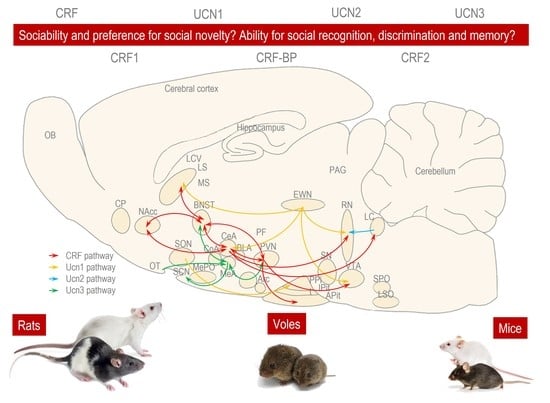
The Role of Corticotropin-Releasing Factor (CRF) and CRF-Related Peptides in the Social Behavior of Rodents
Abstract
1. Introduction
2. Experiments in Rats
3. Experiments in Mice
4. Experiments in Voles
5. Discussion
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
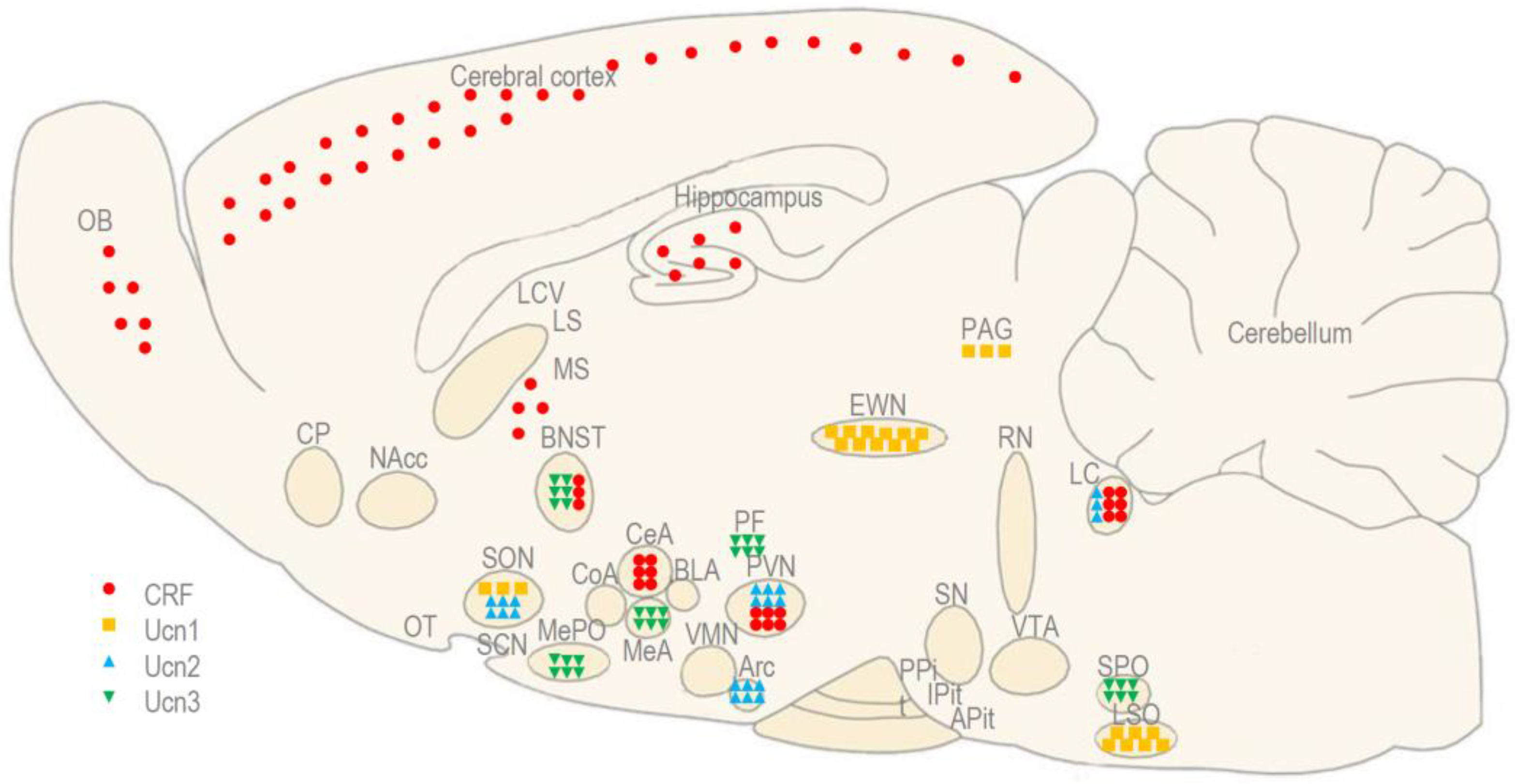
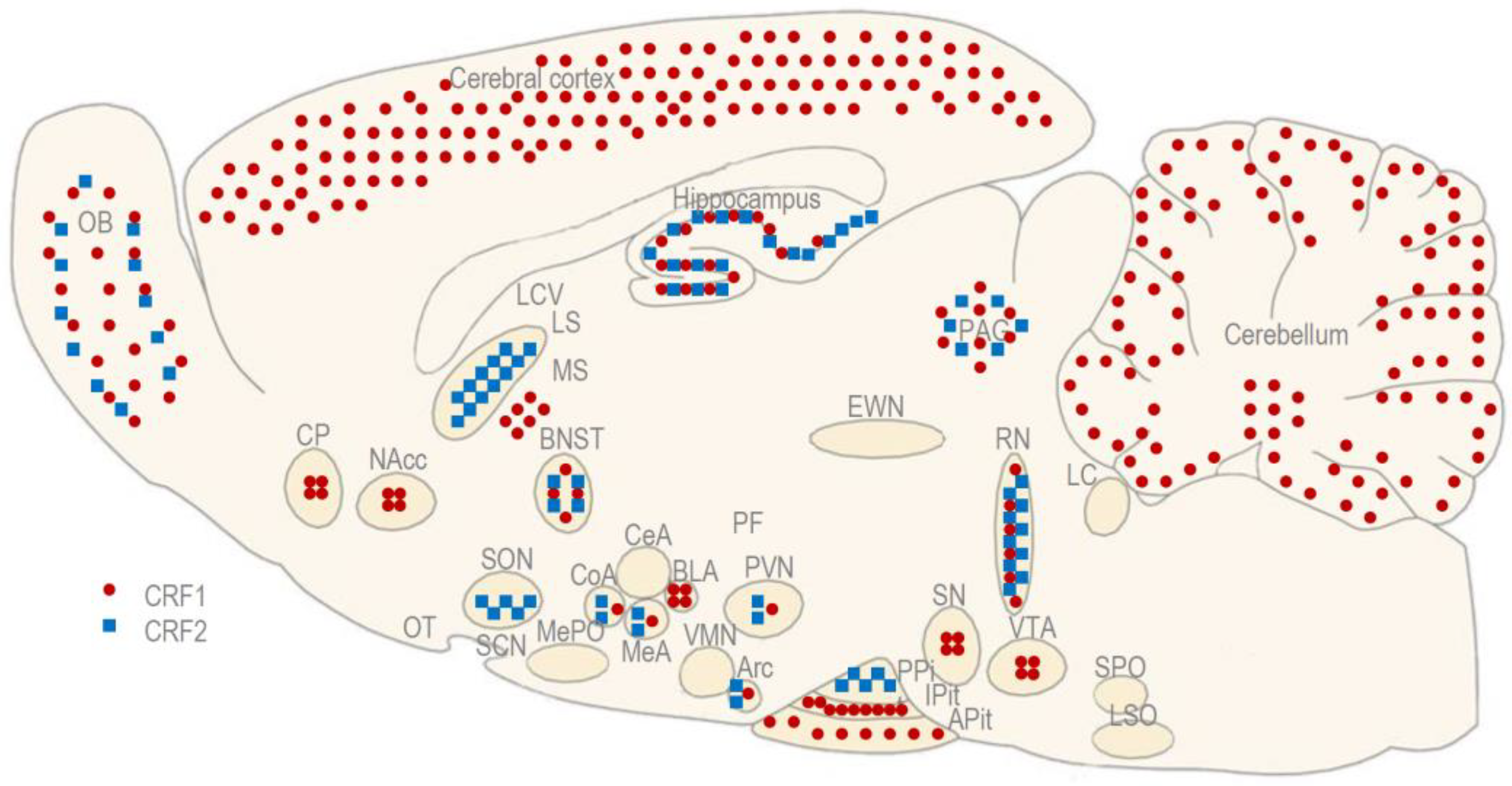
Abbreviations
| Arc | arcuate nucleus of the hypothalamus |
| APit | anterior pituitary |
| BLA | basolateral amygdala |
| BNST | bed nucleus of stria terminalis |
| CeA | central amygdala |
| CFLP | Carworth Farms Lane-Petter |
| cNAcc | core of nucleus accumbens |
| CoA | cortical amygdala |
| CP | caudate-putamen |
| CRF | corticotropin-releasing factor |
| CRF1 | corticotropin-releasing factor receptor type 1 |
| CRF2 | corticotropin-releasing factor receptor type 2 |
| ICV | intracerebroventricular |
| IP | intraperitoneal |
| IV | intravenous |
| EWN | Edinger–Westphal nucleus |
| EWNcp | centrally-projecting Edinger–Westphal nucleus |
| EWNpg | pre-ganglionic Edinger–Westphal nucleus |
| LC | locus coeruleus |
| LCV | lateral cerebral ventricle |
| LS | lateral septum |
| LSO | lateral superior olivary nucleus |
| MeA | medial amygdala |
| MePO | median preoptic area |
| MS | medial septum |
| NAcc | nucleus accumbens |
| OB | olfactory bulb |
| OT | olfactory tubercle |
| PAG | periaqueductal gray matter |
| PF | perifornical area of the hypothalamus |
| PPit | posterior pituitary |
| PVN | paraventricular nucleus of the hypothalamus |
| RN | raphe nuclei |
| SCN | suprachasmatic nucleus |
| SN | substantia nigra |
| SON | supraoptic nucleus of the hypothalamus |
| SPO | superior paraolivary nucleus |
| shNAcc | shell of nucleus accumbens |
| Ucn1 | urocortin 1 |
| Ucn2 | urocortin 2 |
| Ucn3 | urocortin 3 |
| VMN | ventromedial nucleus of the hypothalamus |
| VTA | ventral tegmental area |
References
- Dautzenberg, F.M.; Hauger, R.L. The CRF peptide family and their receptors: Yet more partners discovered. Trends Pharmacol. Sci. 2002, 23, 71–77. [Google Scholar] [CrossRef]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef]
- Vaughan, J.; Donaldson, C.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 1995, 378, 287–292. [Google Scholar] [CrossRef]
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848. [Google Scholar] [CrossRef]
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A.; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575. [Google Scholar] [CrossRef]
- Chang, C.P.; Pearse, R.V., 2nd; O’Connell, S.; Rosenfeld, M.G. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 1993, 11, 1187–1195. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Liaw, C.W.; Grigoriadis, D.E.; Clevenger, W.; Chalmers, D.T.; De Souza, E.B.; Oltersdorf, T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA 1995, 92, 836–840. [Google Scholar] [CrossRef]
- Behan, D.P.; De Souza, E.B.; Lowry, P.J.; Potter, E.; Sawchenko, P.; Vale, W.W. Corticotropin releasing factor (CRF) binding protein: A novel regulator of CRF and related peptides. Front. Neuroendocrinol. 1995, 16, 362–382. [Google Scholar] [CrossRef]
- Reul, J.M.; Holsboer, F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002, 2, 23–33. [Google Scholar] [CrossRef]
- Fekete, E.M.; Zorrilla, E.P. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front. Neuroendocrinol. 2007, 28, 1–27. [Google Scholar] [CrossRef]
- Suda, T.; Kageyama, K.; Sakihara, S.; Nigawara, T. Physiological roles of urocortins, human homologues of fish urotensin I, and their receptors. Peptides 2004, 25, 1689–1701. [Google Scholar] [CrossRef]
- Carrasco, G.A.; Van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Bagosi, Z.; Csabafi, K.; Palotai, M.; Jaszberenyi, M.; Foldesi, I.; Gardi, J.; Szabo, G.; Telegdy, G. The effect of urocortin I on the hypothalamic ACTH secretagogues and its impact on the hypothalamic-pituitary-adrenal axis. Neuropeptides 2014, 48, 15–20. [Google Scholar] [CrossRef]
- Skelton, K.H.; Owens, M.J.; Nemeroff, C.B. The neurobiology of urocortin. Regul. Pept. 2000, 93, 85–92. [Google Scholar] [CrossRef]
- Yamamoto, H.; Maeda, T.; Fujimura, M.; Fujimiya, M. Urocortin-like immunoreactivity in the substantia nigra, ventral tegmental area and Edinger-Westphal nucleus of rat. Neurosci. Lett. 1998, 243, 21–24. [Google Scholar] [CrossRef]
