocrinol. Metab. 2004, 89, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell. Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wang, W.Q.; Zhang, H.; Yang, X.; Fan, Q.; Christopher, T.A.; Lopez, B.L.; Tao, L.; Goldstein, B.J.; Gao, F.; et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1703–E1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Oh, K.H.; Ahn, C.; Kim, S.W.; Bae, E.H. High serum adiponectin as a biomarker of renal dysfunction: Results from the KNOW-CKD study. Sci. Rep. 2020, 10, 5598. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.Y.; Hsu, B.G.; Wu, D.A.; Hou, J.S.; Chen, M.C. Serum fibroblast growth factor 21 levels are positively associated with metabolic syndrome in patients with type 2 diabetes. Int. J. Endocrinol. 2019, 2019, 5163245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Lee, C.J.; Yang, C.F.; Chen, Y.C.; Wang, J.H.; Hsu, B.G. Low serum adiponectin level is associated with metabolic syndrome and is an independent marker of peripheral arterial stiffness in hypertensive patients. Diabetol. Metab. Syndr. 2017, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.; Yen, A.A.; Lin, A.W.; Tanaka, H.; Kleis, S. New indices of endothelial function measured by digital thermal monitoring of vascular reactivity: Data from 6084 patients registry. Int. J. Vasc. Med. 2016, 2016, 1348028. [Google Scholar]
- Lu, C.W.; Lee, C.J.; Hsieh, Y.J.; Hsu, B.G. Empagliflozin attenuates vascular calcification in mice with chronic kidney disease by regulating the NFR2/HO-1 anti-inflammatory pathway through AMPK activation. Int. J. Mol. Sci. 2023, 24, 10016. [Google Scholar] [CrossRef]
