Hypoactive Visual Cortex, Prefrontal Cortex and Insula during Self-Face Recognition in Adults with First-Episode Major Depressive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
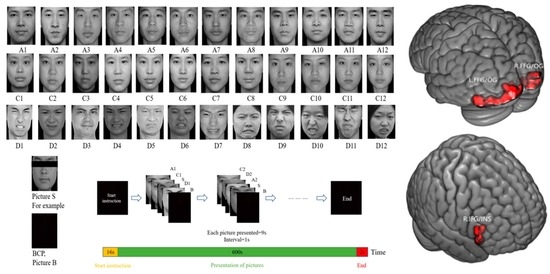
2.3. Experimental Task
2.4. Imaging Acquisition
2.5. Imaging Preprocessing
2.6. Statistical Analysis
2.7. Demographic and Clinical Characteristics
3. Results
3.1. Neuroimaging Results
3.2. Correlations between Abnormal Brain Activation and Clinical Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for united action on depression: A Lancet-World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [Google Scholar] [CrossRef]
- Beck, A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 2008, 165, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, Y.; Fan, H.; Wu, Y.; Liu, L.; Zhang, J.; Li, D.; Tan, Y.; Wang, Z.; Tan, S. Behavioral and electrophysiological analyses of self-referential neural processing in major depressive disorder. Asian J. Psychiatry 2023, 79, 103401. [Google Scholar] [CrossRef]
- Keskin, K.; Eker, M.; Gönül, A.S.; Northoff, G. Abnormal global signal topography of self modulates emotion dysregulation in major depressive disorder. Transl. Psychiatry 2023, 13, 107. [Google Scholar] [CrossRef]
- Devue, C.; Brédart, S. The neural correlates of visual self-recognition. Conscious. Cogn. 2011, 20, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Zahavi, D.; Roepstorff, A. Faces and ascriptions: Mapping measures of the self. Conscious. Cogn. 2011, 20, 141–148. [Google Scholar] [CrossRef]
- Platek, S.M.; Wathne, K.; Tierney, N.G.; Thomson, J.W. Neural correlates of self-face recognition: An effect-location meta-analysis. Brain Res. 2008, 1232, 173–184. [Google Scholar] [CrossRef]
- Ma, Y.; Han, S. Functional dissociation of the left and right fusiform gyrus in self-face recognition. Hum. Brain Mapp. 2012, 33, 2255–2267. [Google Scholar] [CrossRef]
- Chang, L.; Tsao, D.Y. The Code for Facial Identity in the Primate Brain. Cell 2017, 169, 1013–1028.e1014. [Google Scholar] [CrossRef] [Green Version]
- Devue, C.; Collette, F.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Brédart, S. Here I am: The cortical correlates of visual self-recognition. Brain Res. 2007, 1143, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.T.; Aziz-Zadeh, L.; Uddin, L.Q.; Iacoboni, M. The self across the senses: An fMRI study of self-face and self-voice recognition. Soc. Cogn. Affect. Neurosci. 2008, 3, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Di, X.; Eickhoff, S.B.; Zhang, M.; Peng, K.; Guo, H.; Sui, J. Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neurosci. Biobehav. Rev. 2016, 61, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Symons, C.S.; Johnson, B.T. The self-reference effect in memory: A meta-analysis. Psychol. Bull. 1997, 121, 371–394. [Google Scholar] [CrossRef]
- Ferroni, F.; Ardizzi, M.; Sestito, M.; Lucarini, V.; Daniel, B.D.; Paraboschi, F.; Tonna, M.; Marchesi, C.; Gallese, V. Shared multisensory experience affects Others’ boundary: The enfacement illusion in schizophrenia. Schizophr. Res. 2019, 206, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quevedo, K.; Ng, R.; Scott, H.; Martin, J.; Smyda, G.; Keener, M.; Oppenheimer, C.W. The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. J. Abnorm. Psychol. 2016, 125, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, K.; Harms, M.; Sauder, M.; Scott, H.; Mohamed, S.; Thomas, K.M.; Schallmo, M.P.; Smyda, G. The neurobiology of self face recognition among depressed adolescents. J. Affect. Disord. 2018, 229, 22–31. [Google Scholar] [CrossRef]
- Alarcón, G.; Sauder, M.; Teoh, J.Y.; Forbes, E.E.; Quevedo, K. Amygdala Functional Connectivity During Self-Face Processing in Depressed Adolescents with Recent Suicide Attempt. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 221–231. [Google Scholar] [CrossRef]