- Ryabinin, A.E.; Tsivkovskaia, N.O.; Ryabinin, S.A. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience 2005, 134, 1317–1323. [Google Scholar] [CrossRef]
- Vasconcelos, L.A.; Donaldson, C.; Sita, L.V.; Casatti, C.A.; Lotfi, C.F.; Wang, L.; Cadinouche, M.Z.; Frigo, L.; Elias, C.F.; Lovejoy, D.A.; et al. Urocortin in the central nervous system of a primate (Cebus apella): Sequencing, immunohistochemical, and hybridization histochemical characterization. J. Comp. Neurol. 2003, 463, 157–175. [Google Scholar] [CrossRef]
- Bittencourt, J.C.; Vaughan, J.; Arias, C.; Rissman, R.A.; Vale, W.W.; Sawchenko, P.E. Urocortin expression in rat brain: Evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J. Comp. Neurol. 1999, 415, 285–312. [Google Scholar] [CrossRef]
- Takahashi, K.; Totsune, K.; Sone, M.; Murakami, O.; Satoh, F.; Arihara, Z.; Sasano, H.; Iino, K.; Mouri, T. Regional distribution of urocortin-like immunoreactivity and expression of urocortin mRNA in the human brain. Peptides 1998, 19, 643–647. [Google Scholar] [CrossRef]
- Morin, S.M.; Ling, N.; Liu, X.J.; Kahl, S.D.; Gehlert, D.R. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience 1999, 92, 281–291. [Google Scholar] [CrossRef]
- Lim, M.M.; Tsivkovskaia, N.O.; Bai, Y.; Young, L.J.; Ryabinin, A.E. Distribution of corticotropin-releasing factor and urocortin 1 in the vole brain. Brain Behav. Evol. 2006, 68, 229–240. [Google Scholar] [CrossRef]
- Kozicz, T.; Yanaihara, H.; Arimura, A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J. Comp. Neurol. 1998, 391, 1–10. [Google Scholar] [CrossRef]
- Weninger, S.C.; Peters, L.L.; Majzoub, J.A. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology 2000, 141, 256–263. [Google Scholar] [CrossRef]
- Kozicz, T. On the role of urocortin 1 in the non-preganglionic Edinger-Westphal nucleus in stress adaptation. Gen. Comp. Endocrinol. 2007, 153, 235–240. [Google Scholar] [CrossRef]
- Giardino, W.J.; Cocking, D.L.; Kaur, S.; Cunningham, C.L.; Ryabinin, A.E. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PLoS ONE 2011, 6, e26997. [Google Scholar] [CrossRef]
- Giardino, W.J.; Rodriguez, E.D.; Smith, M.L.; Ford, M.M.; Galili, D.; Mitchell, S.H.; Chen, A.; Ryabinin, A.E. Control of chronic excessive alcohol drinking by genetic manipulation of the Edinger-Westphal nucleus urocortin-1 neuropeptide system. Transl. Psychiatry 2017, 7, e1021. [Google Scholar] [CrossRef]
- Korosi, A.; Schotanus, S.; Olivier, B.; Roubos, E.W.; Kozicz, T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005, 1046, 172–179. [Google Scholar] [CrossRef]
- Spiga, F.; Lightman, S.L.; Shekhar, A.; Lowry, C.A. Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience 2006, 138, 1265–1276. [Google Scholar] [CrossRef]
- Sinnayah, P.; Blair-West, J.R.; McBurnie, M.I.; McKinley, M.J.; Oldfield, B.J.; Rivier, J.; Vale, W.W.; Walker, L.L.; Weisinger, R.S.; Denton, D.A. The effect of urocortin on ingestive behaviours and brain Fos immunoreactivity in mice. Eur. J. Neurosci. 2003, 18, 373–382. [Google Scholar] [CrossRef]
- Maillot, C.; Wang, L.; Million, M.; Tache, Y. Intraperitoneal corticotropin-releasing factor and urocortin induce Fos expression in brain and spinal autonomic nuclei and long lasting stimulation of colonic motility in rats. Brain Res. 2003, 974, 70–81. [Google Scholar] [CrossRef]
- Benoit, S.C.; Thiele, T.E.; Heinrichs, S.C.; Rushing, P.A.; Blake, K.A.; Steeley, R.J. Comparison of central administration of corticotropin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-Fos expression. Peptides 2000, 21, 345–351. [Google Scholar] [CrossRef]
- Wang, L.; Martinez, V.; Vale, W.; Tache, Y. Fos induction in selective hypothalamic neuroendocrine and medullary nuclei by intravenous injection of urocortin and corticotropin-releasing factor in rats. Brain Res. 2000, 855, 47–57. [Google Scholar] [CrossRef]
- Yamauchi, N.; Otagiri, A.; Nemoto, T.; Sekino, A.; Oono, H.; Kato, I.; Yanaihara, C.; Shibasaki, T. Distribution of urocortin 2 in various tissues of the rat. J. Neuroendocrinol. 2005, 17, 656–663. [Google Scholar] [CrossRef]
- Mano-Otagiri, A.; Shibasaki, T. Distribution of urocortin 2 and urocortin 3 in rat brain. J. Nippon. Med. Sch. = Nippon. Ika Daigaku Zasshi 2004, 71, 358–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valdez, G.R.; Inoue, K.; Koob, G.F.; Rivier, J.; Vale, W.; Zorrilla, E.P. Human urocortin II: Mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002, 943, 142–150. [Google Scholar] [CrossRef]