- Tseng, T.L.; Chen, M.F.; Hsu, Y.H.; Lee, T.J.F. OroxylinA reverses lipopolysaccharide-induced adhesion molecule expression and endothelial barrier disruption in the rat aorta. Toxicol. Appl. Pharmacol. 2020, 400, 115070. [Google Scholar] [CrossRef]
- Li, H.F.; Liu, H.T.; Chen, P.Y.; Lin, H.; Tseng, T.L. Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3. iScience 2022, 25, 105631. [Google Scholar] [CrossRef]
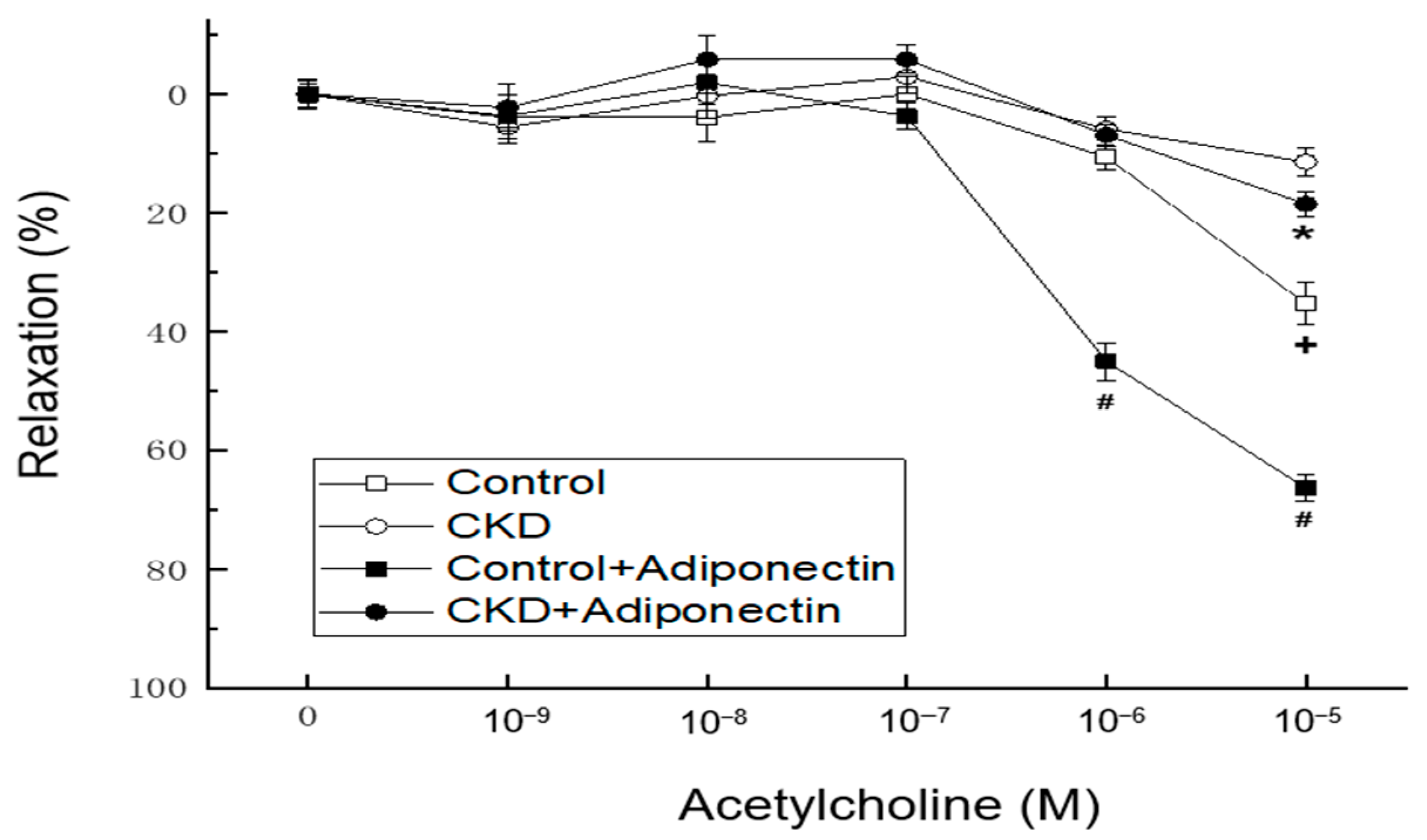
- Du, Y.; Li, R.; Lau, W.B.; Zhao, J.; Lopez, B.; Christopher, T.A.; Ma, X.L.; Wang, Y. Adiponectin at physiologically relevant concentrations enhances the vasorelaxative effect of acetylcholine via Cav-1/AdipoR-1 signaling. PLoS ONE 2016, 11, e0152247. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, E.; Rodríguez-Molina, D.; Bolli, P.; Israili, Z.H.; Faría, J.; Fidilio, E.; Bermúdez, V.; Velasco, M. The role of adiponectin in endothelial dysfunction and hypertension. Curr. Hypertens. Rep. 2014, 16, 463. [Google Scholar] [CrossRef]
- Hirata, Y.; Sugiyama, S.; Yamamoto, E.; Matsuzawa, Y.; Akiyama, E.; Kusaka, H.; Fujisue, K.; Kurokawa, H.; Matsubara, J.; Sugamura, K.; et al. Endothelial function and cardiovascular events in chronic kidney disease. Int. J. Cardiol. 2014, 173, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, U.; Randin, D.; Vollenweider, P.; Vollenweider, L.; Nicod, P. Nitric oxide release accounts for insulin’s vascular effects in humans. J. Clin. Investig. 1994, 94, 2511–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cersosimo, E.; DeFronzo, R.A. Insulin resistance and endothelial dysfunction: The road map to cardiovascular diseases. Diabetes Metab. Res. Rev. 2006, 22, 423–436. [Google Scholar] [CrossRef]