- Quevedo, K.; Liu, G.; Teoh, J.Y.; Ghosh, S.; Zeffiro, T.; Ahrweiler, N.; Zhang, N.; Wedan, R.; Oh, S.; Guercio, G.; et al. Neurofeedback and neuroplasticity of visual self-processing in depressed and healthy adolescents: A preliminary study. Dev. Cogn. Neurosci. 2019, 40, 100707. [Google Scholar] [CrossRef]
- Thapar, A.; Eyre, O.; Patel, V.; Brent, D. Depression in young people. Lancet 2022, 400, 617–631. [Google Scholar] [CrossRef]
- Rodger, H.; Vizioli, L.; Ouyang, X.; Caldara, R. Mapping the development of facial expression recognition. Dev. Sci. 2015, 18, 926–939. [Google Scholar] [CrossRef]
- Karakale, O.; Moore, M.R.; Kirk, I.J. Mental Simulation of Facial Expressions: Mu Suppression to the Viewing of Dynamic Neutral Face Videos. Front. Hum. Neurosci. 2019, 13, 34. [Google Scholar] [CrossRef]
- Leppänen, J.M.; Hietanen, J.K. Positive facial expressions are recognized faster than negative facial expressions, but why? Psychol. Res. 2004, 69, 22–29. [Google Scholar] [CrossRef]
- Leppänen, J.M.; Milders, M.; Bell, J.S.; Terriere, E.; Hietanen, J.K. Depression biases the recognition of emotionally neutral faces. Psychiatry Res. 2004, 128, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.; Douglas, K.; Porter, R. Processing of facial emotion expression in major depression: A review. Aust. New Zealand J. Psychiatry 2010, 44, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Simpson, J.; Varese, F. A systematic review of the clinical utility of the concept of self-disgust. Clin. Psychol. Psychother. 2019, 26, 110–134. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zhang, L.; Yao, X.; Lin, J.; Meng, X. Associations between self-disgust, depression, and anxiety: A three-level meta-analytic review. Acta Psychol. 2022, 228, 103658. [Google Scholar] [CrossRef] [PubMed]
- Wabnegger, A.; Schlintl, C.; Schienle, A. The association between local brain structure and disgust propensity. Sci. Rep. 2022, 12, 1327. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The brief psychiatric rating scale. Psychol. Rep. 2011, 10, 799–812. [Google Scholar] [CrossRef]
- Overton, P.G.; Markland, F.E.; Taggart, H.S.; Bagshaw, G.L.; Simpson, J. Self-disgust mediates the relationship between dysfunctional cognitions and depressive symptomatology. Emotion 2008, 8, 379–385. [Google Scholar] [CrossRef]
- Xiong, G.; Yang, W.; Fan, Z.; Liu, Z.; Tao, H. The Validity and Reliability of Self-Disgust Scale of Chinese Version among Patients with Major Depressive Disorder. Chin. J. Clin. Psychol. 2022, 30, 1143–1148. [Google Scholar] [CrossRef]
- Zimmerman, M.; Galione, J.N. Screening for bipolar disorder with the Mood Disorders Questionnaire: A review. Harv. Rev. Psychiatry 2011, 19, 219–228. [Google Scholar] [CrossRef]
- Lv, S.; Zhong, S.; Zhang, S.; Lai, S.; Wang, Y.; Zhao, H.; Zhang, Y.; Luo, Y.; Yan, S.; Ran, H.; et al. Correlations between facial emotion processing and biochemical abnormalities in untreated adolescent patients with major depressive disorder: A proton magnetic resonance spectroscopy study. J. Affect. Disord. 2022, 296, 408–417. [Google Scholar] [CrossRef]
- Kruggel, F.; von Cramon, D.Y. Temporal properties of the hemodynamic response in functional MRI. Hum. Brain Mapp. 1999, 8, 259–271. [Google Scholar] [CrossRef]
- Zeki, S.; Romaya, J.P. Neural correlates of hate. PLoS ONE 2008, 3, e3556. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- Bandettini, P.A.; Jesmanowicz, A.; Van Kylen, J.; Birn, R.M.; Hyde, J.S. Functional MRI of brain activation induced by scanner acoustic noise. Magn. Reson. Med. 1998, 39, 410–416. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000, 4, 223–233. [Google Scholar] [CrossRef]
- Sugiura, M. Three faces of self-face recognition: Potential for a multi-dimensional diagnostic tool. Neurosci. Res. 2015, 90, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Sui, J.; Chechlacz, M.; Rotshtein, P.; Humphreys, G.W. Lesion-symptom mapping of self-prioritization in explicit face categorization: Distinguishing hypo- and hyper-self-biases. Cereb. Cortex 2015, 25, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, M.; Watanabe, J.; Maeda, Y.; Matsue, Y.; Fukuda, H.; Kawashima, R. Cortical mechanisms of visual self-recognition. NeuroImage 2005, 24, 143–149. [Google Scholar] [CrossRef]