- Inoue, K.; Valdez, G.R.; Reyes, T.M.; Reinhardt, L.E.; Tabarin, A.; Rivier, J.; Vale, W.W.; Sawchenko, P.E.; Koob, G.F.; Zorrilla, E.P. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J. Pharmacol. Exp. Ther. 2003, 305, 385–393. [Google Scholar] [CrossRef]
- Battagello, D.S.; Diniz, G.B.; Candido, P.L.; da Silva, J.M.; de Oliveira, A.R.; Torres da Silva, K.R.; Lotfi, C.F.P.; de Oliveira, J.A.; Sita, L.V.; Casatti, C.A.; et al. Anatomical Organization of Urocortin 3-Synthesizing Neurons and Immunoreactive Terminals in the Central Nervous System of Non-Human Primates [Sapajus spp.]. Front. Neuroanat. 2017, 11, 57. [Google Scholar] [CrossRef]
- Valdez, G.R.; Zorrilla, E.P.; Rivier, J.; Vale, W.W.; Koob, G.F. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003, 980, 206–212. [Google Scholar] [CrossRef]
- Ushikai, M.; Asakawa, A.; Sakoguchi, T.; Tanaka, C.; Inui, A. Centrally administered urocortin 3 inhibits food intake and gastric emptying in mice. Endocrine 2011, 39, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, D.K.; Randeva, H.S.; Levine, M.A.; Kanellopoulou, K.A.; Hillhouse, E.W. Rat cerebral cortex corticotropin-releasing hormone receptors: Evidence for receptor coupling to multiple G-proteins. J. Neurochem. 2001, 76, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Van Pett, K.; Viau, V.; Bittencourt, J.C.; Chan, R.K.; Li, H.Y.; Arias, C.; Prins, G.S.; Perrin, M.; Vale, W.; Sawchenko, P.E. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000, 428, 191–212. [Google Scholar] [CrossRef]
- Behan, D.P.; Grigoriadis, D.E.; Lovenberg, T.; Chalmers, D.; Heinrichs, S.; Liaw, C.; De Souza, E.B. Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: Implications for the treatment of CNS disorders. Mol. Psychiatry 1996, 1, 265–277. [Google Scholar]
- Xu, J.; Hennebold, J.D.; Stouffer, R.L. Dynamic expression and regulation of the corticotropin-releasing hormone/urocortin-receptor-binding protein system in the primate ovary during the menstrual cycle. J. Clin. Endocrinol. Metab. 2006, 91, 1544–1553. [Google Scholar] [CrossRef]
- Xu, J.; Xu, F.; Hennebold, J.D.; Molskness, T.A.; Stouffer, R.L. Expression and role of the corticotropin-releasing hormone/urocortin-receptor-binding protein system in the primate corpus luteum during the menstrual cycle. Endocrinology 2007, 148, 5385–5395. [Google Scholar] [CrossRef] [PubMed]
- Weissheimer, K.V.; Herod, S.M.; Cameron, J.L.; Bethea, C.L. Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorrhea. Neuroendocrinology 2010, 92, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Potter, E.; Behan, D.P.; Linton, E.A.; Lowry, P.J.; Sawchenko, P.E.; Vale, W.W. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc. Natl. Acad. Sci. USA 1992, 89, 4192–4196. [Google Scholar] [CrossRef] [PubMed]
- Slater, P.G.; Cerda, C.A.; Pereira, L.A.; Andres, M.E.; Gysling, K. CRF binding protein facilitates the presence of CRF type 2alpha receptor on the cell surface. Proc. Natl. Acad. Sci. USA 2016, 113, 4075–4080. [Google Scholar] [CrossRef]
- Behan, D.P.; Khongsaly, O.; Liu, X.J.; Ling, N.; Goland, R.; Nasman, B.; Olsson, T.; De Souza, E.B. Measurement of corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in human plasma by two-site enzyme-linked immunoabsorbant assay. J. Clin. Endocrinol. Metab. 1996, 81, 2579–2586. [Google Scholar] [CrossRef][Green Version]
- Bale, T.L.; Vale, W.W. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L.; Lee, K.F.; Vale, W.W. The role of corticotropin-releasing factor receptors in stress and anxiety. Integr. Comp. Biol. 2002, 42, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Bagosi, Z.; Csabafi, K.; Karasz, G.; Jaszberenyi, M.; Foldesi, I.; Siska, A.; Szabo, G.; Telegdy, G. The effects of the urocortins on the hypothalamic-pituitary-adrenal axis—Similarities and discordancies between rats and mice. Peptides 2019, 112, 1–13. [Google Scholar] [CrossRef]
- Henckens, M.J.; Deussing, J.M.; Chen, A. Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nature reviews. Neuroscience 2016, 17, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.; Kozicz, T. Is it really a matter of simple dualism? Corticotropin-releasing factor receptors in body and mental health. Front. Endocrinol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Hostetler, C.M.; Ryabinin, A.E. The CRF system and social behavior: A review. Front. Neurosci. 2013, 7, 92. [Google Scholar] [CrossRef]
