The Effect of Different Output Powers of Blue Diode Laser along with Curcumin and Riboflavin against Streptococcus mutans around Orthodontic Brackets: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Sample Preparation
2.3. Microbial Suspension
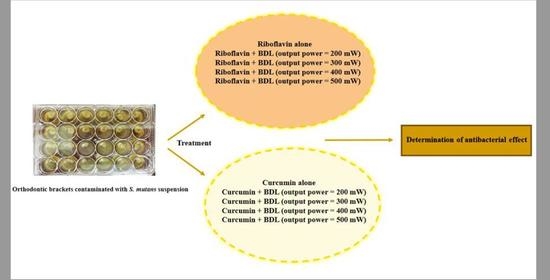
2.4. Experimental Groups
- Control group;
- Riboflavin alone;
- Riboflavin + BDL (output power = 200 mW);
- Riboflavin + BDL (output power = 300 mW);
- Riboflavin + BDL (output power = 400 mW);
- Riboflavin + BDL (output power = 500 mW);
- Curcumin alone;
- Curcumin + BDL (output power = 200 mW);
- Curcumin + BDL (output power = 300 mW);
- Curcumin + BDL (output power = 400 mW);
- Curcumin + BDL (output power = 500 mW);
- 0.2% chlorhexidine mouthwash (CHX; Vi-One, Tabriz, Iran) as positive control.
2.5. Photosensitizers, Light Source, and aPDT
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wishney, M. Potential risks of orthodontic therapy: A critical review and conceptual framework. Aust. Dent. J. 2017, 62, 86–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahirwar, S.S.; Gupta, M.; Snehi, S.K. Dental caries and lactobacillus: Role and ecology in the oral cavity. Int. J. Pharm. Sci. Res. 2019, 11, 4818–4829. [Google Scholar]
- Qin, X.; Zi, H.; Zeng, X. Changes in the global burden of untreated dental caries from 1990 to 2019: A systematic analysis for the Global Burden of Disease study. Heliyon 2022, 8, e10714. [Google Scholar] [CrossRef] [PubMed]
- Abrar, E.; Naseem, M.; Baig, Q.A.; Vohra, F.; Maawadh, A.M.; Almohareb, T.; AlRifaiy, M.Q.; Abduljabbar, T. Antimicrobial efficacy of silver diamine fluoride in comparison to photodynamic therapy and chlorhexidine on canal disinfection and bond strength to radicular dentin. Photodiagn. Photodyn. Ther. 2020, 32, 102066. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic therapy in dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Moro, G.G.; Massat, N.C.; Grandizoli, D.R.P.; Junior, A.E.; Degasperi, G.R.; Fontana, C.E.; Pinheiro, S.L. Effect of cetrimide 2% with and without photodynamic therapy to reduce Streptococcus mutans burden in dentinal carious lesions. Lasers Med. Sci. 2021, 36, 1935–1940. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Kamran, M.A.; Qasim, M.; Udeabor, S.E.; Hameed, M.S.; Mannakandath, M.L.; Alshahrani, I. Impact of riboflavin mediated photodynamic disinfection around fixed orthodontic system infected with oral bacteria. Photodiagn. Photodyn. Ther. 2021, 34, 102232. [Google Scholar] [CrossRef]
- Comeau, P.; Burgess, J.; Qomi, N.R.; Lee, A.; Manso, A. The antimicrobial, physical, and chemical properties of a riboflavin-loaded dental resin intended for antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2022, 40, 103124. [Google Scholar] [CrossRef]
- Fawzy, A.S.; Nitisusanta, L.I.; Iqbal, K.; Daood, U.; Neo, J. Riboflavin as a dentin crosslinking agent: Ultraviolet A versus blue light. Dent. Mater. 2012, 28, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Alam, K.; Rahaman, M.S.; Rahman, M.A.; Awal, R. Synthesis and characterization of metal complexes containing curcumin (C21H20O6) and study of their anti-microbial activities and DNA-binding properties. J. Sci. Res. 2013, 6, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Leite, D.P.V.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef]
- Azizi, A.; Shohrati, P.; Goudarzi, M.; Lawaf, S.; Rahimi, A. Comparison of the effect of photodynamic therapy with curcumin and methylene Blue on streptococcus mutans bacterial colonies. Photodiagn. Photodyn. Ther. 2019, 27, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kang, S.M.; Jeong, S.H.; Chung, K.H.; Kim, B.I. Antibacterial photodynamic therapy with curcumin and Curcuma xanthorrhiza extract against Streptococcus mutans. Photodiagn. Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Tonon, C.C.; Paschoal, M.A.; Correia, M.; Spolidório, D.M.; Bagnato, V.S.; Giusti, J.S.; Santos-Pinto, L. Comparative effects of photodynamic therapy mediated by curcumin on standard and clinical isolate of Streptococcus mutans. J. Contemp. Dent. Pract. 2015, 16, 1–6. [Google Scholar] [PubMed]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 2009, 38, 732–749. [Google Scholar] [CrossRef] [Green Version]
- Antoun, J.S.; Mei, L.; Gibbs, K.; Farella, M. Effect of orthodontic treatment on the periodontal tissues. Periodontol. 2000 2017, 74, 140–157. [Google Scholar] [CrossRef]
- Papaioannou, W.; Panagopoulos, A.; Koletsi-Kounari, H.; Kontou, E.; Makou, M. Adhesion of Porphyromonas gingivalis and biofilm formation on different types of orthodontic brackets. Int. J. Dent. 2012, 2012, 471380. [Google Scholar] [CrossRef] [Green Version]