- Ran, J.; Xiong, X.; Liu, W.; Guo, S.; Li, Q.; Zhang, R.; Lao, G. Increased plasma adiponectin closely associates with vascular endothelial dysfunction in type 2 diabetic patients with diabetic nephropathy. Diabetes Res. Clin. Pract. 2010, 88, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Hirayama, A.; Nishio, Y.; Yoshida, Y.; Ohtani, T.; Okamura, T.; Masada, M.; Kikkawa, R.; Kodama, K.; Kashiwagi, A. Coronary endothelial dysfunction in the insulin-resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J. Am. Coll. Cardiol. 2001, 38, 1821–1828. [Google Scholar] [CrossRef] [Green Version]
- Coimbra, S.; Rocha, S.; Valente, M.J.; Catarino, C.; Bronze-da-Rocha, E.; Belo, L.; Santos-Silva, A. New insights into adiponectin and leptin roles in chronic kidney disease. Biomedicines 2022, 10, 2642. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Arakawa, K. Salt-induced hemodynamic regulation mediated by nitric oxide. J. Hypertens. 2011, 29, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Long, Y.; Yu, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobashi, C.; Urakaze, M.; Kishida, M.; Kibayashi, E.; Kobayashi, H.; Kihara, S.; Funahashi, T.; Takata, M.; Temaru, R.; Sato, A.; et al. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ. Res. 2005, 97, 1245–1252. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [PubMed] [Green Version]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Lo, M.M.; Salisbury, S.; Scherer, P.E.; Furth, S.L.; Warady, B.A.; Mitsnefes, M.M. Serum adiponectin complexes and cardiovascular risk in children with chronic kidney disease. Pediatr. Nephrol. 2011, 26, 2009–2017. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, R.; Yamane, K.; Kamei, N.; Nakanishi, S.; Kohno, N. Low serum levels of total and high-molecular-weight adiponectin predict the development of metabolic syndrome in Japanese-Americans. J. Endocrinol. Investig. 2011, 34, 615–619. [Google Scholar]