- Backstrom, T.; Winberg, S. Central corticotropin releasing factor and social stress. Front. Neurosci. 2013, 7, 117. [Google Scholar] [CrossRef]
- Wagner, S. Urocortins and their unfolding role in mammalian social behavior. Cell Tissue Res. 2019, 375, 133–142. [Google Scholar] [CrossRef]
- Goodson, J.L.; Kingsbury, M.A. What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm. Behav. 2013, 64, 103–112. [Google Scholar] [CrossRef]
- Goodson, J.L. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 2005, 48, 11–22. [Google Scholar] [CrossRef]
- Dunn, A.J.; File, S.E. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm. Behav. 1987, 21, 193–202. [Google Scholar] [CrossRef]
- Sajdyk, T.J.; Schober, D.A.; Gehlert, D.R.; Shekhar, A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav. Brain Res. 1999, 100, 207–215. [Google Scholar] [CrossRef]
- Sajdyk, T.J.; Gehlert, D.R. Astressin, a corticotropin releasing factor antagonist, reverses the anxiogenic effects of urocortin when administered into the basolateral amygdala. Brain Res. 2000, 877, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, D.R.; Shekhar, A.; Morin, S.M.; Hipskind, P.A.; Zink, C.; Gackenheimer, S.L.; Shaw, J.; Fitz, S.D.; Sajdyk, T.J. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur. J. Pharmacol. 2005, 509, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rainnie, D.G.; Bergeron, R.; Sajdyk, T.J.; Patil, M.; Gehlert, D.R.; Shekhar, A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 3471–3479. [Google Scholar] [CrossRef]
- Lee, Y.; Fitz, S.; Johnson, P.L.; Shekhar, A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology 2008, 33, 2586–2594. [Google Scholar] [CrossRef]
- Shekhar, A.; Johnson, P.L.; Fitz, S.D.; Nakazato, A.; Chaki, S.; Steckler, T.; Schmidt, M. A selective, non-peptide CRF receptor 1 antagonist prevents sodium lactate-induced acute panic-like responses. Int. J. Neuropsychopharmacol. 2011, 14, 355–365. [Google Scholar] [CrossRef]
- Zhao, Y.; Valdez, G.R.; Fekete, E.M.; Rivier, J.E.; Vale, W.W.; Rice, K.C.; Weiss, F.; Zorrilla, E.P. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J. Pharmacol. Exp. Ther. 2007, 323, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C. Modulation of social learning in rats by brain corticotropin-releasing factor. Brain Res. 2003, 994, 107–114. [Google Scholar] [CrossRef]
- File, S.E.; Hyde, J.R. Can social interaction be used to measure anxiety? Br. J. Pharmacol. 1978, 62, 19–24. [Google Scholar] [CrossRef]
- Bagosi, Z.; Karasz, G.; Czebely-Lenart, A.; Csabafi, K.; Jaszberenyi, M.; Telegdy, G. The effects of CRF and urocortins on the sociability of mice. Brain Res. 2017, 1663, 114–122. [Google Scholar] [CrossRef]
- Bagosi, Z.; Czebely-Lenart, A.; Karasz, G.; Csabafi, K.; Jaszberenyi, M.; Telegdy, G. The effects of CRF and urocortins on the preference for social novelty of mice. Behav. Brain Res. 2017, 324, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, Y.; Forkosh, O.; Mahn, M.; Anpilov, S.; Sztainberg, Y.; Manashirov, S.; Shlapobersky, T.; Elliott, E.; Tabouy, L.; Ezra, G.; et al. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat. Neurosci. 2016, 19, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N.; Chen, T.; Puri, A.; Washburn, R.; Sullivan, T.L.; Hill, J.M.; Young, N.B.; Nadler, J.J.; Moy, S.S.; Young, L.J.; et al. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides 2007, 41, 145–163. [Google Scholar] [CrossRef]
- Gammie, S.C.; Bethea, E.D.; Stevenson, S.A. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Gammie, S.C.; Hasen, N.S.; Stevenson, S.A.; Bale, T.L.; D’Anna, K.L. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behav. Brain Res. 2005, 160, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gammie, S.C.; Negron, A.; Newman, S.M.; Rhodes, J.S. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav. Neurosci. 2004, 118, 805–814. [Google Scholar] [CrossRef]
- Gammie, S.C.; Seasholtz, A.F.; Stevenson, S.A. Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience 2008, 157, 502–512. [Google Scholar] [CrossRef]
- Gammie, S.C.; Stevenson, S.A. Intermale aggression in corticotropin-releasing factor receptor 1 deficient mice. Behav. Brain Res. 2006, 171, 63–69. [Google Scholar] [CrossRef]
- Deussing, J.M.; Breu, J.; Kuhne, C.; Kallnik, M.; Bunck, M.; Glasl, L.; Yen, Y.C.; Schmidt, M.V.; Zurmuhlen, R.; Vogl, A.M.; et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 9103–9116. [Google Scholar] [CrossRef]