- Pitcher, D.; Walsh, V.; Duchaine, B. The role of the occipital face area in the cortical face perception network. Exp. Brain Res. 2011, 209, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Palejwala, A.H.; O’Connor, K.P.; Pelargos, P.; Briggs, R.G.; Milton, C.K.; Conner, A.K.; Milligan, T.M.; O’Donoghue, D.L.; Glenn, C.A.; Sughrue, M.E. Anatomy and white matter connections of the lateral occipital cortex. Surg. Radiol. Anat. SRA 2020, 42, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Weiner, K.S.; Zilles, K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 2016, 83, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, N.; Yang, X.R.; Knott, V.; MacQueen, G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2015, 16, 448–471. [Google Scholar] [CrossRef] [PubMed]
- Teffer, K.; Semendeferi, K. Human prefrontal cortex: Evolution, development, and pathology. Prog. Brain Res. 2012, 195, 191–218. [Google Scholar] [CrossRef]
- Van Veluw, S.J.; Chance, S.A. Differentiating between self and others: An ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2014, 8, 24–38. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, N.; Teoh, J.Y.; Egan, C.; Zeffiro, T.A.; Davidson, R.J.; Quevedo, K. Self-compassion and dorsolateral prefrontal cortex activity during sad self-face recognition in depressed adolescents. Psychol. Med. 2022, 52, 864–873. [Google Scholar] [CrossRef]
- Morita, T.; Tanabe, H.C.; Sasaki, A.T.; Shimada, K.; Kakigi, R.; Sadato, N. The anterior insular and anterior cingulate cortices in emotional processing for self-face recognition. Soc. Cogn. Affect. Neurosci. 2014, 9, 570–579. [Google Scholar] [CrossRef] [Green Version]
- Morita, T.; Itakura, S.; Saito, D.N.; Nakashita, S.; Harada, T.; Kochiyama, T.; Sadato, N. The role of the right prefrontal cortex in self-evaluation of the face: A functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008, 20, 342–355. [Google Scholar] [CrossRef]
- Lou, H.C.; Changeux, J.P.; Rosenstand, A. Towards a cognitive neuroscience of self-awareness. Neurosci. Biobehav. Rev. 2017, 83, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Hutcherson, C.A.; Tusche, A. Evidence accumulation, not ‘self-control’, explains dorsolateral prefrontal activation during normative choice. eLife 2022, 11, e65661. [Google Scholar] [CrossRef]
- Wu, F.; Lu, Q.; Kong, Y.; Zhang, Z. A Comprehensive Overview of the Role of Visual Cortex Malfunction in Depressive Disorders: Opportunities and Challenges. Neurosci. Bull. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Z.; Yin, X.; Tang, Y.; Ji, R.; Chen, H.; Guang, Y.; Gong, X.; He, Y.; Zhou, W.; et al. An entorhinal-visual cortical circuit regulates depression-like behaviors. Mol. Psychiatry 2022, 27, 3807–3820. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Saito, D.N.; Ban, M.; Shimada, K.; Okamoto, Y.; Kosaka, H.; Okazawa, H.; Asada, M.; Naito, E. Self-face recognition shares brain regions active during proprioceptive illusion in the right inferior fronto-parietal superior longitudinal fasciculus III network. Neuroscience 2017, 348, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Molnar-Szakacs, I.; Uddin, L.Q. Anterior insula as a gatekeeper of executive control. Neurosci. Biobehav. Rev. 2022, 139, 104736. [Google Scholar] [CrossRef]
- Xie, H.; Guo, Q.; Duan, J.; Jia, X.; Zhou, W.; Sun, H.; Fang, P.; Yang, H. Disrupted Causal Connectivity Anchored on the Right Anterior Insula in Drug-Naive First-Episode Patients with Depressive Disorder. Front. Psychiatry 2022, 13, 858768. [Google Scholar] [CrossRef]
- Zeng, M.; Li, J.; Wang, C.; Deng, C.; Li, R.; Chen, H.; Yang, J. Neural processing of personal, relational, and collective self-worth reflected individual differences of self-esteem. J. Personal. 2022, 90, 133–151. [Google Scholar] [CrossRef]