- Ciccone, M.M.; Bilianou, E.; Balbarini, A.; Gesualdo, M.; Ghiadoni, L.; Metra, M.; Palmiero, P.; Pedrinelli, R.; Salvetti, M.; Scicchitano, P.; et al. Task force on: ‘Early markers of atherosclerosis: Influence of age and sex’. J. Cardiovasc. Med. 2013, 14, 757–766. [Google Scholar] [CrossRef]
- Hellman, T.; Lankinen, R.; Järvisalo, M.J.; Hakamäki, M.; Koivuviita, N.S.; Raitakari, O.T.; Metsärinne, K. Arterial endothelial function, carotid artery intima-media thickness and abdominal aortic calcification in diabetic and nondiabetic CKD stage 4–5 patients not on dialysis. Diabetes Res. Clin. Pract. 2021, 171, 108559. [Google Scholar] [CrossRef]

| Characteristics | All Patients (n = 130) | Good Vascular Reactivity (n = 69) | Intermediate Vascular Reactivity (n = 39) | Poor Vascular Reactivity (n = 22) | p Value |
|---|---|---|---|---|---|
| Age (years) | 65.35 ± 11.94 | 63.65 ± 10.36 | 67.87 ± 13.40 | 66.23 ± 13.42 | 0.197 |
| Height (cm) | 159.69 ± 8.49 | 160.61 ± 8.11 | 158.59 ± 9.45 | 158.75 ± 7.91 | 0.423 |
| Body weight (kg) | 68.70 ± 14.55 | 69.12 ± 14.31 | 70.05 ± 16.31 | 64.99 ± 11.71 | 0.405 |
| Body mass index (kg/m2) | 26.79 ± 4.38 | 26.65 ± 4.39 | 27.65 ± 4.61 | 25.72 ± 3.80 | 0.239 |
| Waist circumference (cm) | 88.44 ± 11.27 | 87.87 ± 11.83 | 90.05 ± 10.91 | 87.36 ± 10.27 | 0.456 |
| Body fat mass (%) | 30.94 ± 7.60 | 30.81 ± 7.69 | 31.41 ± 7.40 | 30.50 ± 7.99 | 0.887 |
| VRI | 2.02 (1.40–2.45) | 2.39 (2.21–2.66) | 1.73 (1.36–1.83) | 0.42 (0.18–0.78) | <0.001 * |