- Breu, J.; Touma, C.; Holter, S.M.; Knapman, A.; Wurst, W.; Deussing, J.M. Urocortin 2 modulates aspects of social behaviour in mice. Behav. Brain Res. 2012, 233, 331–336. [Google Scholar] [CrossRef]
- Piccin, A.; Contarino, A. Sex-linked roles of the CRF(1) and the CRF(2) receptor in social behavior. J. Neurosci. Res. 2020, 98, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, M.; Wotjak, C.T.; Landgraf, R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol. Behav. 1995, 58, 315–321. [Google Scholar] [CrossRef]
- DeVries, A.C.; Guptaa, T.; Cardillo, S.; Cho, M.; Carter, C.S. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology 2002, 27, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.M.; Liu, Y.; Ryabinin, A.E.; Bai, Y.; Wang, Z.; Young, L.J. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm. Behav. 2007, 51, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L. CRF as the key component of stress response systems. Front. Neuroendocrinol. 2014, 35, 159–160. [Google Scholar] [CrossRef]
- McGraw, L.A.; Young, L.J. The prairie vole: An emerging model organism for understanding the social brain. Trends Neurosci. 2010, 33, 103–109. [Google Scholar] [CrossRef]
- Newman, S.W. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef]
- Kabitzke, P.A.; Simpson, E.H.; Kandel, E.R.; Balsam, P.D. Social behavior in a genetic model of dopamine dysfunction at different neurodevelopmental time points. Genes Brain Behav. 2015, 14, 503–515. [Google Scholar] [CrossRef]
- Plaven-Sigray, P.; Gustavsson, P.; Farde, L.; Borg, J.; Stenkrona, P.; Nyberg, L.; Backman, L.; Cervenka, S. Dopamine D1 receptor availability is related to social behavior: A positron emission tomography study. Neuroimage 2014, 102 Pt 2, 590–595. [Google Scholar] [CrossRef]
- Young, K.A.; Gobrogge, K.L.; Wang, Z. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci. Biobehav. Rev. 2011, 35, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, T.; Yonaga, M.; Furuya, Y.; Hashimoto, T.; Kuroki, J.; Nishizawa, Y. Dopamine D3 agonists disrupt social behavior in rats. Brain Res. 1996, 721, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.T.; Liu, Y.; Aragona, B.J.; Wang, Z. Dopamine and monogamy. Brain Res. 2006, 1126, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Self, D.W. Monogamy: Dopamine ties the knot. Nat. Neurosci. 2006, 9, 7–8. [Google Scholar] [CrossRef]
- Gould, G.G.; Hensler, J.G.; Burke, T.F.; Benno, R.H.; Onaivi, E.S.; Daws, L.C. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J. Neurochem. 2011, 116, 291–303. [Google Scholar] [CrossRef]
- Moskowitz, D.S.; Pinard, G.; Zuroff, D.C.; Annable, L.; Young, S.N. Tryptophan, serotonin and human social behavior. Adv. Exp. Med. Biol. 2003, 527, 215–224. [Google Scholar]
- Miczek, K.A.; Fish, E.W.; De Bold, J.F.; De Almeida, R.M. Social and neural determinants of aggressive behavior: Pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology 2002, 163, 434–458. [Google Scholar] [CrossRef]
- Caldwell, H.K. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist 2017, 23, 517–528. [Google Scholar] [CrossRef]
- Harony, H.; Wagner, S. The contribution of oxytocin and vasopressin to mammalian social behavior: Potential role in autism spectrum disorder. Neurosignals 2010, 18, 82–97. [Google Scholar] [CrossRef]
- Ebstein, R.P.; Israel, S.; Lerer, E.; Uzefovsky, F.; Shalev, I.; Gritsenko, I.; Riebold, M.; Salomon, S.; Yirmiya, N. Arginine vasopressin and oxytocin modulate human social behavior. Ann. N. Y. Acad. Sci. 2009, 1167, 87–102. [Google Scholar] [CrossRef]
- Heinrichs, M.; von Dawans, B.; Domes, G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009, 30, 548–557. [Google Scholar] [CrossRef]
- Lim, M.M.; Nair, H.P.; Young, L.J. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J. Comp. Neurol. 2005, 487, 75–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ni, X.; Nicholson, R.C. Steroid hormone mediated regulation of corticotropin-releasing hormone gene expression. Front. Biosci. A J. Virtual Libr. 2006, 11, 2909–2917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seale, J.V.; Wood, S.A.; Atkinson, H.C.; Bate, E.; Lightman, S.L.; Ingram, C.D.; Jessop, D.S.; Harbuz, M.S. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J. Neuroendocrinol. 2004, 16, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki-Sekino, A.; Mano-Otagiri, A.; Ohata, H.; Yamauchi, N.; Shibasaki, T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 2009, 34, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zorrilla, E.; Smith, S.; Rousso, D.; Levy, C.; Vaughan, J.; Donaldson, C.; Roberts, A.; Lee, K.F.; Vale, W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Choleris, E.; Galea, L.A.M.; Sohrabji, F.; Frick, K.M. Sex differences in the brain: Implications for behavioral and biomedical research. Neurosci. Biobehav. Rev. 2018, 85, 126–145. [Google Scholar] [CrossRef]