- Kircher, T.T.; Senior, C.; Phillips, M.L.; Rabe-Hesketh, S.; Benson, P.J.; Bullmore, E.T.; Brammer, M.; Simmons, A.; Bartels, M.; David, A.S. Recognizing one’s own face. Cognition 2001, 78, B1–B15. [Google Scholar] [CrossRef]
- Gan, X.; Zhou, X.; Li, J.; Jiao, G.; Jiang, X.; Biswal, B.; Yao, S.; Klugah-Brown, B.; Becker, B. Common and distinct neurofunctional representations of core and social disgust in the brain: Coordinate-based and network meta-analyses. Neurosci. Biobehav. Rev. 2022, 135, 104553. [Google Scholar] [CrossRef]

| Variables | FEMDD (n = 59) | HC (n = 36) | Analysis | |
|---|---|---|---|---|
| F/χ2 | p Value | |||
| Age (years) | 25.68 ± 9.61 | 28.42 ± 8.55 | 1.97 | 0.16 |
| Self-report sex (male/female) | 19/40 | 14/22 | 0.44 | 0.51 |
| Education (years) | 13.88 ± 2.24 | 16.28 ± 2.87 | 20.68 | <0.01 |
| Illness course (months) | 15.03 ± 19.89 | - | - | - |
| Duration of medication (days) | 2.53 ± 4.75 | - | - | - |
| HAMD score | 22.00 ± 4.53 | 1.02 ± 1.90 | 694.90 | <0.01 |
| HAMA score | 18.03 ± 6.44 | - | - | - |
| BPRS total score | 35.15 ± 7.17 | - | - | - |
| SDS score | 58.66 ± 11.37 | 31.35 ± 8.46 | 155.03 | <0.01 |
| Head movement index * | 0.05 ± 0.03 | 0.06 ± 0.02 | 0.03 | 0.87 |
| Brain Areas | Voxels | Peak MNI Coordinates (x, y, z) | F/t Value | |
|---|---|---|---|---|
| Main effect of the two diagnostic groups | ||||
| Left | Fusiform gyrus/Inferior occipital gyrus | 62 | −42, −69, −18 | 18.33 |
| Left | Superior frontal gyrus/Middle frontal gyrus | 57 | −21, 54, 9 | 18.26 |
| Right | Superior frontal gyrus/Middle frontal gyrus | 53 | 18, 60, 27 | 16.35 |
| Main effect of three conditions | ||||
| Bilateral | Occipital gyrus/Precuneus/Fusiform gyrus | 12,007 | −18, −96, 3 | 218.70 |
| Left | Hippocampus/Putamen | 667 | −21, −27, −9 | 36.92 |
| Right | Middle frontal gyrus/Superior frontal gyrus | 171 | 27, 33, 36 | 19.55 |
| Left | Postcentral gyrus/Precentral gyrus/Frontal gyrus | 2760 | −54, −21, 54 | 42.54 |
| Interaction effect: two groups by three conditions | ||||
| Left | Fusiform gyrus/Inferior occipital gyrus | 41 | −36, −84, −12 | 10.06 |
| Left | Calcarine/ Middle occipital gyrus /Inferior occipital gyrus | 100 | −21, −99, −9 | 12.02 |
| t-test between FEMDD vs. HC in condition 1 | ||||
| Left | Occipital gyrus/Fusiform gyrus | 436 | −42, −75, −18 | −5.14 |
| Right | Occipital gyrus/Fusiform gyrus | 248 | 45, −78, −9 | −4.57 |
| t-test between FEMDD vs. HC in condition 2 | ||||
| Right | Inferior frontal gyrus/Insula | 89 | 45, 21, −9 | −4.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Liu, Z.; Yang, J.; Yang, J.; Sun, F.; Tang, S.; Wu, G.; Guo, S.; Ouyang, X.; Tao, H. Hypoactive Visual Cortex, Prefrontal Cortex and Insula during Self-Face Recognition in Adults with First-Episode Major Depressive Disorder. Biomedicines 2023, 11, 2200. https://doi.org/10.3390/biomedicines11082200
Fan Z, Liu Z, Yang J, Yang J, Sun F, Tang S, Wu G, Guo S, Ouyang X, Tao H. Hypoactive Visual Cortex, Prefrontal Cortex and Insula during Self-Face Recognition in Adults with First-Episode Major Depressive Disorder. Biomedicines. 2023; 11(8):2200. https://doi.org/10.3390/biomedicines11082200
Chicago/Turabian StyleFan, Zebin, Zhening Liu, Jie Yang, Jun Yang, Fuping Sun, Shixiong Tang, Guowei Wu, Shuixia Guo, Xuan Ouyang, and Haojuan Tao. 2023. "Hypoactive Visual Cortex, Prefrontal Cortex and Insula during Self-Face Recognition in Adults with First-Episode Major Depressive Disorder" Biomedicines 11, no. 8: 2200. https://doi.org/10.3390/biomedicines11082200
APA StyleFan, Z., Liu, Z., Yang, J., Yang, J., Sun, F., Tang, S., Wu, G., Guo, S., Ouyang, X., & Tao, H. (2023). Hypoactive Visual Cortex, Prefrontal Cortex and Insula during Self-Face Recognition in Adults with First-Episode Major Depressive Disorder. Biomedicines, 11(8), 2200. https://doi.org/10.3390/biomedicines11082200