| SBP (mmHg) | 135.92 ± 16.27 | 135.86 ± 14.81 | 135.49 ± 16.28 | 136.91 ± 20.84 | 0.947 |
| DBP (mmHg) | 76.91 ± 11.28 | 78.49 ± 10.08 | 73.87 ± 11.25 | 77.32 ± 14.07 | 0.121 |
| TCH (mg/dL) | 157.14 ± 34.95 | 155.32 ± 33.21 | 156.74 ± 37.47 | 163.55 ± 36.62 | 0.631 |
| TG (mg/dL) | 121.50 (94.00–172.50) | 117.00 (95.00–170.50) | 122.00 (86.00–162.00) | 125.00 (93.25–214.50) | 0.646 |
| LDL-C (mg/dL) | 90.21 ± 31.23 | 90.23 ± 30.49 | 88.38 ± 32.51 | 93.36 ± 32.42 | 0.838 |
| Fasting glucose (mg/dL) | 101.50 (98.00–130.50) | 106.00 (100.00–130.50) | 100.00 (98.00–150.00) | 100.50 (94.75–150.75) | 0.195 |
| BUN (mg/dL) | 27.00 (20.00–41.00) | 24.00 (19.00–38.00) | 30.00 (21.00–51.00) | 30.50 (24.50–48.50) | 0.037 * |
| Creatinine (mg/dL) | 1.60 (1.20–2.4) | 1.50 (1.20–2.35) | 1.60 (1.20–2.40) | 2.20 (1.38–2.70) | 0.129 |
| eGFR (mL/min) | 41.51 ± 21.98 | 45.65 ± 22.99 | 38.56 ± 21.69 | 33.55 ± 16.01 | 0.047 * |
| Uric acid (mg/dL) | 5.97 ± 1.73 | 5.80 ± 1.57 | 6.25 ± 2.04 | 5.97 ± 1.62 | 0.437 |
| Total calcium (mg/dL) | 9.58 ± 1.52 | 9.70 ± 1.51 | 9.21 ± 1.32 | 9.87 ± 1.81 | 0.175 |
| Phosphorus (mg/dL) | 3.86 ± 0.66 | 3.88 ± 0.68 | 3.89 ± 0.59 | 3.73 ± 0.73 | 0.592 |
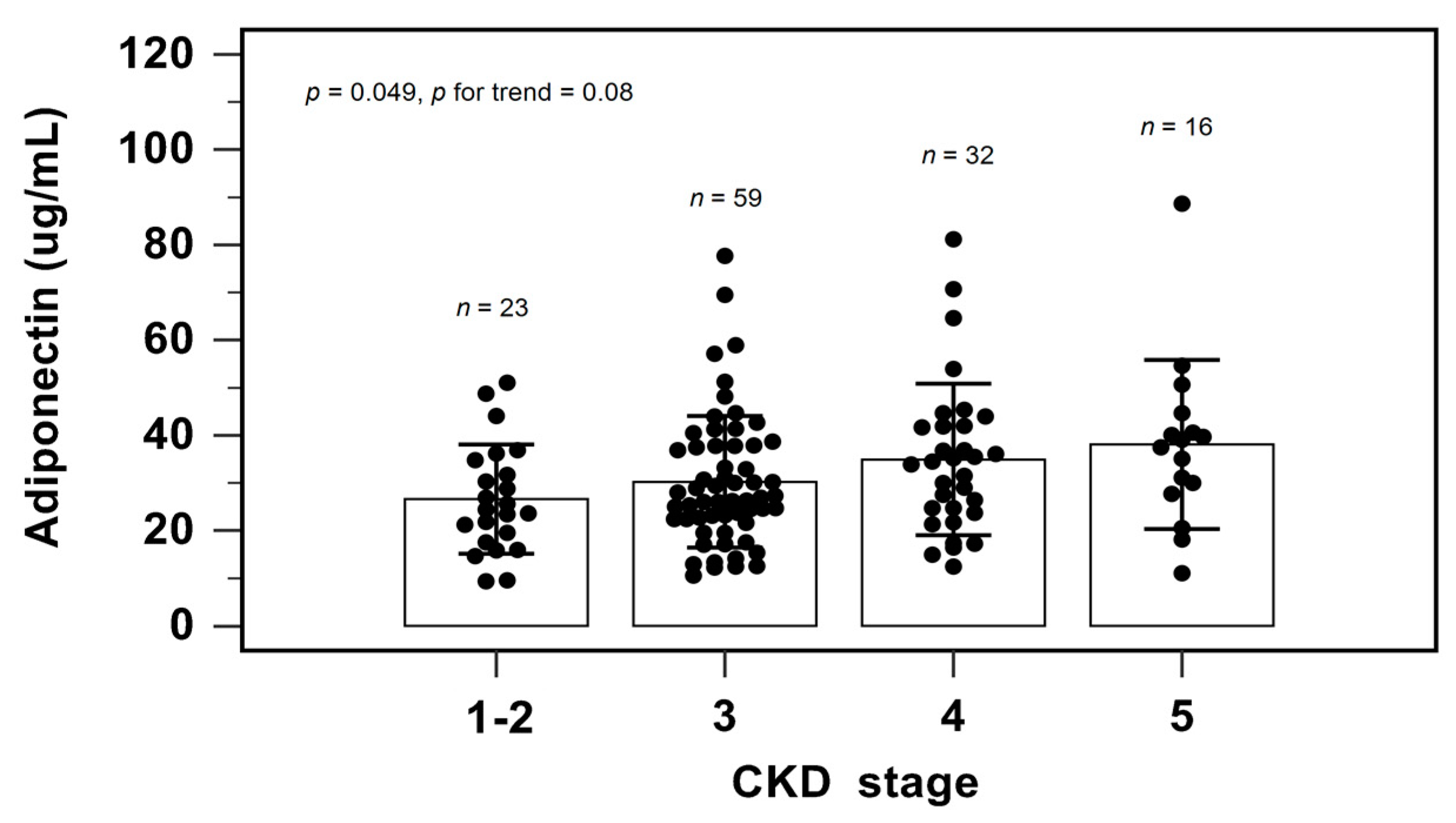
| Adiponectin (μg/mL) | 31.74 ± 14.81 | 37.65 ± 16.12 | 28.73 ± 8.93 | 18.53 ± 6.81 | <0.001 * |
| Female, n (%) | 55 (42.3) | 29 (42.0) | 17 (43.6) | 9 (40.9) | 0.977 |
| DM, n (%) | 65 (50.0) | 38 (55.1) | 18 (46.2) | 9 (40.9) | 0.434 |
| HTN, n (%) | 105 (80.8) | 53 (76.8) | 34 (87.2) | 18 (81.8) | 0.418 |
| CKD stage 1, n (%) | 5 (3.9) | 4 (5.8) | 1 (2.6) | 0 | 0.263 |
| CKD stage 2, n (%) | 18 (13.8) | 13 (18.8) | 4 (10.3) | 1 (4.5) | |