- Crawley, J.N. What’s Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; pp. x–vi, 1–523. [Google Scholar]
- Crawley, J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef]
- Rotzinger, S.; Lovejoy, D.A.; Tan, L.A. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 2010, 31, 736–756. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the role of neuropeptides in depression and anxiety. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 114, 110478. [Google Scholar] [CrossRef]
- Okdeh, N.; Mahfouz, G.; Harb, J.; Sabatier, J.M.; Roufayel, R.; Gazo Hanna, E.; Kovacic, H.; Fajloun, Z. Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview. Bioengineering 2023, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Madaan, V.; Wilson, D.R. Neuropeptides: Relevance in treatment of depression and anxiety disorders. Drug News Perspect. 2009, 22, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R. Neuropeptides in anxiety and depression. Amino Acids 2006, 31, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Kupcova, I.; Danisovic, L.; Grgac, I.; Harsanyi, S. Anxiety and Depression: What Do We Know of Neuropeptides? Behav. Sci. 2022, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: From animals to humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C.; Yalcin, I.; Griebel, G.; Surget, A.; Leman, S. Neuropeptides in psychiatric diseases: An overview with a particular focus on depression and anxiety disorders. CNS Neurol. Disord. Drug Targets 2006, 5, 135–145. [Google Scholar] [CrossRef]
- Alldredge, B. Pathogenic involvement of neuropeptides in anxiety and depression. Neuropeptides 2010, 44, 215–224. [Google Scholar] [CrossRef]
- Spierling, S.R.; Zorrilla, E.P. Don’t stress about CRF: Assessing the translational failures of CRF(1)antagonists. Psychopharmacology 2017, 234, 1467–1481. [Google Scholar] [CrossRef]
- Shaham, Y.; de Wit, H. Lost in Translation: CRF1 Receptor Antagonists and Addiction Treatment. Neuropsychopharmacology 2016, 41, 2795–2797. [Google Scholar] [CrossRef]
- File, S.E.; Seth, P. A review of 25 years of the social interaction test. Eur. J. Pharmacol. 2003, 463, 35–53. [Google Scholar] [CrossRef]
- Amaral, I.M.; Lemos, C.; Cera, I.; Dechant, G.; Hofer, A.; El Rawas, R. Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward. Int. J. Mol. Sci. 2020, 22, 345. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.H.; Glasper, E.R.; Neigh, G.N. SSRI or CRF antagonism partially ameliorate depressive-like behavior after adolescent social defeat. Behav. Brain Res. 2014, 270, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Boyson, C.O.; Holly, E.N.; Shimamoto, A.; Albrechet-Souza, L.; Weiner, L.A.; DeBold, J.F.; Miczek, K.A. Social stress and CRF-dopamine interactions in the VTA: Role in long-term escalation of cocaine self-administration. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 6659–6667. [Google Scholar] [CrossRef] [PubMed]
- Boyson, C.O.; Miguel, T.T.; Quadros, I.M.; Debold, J.F.; Miczek, K.A. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology 2011, 218, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.R.; DeBold, J.F.; Miczek, K.A. CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: Prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology 2016, 233, 2727–2736. [Google Scholar] [CrossRef]
- Faria, M.P.; Laverde, C.F.; Nunes-de-Souza, R.L. Anxiogenesis induced by social defeat in male mice: Role of nitric oxide, NMDA, and CRF(1) receptors in the medial prefrontal cortex and BNST. Neuropharmacology 2020, 166, 107973. [Google Scholar] [CrossRef]
- Ferrer-Perez, C.; Reguilon, M.D.; Manzanedo, C.; Aguilar, M.A.; Minarro, J.; Rodriguez-Arias, M. Antagonism of corticotropin-releasing factor CRF(1) receptors blocks the enhanced response to cocaine after social stress. Eur. J. Pharmacol. 2018, 823, 87–95. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, L.; Yuan, W.; Li, L.; Zhang, J.; Hou, W.; Yang, Y.; Zhang, X.; Cai, W.; Ma, H.; et al. Different effects of chronic social defeat on social behavior and the brain CRF system in adult male C57 mice with different susceptibilities. Behav. Brain Res. 2020, 384, 112553. [Google Scholar] [CrossRef]