- Papaioannou, W.; Gizani, S.; Nassika, M.; Kontou, E.; Nakou, M. Adhesion of Streptococcus mutans to Different Types of Brackets. Angle Orthod. 2007, 77, 1090–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balagopal, S.; Arjunkumar, R. Chlorhexidine: The Gold Standard Antiplaque Agent. J. Pharm. Sci. 2013, 5, 270. [Google Scholar]
- Polizzi, E.; TetÃ, G.; Bova, F.; Pantaleo, G.; Gastaldi, G.; CapparÃ, P.; Gherlone, E. Antibacterial properties and side effects of chlorhexidine-based mouthwashes. A prospective, randomized clinical study. J. Osseointegr. 2019, 12, 2–7. [Google Scholar]
- Araújo, N.C.; Fontana, C.R.; Gerbi, M.E.; Bagnato, V.S. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed. Laser Surg. 2012, 30, 96–101. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Tonon, C.C.; Spolidório, D.M.; Bagnato, V.S.; Giusti, J.S.; Santos-Pinto, L. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagn. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Chiniforush, N. Antibacterial potential of riboflavin mediated blue diode laser photodynamic inactivation against Enterococcus faecalis: A laboratory investigation. Photodiagn. Photodyn. Ther. 2023, 41, 103291. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.K.; Garcia, J.; Væth, M.; Schlafer, S. Comparison of riboflavin and toluidine Blue O as photosensitizers for photoactivated disinfection on endodontic and periodontal pathogens in vitro. PLoS ONE 2015, 10, e0140720. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Ghosh, D.; Vishakha, K.; Das, S.; Mondal, S.; Ganguli, A. Photodynamic antimicrobial chemotherapy (PACT) using riboflavin inhibits the mono and dual species biofilm produced by antibiotic resistant Staphylococcus aureus and Escherichia coli. Photodiagn. Photodyn. Ther. 2020, 32, 102002. [Google Scholar] [CrossRef]
- Morelato, L.; Budimir, A.; Smojver, I.; Katalinić, I.; Vuletić, M.; Ajanović, M.; Gabrić, D. A novel technique for disinfection treatment of contaminated dental implant surface using 0.1% riboflavin and 445 nm diode laser-an in vitro study. Bioengineering 2022, 9, 308. [Google Scholar] [CrossRef]
- Etemadi, A.; Hamidain, M.; Parker, S.; Chiniforush, N. Blue light photodynamic therapy with curcumin and riboflavin in the management of periodontitis: A Systematic Review. J. Lasers Med. Sci. 2021, 12, e15. [Google Scholar] [CrossRef]

| Group 1 | Group 2 | p-Value |
|---|---|---|
| Riboflavin | Riboflavin + BDL 200 mW | 0.85 |
| Riboflavin + BDL 300 mW | 0.03 * | |
| Riboflavin + BDL 400 mW | 0.00 * | |
| Riboflavin + BDL 500 mW | 0.00 * | |
| CHX | 0.00 * | |
| Riboflavin + BDL 200 mW | Riboflavin + BDL 300 mW | 0.67 |
| Riboflavin + BDL 400 mW | 0.07 | |
| Riboflavin + BDL 500 mW | 0.00 * | |
| CHX | 0.00 * | |
| Riboflavin + BDL 300 mW | Riboflavin + BDL 400 mW | 0.95 |
| Riboflavin + BDL 500 mW | 0.00 * | |
| CHX | 0.00 * | |
| Riboflavin + BDL 400 mW | Riboflavin + BDL 500 mW | 0.06 |
| CHX | 0.00 * | |
| Riboflavin + BDL 500 mW | CHX | 0.00 * |
| Curcumin | Curcumin + BDL 200 mW | 0.00 * |
| Curcumin + BDL 300 mW | 0.00 * | |
| Curcumin + BDL 400 mW | 0.00 * | |
| Curcumin + BDL 500 mW | 0.00 * | |
| CHX * | 0.00 * | |
| Curcumin + BDL 200 mW | Curcumin + BDL 300 mW | 0.10 |
| Curcumin + BDL 400 mW | 0.01 * | |
| Curcumin + BDL 500 mW | 0.00 * | |
| CHX | 0.00 * | |
| Curcumin + BDL 300 mW | Curcumin + BDL 400 mW | 0.99 |
| Curcumin + BDL 500 mW | 0.87 | |
| CHX | 0.89 | |
| Curcumin + BDL 400 mW | Curcumin + BDL 500 mW | 1.00 |
| CHX | 1.00 | |
| Curcumin + BDL 500 mW | CHX | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pordel, E.; Ghasemi, T.; Afrasiabi, S.; Benedicenti, S.; Signore, A.; Chiniforush, N. The Effect of Different Output Powers of Blue Diode Laser along with Curcumin and Riboflavin against Streptococcus mutans around Orthodontic Brackets: An In Vitro Study. Biomedicines 2023, 11, 2248. https://doi.org/10.3390/biomedicines11082248
Pordel E, Ghasemi T, Afrasiabi S, Benedicenti S, Signore A, Chiniforush N. The Effect of Different Output Powers of Blue Diode Laser along with Curcumin and Riboflavin against Streptococcus mutans around Orthodontic Brackets: An In Vitro Study. Biomedicines. 2023; 11(8):2248. https://doi.org/10.3390/biomedicines11082248
Chicago/Turabian StylePordel, Edris, Trife Ghasemi, Shima Afrasiabi, Stefano Benedicenti, Antonio Signore, and Nasim Chiniforush. 2023. "The Effect of Different Output Powers of Blue Diode Laser along with Curcumin and Riboflavin against Streptococcus mutans around Orthodontic Brackets: An In Vitro Study" Biomedicines 11, no. 8: 2248. https://doi.org/10.3390/biomedicines11082248