| CKD stage 3, n (%) | 60 (46.2) | 32 (46.4) | 18 (46.2) | 10 (45.5) | |
| CKD stage 4, n (%) | 31 (23.8) | 15 (21.7) | 8 (20.5) | 8 (36.4) | |
| CKD stage 5, n (%) | 16 (12.3) | 5 (7.2) | 8 (20.5) | 3 (13.6) |
| Variables | Vascular Reactivity Index | ||||
|---|---|---|---|---|---|
| Simple Linear Regression | Multivariable Linear Regression | ||||
| r | p Value | Beta | Adjusted R2 Change | p Value | |
| Female | 0.040 | 0.655 | – | – | – |
| DM | 0.066 | 0.454 | – | – | – |
| HTN | −0.031 | 0.723 | – | – | – |
| Age (years) | −0.121 | 0.171 | – | – | – |
| Height (cm) | 0.038 | 0.677 | – | – | – |
| Body weight (kg) | 0.031 | 0.728 | – | – | – |
| Body mass index (kg/m2) | 0.019 | 0.829 | – | – | – |
| Waist circumference (cm) | −0.087 | 0.327 | – | – | – |
| Body fat mass (%) | 0.032 | 0.718 | – | – | – |
| Systolic blood pressure (mmHg) | −0.028 | 0.754 | – | – | – |
| Diastolic blood pressure (mmHg) | 0.092 | 0.300 | – | – | – |
| TCH (mg/dL) | 0.010 | 0.914 | – | – | – |
| Log-TG (mg/dL) | -0.012 | 0.891 | – | – | – |
| LDL-C (mg/dL) | 0.051 | 0.563 | – | – | – |
| Log-Glucose (mg/dL) | 0.056 | 0.527 | – | – | – |
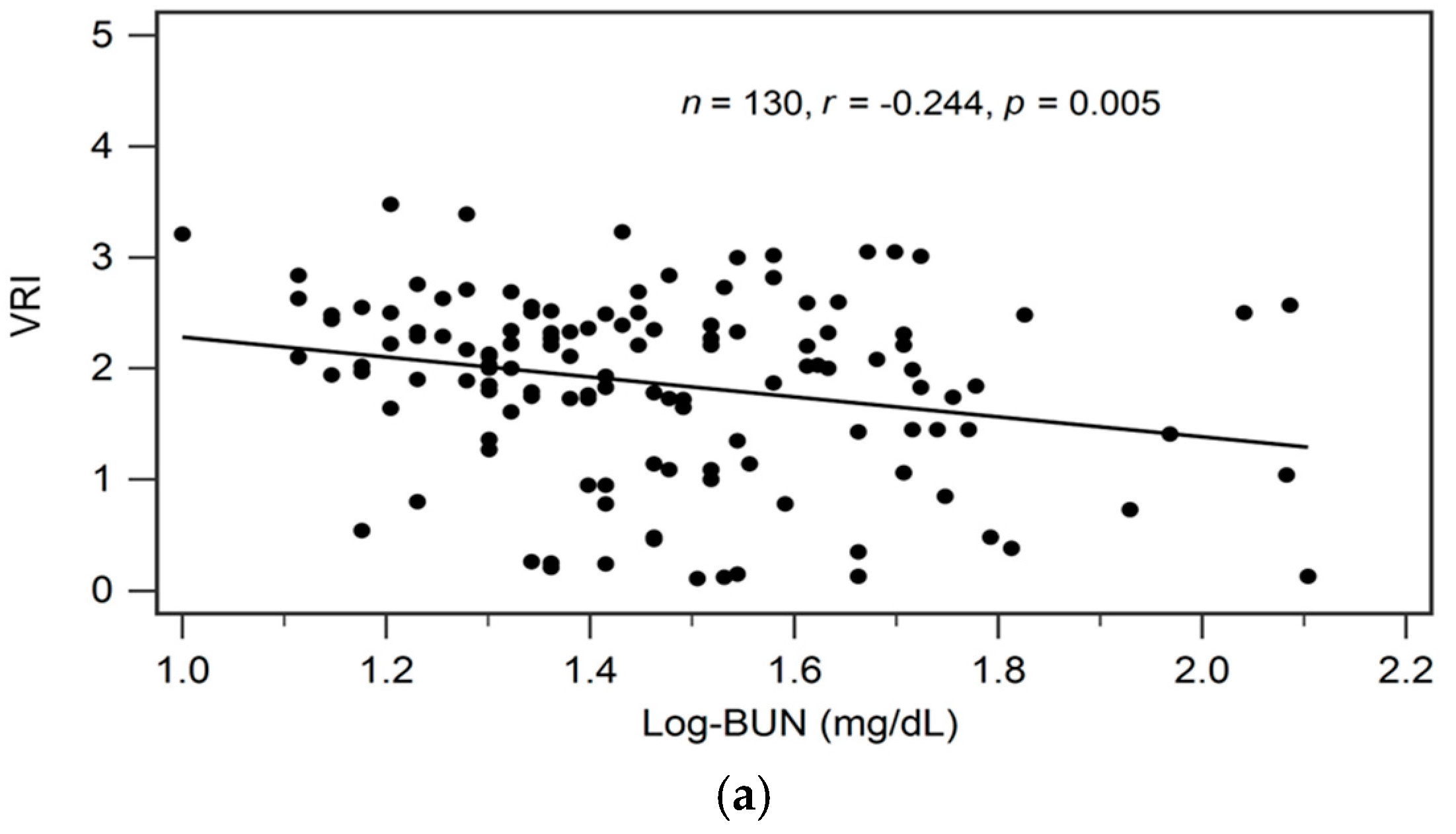
| Log-BUN (mg/dL) | −0.244 | 0.005 * | −0.176 | 0.025 | 0.021 * |
| Log-Creatinine (mg/dL) | −0.200 | 0.023 * | – | – | – |
| eGFR (mL/min) | 0.230 | 0.008 * | – | – | – |
| Uric acid (mg/dL) | −0.034 | 0.699 | – | – | – |
| Total calcium (mg/dL) | 0.043 | 0.631 | – | – | – |
| Phosphorus (mg/dL) | 0.050 | 0.574 | – | – | – |
| Adiponectin (μg/mL) | 0.512 | <0.001 * | 0.487 | 0.256 | <0.001 * |
| Variables | Spearman’s Correlation Coefficient | p Value |
|---|---|---|
| VRI | 0.512 | <0.001 * |