- Lemos, C.; Salti, A.; Amaral, I.M.; Fontebasso, V.; Singewald, N.; Dechant, G.; Hofer, A.; El Rawas, R. Social interaction reward in rats has anti-stress effects. Addict. Biol. 2021, 26, e12878. [Google Scholar] [CrossRef]
- Leonard, M.Z.; DeBold, J.F.; Miczek, K.A. Escalated cocaine “binges” in rats: Enduring effects of social defeat stress or intra-VTA CRF. Psychopharmacology 2017, 234, 2823–2836. [Google Scholar] [CrossRef]
- Newman, E.L.; Albrechet-Souza, L.; Andrew, P.M.; Auld, J.G.; Burk, K.C.; Hwa, L.S.; Zhang, E.Y.; DeBold, J.F.; Miczek, K.A. Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress: Suppression by CRF-R1 antagonism. Psychopharmacology 2018, 235, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.L.; Leonard, M.Z.; Arena, D.T.; de Almeida, R.M.M.; Miczek, K.A. Social defeat stress and escalation of cocaine and alcohol consumption: Focus on CRF. Neurobiol. Stress 2018, 9, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Diaz, J.; Pomrenze, M.B.; Kan, R.; Pahlavan, B.; Morikawa, H. Cooperative CRF and alpha1 Adrenergic Signaling in the VTA Promotes NMDA Plasticity and Drives Social Stress Enhancement of Cocaine Conditioning. Cell Rep. 2018, 22, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, G. Is the neuropeptide urocortin, a member of the corticotropin-releasing factor family, involved in schizophrenia? Schizophr. Res. 2000, 42, 165–166. [Google Scholar] [CrossRef]
- Brune, M.; Schaub, D.; Juckel, G.; Langdon, R. Social skills and behavioral problems in schizophrenia: The role of mental state attribution, neurocognition and clinical symptomatology. Psychiatry Res. 2011, 190, 9–17. [Google Scholar] [CrossRef]
- de la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef]
- Hirschfeld, R.M.; Montgomery, S.A.; Keller, M.B.; Kasper, S.; Schatzberg, A.F.; Moller, H.J.; Healy, D.; Baldwin, D.; Humble, M.; Versiani, M.; et al. Social functioning in depression: A review. J. Clin. Psychiatry 2000, 61, 268–275. [Google Scholar] [CrossRef]
- Hoaken, P.N.; Stewart, S.H. Drugs of abuse and the elicitation of human aggressive behavior. Addict. Behav. 2003, 28, 1533–1554. [Google Scholar] [CrossRef]
- Young, L.J. Can understanding social preferences in rodents lead to novel pharmacotherapies for social anxiety and avoidance in psychiatric disorders? Neuropsychopharmacology 2011, 36, 2151–2152. [Google Scholar] [CrossRef]

| Animals | Materials | Methods | Results | References |
|---|---|---|---|---|
| Male Lister Hooded rats | CRF | ICV administration followed by social interaction test | CRF decreased the time of active social interaction. | Dunn and File, 1987 [61] |
| Male Wistar rats | CRF and Ucn1 | Single and repeated administration into the BLA followed by social interaction test | CRF and Ucn1 decreased the active social interaction time and induced cardiovascular and behavioral “priming” to the panicogenic effect of IV sodium lactate. | Sajdyk et al., 1999 [62], Sajdyk and Gehlert, 2000 [63], Rainnie et al., 2004 [65], Gehlert et al., 2005 [64], Spiga et al., 2006 [30], Shekhar et al., 2011 [67] |
| Male Wistar rats | Ucn1 | Single and repeated administration into the BNST followed by social interaction test | Ucn1 decreased the time of active social interaction, but did not induce cardiovascular, but only behavioral “priming” to the panicogenic effect of IV sodium lactate. | Lee et al., 2008 [66] |
| Male Wistar rats | Stressin1-A and Ucn3 | ICV administration followed by several tests, including social interaction test | Stressin1-A, but not Ucn3, decreased the time in active social interaction. | Zhao et al., 2007 [68] |
| Female CD rats | CRF1, CRF2, and CRF-BP | ICV administration followed by social recognition test | D-Phe CRF (12–41) and r/h CRF(6–33) induced similar alterations in recognition abilities. | Heinrichs et al., 2003 [69] |
| Animals | Materials | Methods | Results | References |
|---|---|---|---|---|
| Male CFLP mice | CRF, Ucn1, Ucn2, and Ucn3 | ICV administration followed by sociability test | CRF decreased the sociability, whereas Ucn1 increased the sociability. Ucn2 and Ucn3 did not influence the number of entries, but decreased the time of social interaction with stranger males. | Bagosi et al., 2017 [71] |