| Age (years) | −0.078 | 0.379 |
| BMI (kg/m2) | −0.197 | 0.025 * |
| Waist circumference (cm) | −0.285 | 0.001 * |
| Body fat mass (%) | −0.185 | 0.035 * |
| TCH (mg/dL) | 0.044 | 0.618 |
| Log-TG (mg/dL) | −0.182 | 0.038 * |
| LDL-C (mg/dL) | 0.005 | 0.959 |
| Log-Glucose (mg/dL) | −0.183 | 0.037 * |
| eGFR (mL/min) | −0.277 | 0.001 * |
| Uric acid (mg/dL) | −0.026 | 0.772 |
| Total calcium (mg/dL) | −0.008 | 0.931 |
| Phosphorus (mg/dL) | 0.084 | 0.343 |
| SBP (mmHg) | 0.053 | 0.548 |
| DBP (mmHg) | 0.132 | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-C.; Lee, C.-J.; Lin, Y.-L.; Wang, C.-H.; Hsu, B.-G. The Association between Serum Adiponectin Levels and Endothelial Function in Non-Dialysis-Dependent Chronic Kidney Disease Patients. Biomedicines 2023, 11, 2174. https://doi.org/10.3390/biomedicines11082174
Chen M-C, Lee C-J, Lin Y-L, Wang C-H, Hsu B-G. The Association between Serum Adiponectin Levels and Endothelial Function in Non-Dialysis-Dependent Chronic Kidney Disease Patients. Biomedicines. 2023; 11(8):2174. https://doi.org/10.3390/biomedicines11082174
Chicago/Turabian StyleChen, Ming-Chun, Chung-Jen Lee, Yu-Li Lin, Chih-Hsien Wang, and Bang-Gee Hsu. 2023. "The Association between Serum Adiponectin Levels and Endothelial Function in Non-Dialysis-Dependent Chronic Kidney Disease Patients" Biomedicines 11, no. 8: 2174. https://doi.org/10.3390/biomedicines11082174
APA StyleChen, M.-C., Lee, C.-J., Lin, Y.-L., Wang, C.-H., & Hsu, B.-G. (2023). The Association between Serum Adiponectin Levels and Endothelial Function in Non-Dialysis-Dependent Chronic Kidney Disease Patients. Biomedicines, 11(8), 2174. https://doi.org/10.3390/biomedicines11082174