| Male CFLP mice | CRF, Ucn1, Ucn2, and Ucn3 | ICV administration followed by preference for social novelty test | CRF and Ucn1 similarly decreased the preference for social novelty. Ucn2 and Ucn3 did not influence the number of entries and the time of social interaction with stranger females. | Bagosi et al., 2017 [72] |
| Male and female C57BL/6 mice | Ucn3 | Single administration into the MeA followed by a preference for social novelty test | Ucn3 decreased the time of social interaction with the familiar male, and increased the time of social interaction with stranger males. | Shemesh et al., 2016 [73] |
| Animals | Materials | Methods | Results | References |
|---|---|---|---|---|
| Male and female C57BL/6J mice | Ucn2 | Generation of Ucn2, Ucn3, and CRF2 knock-out mice followed by social interaction test | Male, but not female, Ucn2 knock-out mice exhibited more passive social interactions and less aggression. | Breu et al., 2012 [81] |
| Male and female C57BL/6J mice | Ucn2, Ucn3 and CRF2 | Generation of Ucn2, Ucn3, and CRF2 knock-out mice followed by several tests, including a social recognition and discrimination test | Both male and female Ucn3 and CRF2 knock-out mice, but not Ucn2 knock-out mice, expressed enhanced discrimination abilities. | Deussing et al., 2010 [80] |
| Male and female C57BL/6J mice | Ucn3 and CRF2 | Generation of global Ucn3 and CRF2 knock-out mice, as well as MeA-specific Ucn3 knock-out mice, followed by several tests, including sociability and preference for social novelty tests | Global Ucn3 and CRF2 knock-out mice, as well as MeA-specific Ucn3 knock-out mice displayed decreased preference for social novelty. | Shemesh et al., 2016 [73] |
| Male and female C57BL/6J mice | CRF1 and CRF2 | Generation of CRF2 knock-out mice and the ICV administration of the CRF1 antagonist antalarmin, followed by sociability and preference for social novelty tests | CRF2 deficiency decreased sociability in female, but increased it in male mice. CRF1 antagonism induced sociability in non-social mice, but disrupted it in social mice. | Piccin and Contarino, 2020 [82] |
| Male and female C57BL/6J mice | CRF1, CRF2 and CRF-BP | Generation of CRF1, CRF2, and CRF-BP knockout mice, followed by a series of tests based on the resident–intruder paradigm | CRF1 knock-out mice exhibited significant deficits in nurturing and non-significant deficits in maternal aggression. CRF2 and CRF-BP knock-out mice expressed significant deficits in maternal aggression. Inter-male aggression was unaltered in all knock-out mice. | Gammie and Stevenson, 2006 [79], Gammie et al., 2007 [75], Gammie et al., 2005 [76], Gammie et al., 2008 [78] |
| Animals | Materials | Methods | Results | References |
|---|---|---|---|---|
| Male prairie voles | CRF | ICV administration followed by preference for social novelty test | CRF increased the preference for a partner during the social interaction period in monogamous prairie voles. | DeVries et al., 2002 [84] |
| Male prairie and meadow voles | CRF | Administration into the NAcc and CP followed by preference for social novelty test | CRF increased the preference for partners during the cohabitation period when injected into the NAcc (but not the CP) of monogamous prairie voles (but not non-monogamous meadow voles). | Lim et al., 2007 [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagosi, Z.; Megyesi, K.; Ayman, J.; Rudersdorf, H.; Ayaz, M.K.; Csabafi, K. The Role of Corticotropin-Releasing Factor (CRF) and CRF-Related Peptides in the Social Behavior of Rodents. Biomedicines 2023, 11, 2217. https://doi.org/10.3390/biomedicines11082217
Bagosi Z, Megyesi K, Ayman J, Rudersdorf H, Ayaz MK, Csabafi K. The Role of Corticotropin-Releasing Factor (CRF) and CRF-Related Peptides in the Social Behavior of Rodents. Biomedicines. 2023; 11(8):2217. https://doi.org/10.3390/biomedicines11082217
Chicago/Turabian StyleBagosi, Zsolt, Kíra Megyesi, Jázmin Ayman, Hanna Rudersdorf, Maieda Khan Ayaz, and Krisztina Csabafi. 2023. "The Role of Corticotropin-Releasing Factor (CRF) and CRF-Related Peptides in the Social Behavior of Rodents" Biomedicines 11, no. 8: 2217. https://doi.org/10.3390/biomedicines11082217
APA StyleBagosi, Z., Megyesi, K., Ayman, J., Rudersdorf, H., Ayaz, M. K., & Csabafi, K. (2023). The Role of Corticotropin-Releasing Factor (CRF) and CRF-Related Peptides in the Social Behavior of Rodents. Biomedicines, 11(8), 2217. https://doi.org/10.3390/biomedicines11082217