Hsp70—A Universal Biomarker for Predicting Therapeutic Failure in Human Female Cancers and a Target for CTC Isolation in Advanced Cancers
Abstract
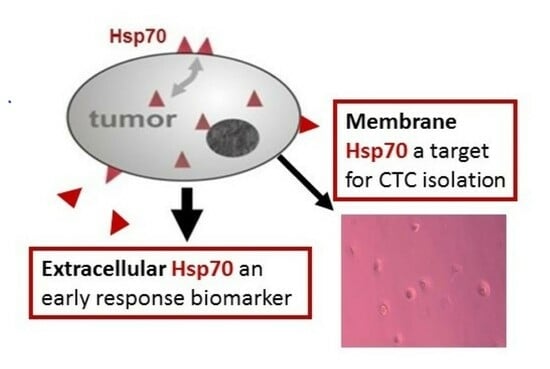
:1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Sample Collection
2.2. Measurement of Circulating Hsp70 Levels Using the compHSP70 ELISA [21]
2.3. Isolation of CTCs with cmHsp70.1 and EpCAM Antibody-Coupled S-PluriSelect Beads
2.4. Statistical Tests
3. Results
3.1. Hsp70 Concentrations in the Blood of Patients with Breast Cancer during Different Therapies and in the Follow-Up Period
3.2. Hsp70 Concentrations in the Blood of Patients with Breast Cancer with and without Recurrence
3.3. Hsp70 as a Target for CTC Isolation in the Peripheral Blood of Patients with Metastatic Tumors
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel, M. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Vardaki, I.; Ozcan, S.S.; Fonseca, P.; Lin, S.H.; Logothetis, C.J.; Yachnin, J.; Ullen, A.; Panaretakis, T. Transcriptomic analysis of plasma exosomes provides molecular information of response to cabazitaxel treatment in men with metastatic castration-resistant prostate cancer. Prostate 2023, 83, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Gang, X.; Wang, G. Liquid biopsy in prostate cancer: Current status and future challenges of clinical application. Aging Male 2021, 24, 58–71. [Google Scholar] [CrossRef]
- Fu, X.; Liu, J.; Yan, X.; Disanto, M.E.; Zhang, X. Heat shock protein 70 and 90 family in prostate cancer. Life 2022, 12, 1489. [Google Scholar] [CrossRef]
- Radons, J. The human hsp70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–579. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V. Heat Shock Proteins and Cytoprotection: Atp-Deprived Mammalian Cells; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Ritossa, F.M. A new puffing pattern induced by temperature shock and dnp in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Richard, V.; Kaeffer, N.; Thuillez, C. Delayed protection of the ischemic heart—From pathophysiology to therapeutic applications. Fundam. Clin. Pharmacol. 1996, 10, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.C.; Kontos, M.C.; Loesser, K.E.; Batra, S.K.; Qian, Y.Z.; Gbur, C.J., Jr.; Naseem, S.A.; Jesse, R.L.; Hess, M.L. Oxidant stress increases heat shock protein 70 mrna in isolated perfused rat heart. Am. J. Physiol. 1994, 267, H2213–H2219. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting membrane heat-shock protein 70 (hsp70) on tumors by cmhsp70.1 antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem.Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef]
- Athanassiadou, P.; Petrakakou, E.; Sakelariou, V.; Zerva, C.; Liossi, A.; Michalas, S.; Athanassiades, P. Expression of p53, bcl-2 and heat shock protein (hsp72) in malignant and benign ovarian tumours. Eur. J Cancer Prev. 1998, 7, 225–231. [Google Scholar] [CrossRef]
- De Maio, A. Heat shock proteins: Facts, thoughts, and dreams. Shock 1999, 11, 1–12. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. Hsp70 multi-functionality in cancer. Cells. 2020, 9, 587. [Google Scholar] [CrossRef]
- Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Tholke, D.; Ostheimer, C.; et al. Hsp70 in liquid biopsies-a tumor-specific biomarker for detection and response monitoring in cancer. Cancers 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.E.; Peltz, F.; Jung, M.P.; et al. Correlation of hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front Immunol. 2015, 6, 556. [Google Scholar] [CrossRef] [PubMed]
- Botzler, C.; Schmidt, J.; Luz, A.; Jennen, L.; Issels, R.; Multhoff, G. Differential hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int. J. Cancer 1998, 77, 942–948. [Google Scholar] [CrossRef]
- Viswanath, B.; Kim, S. Influence of nanotoxicity on human health and environment: The alternative strategies. Rev. Env. Contam. Toxicol. 2017, 242, 61–104. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell. Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- He, P.; Dai, Q.; Wu, X. New insight in urological cancer therapy: From epithelial-mesenchymal transition (emt) to application of nano-biomaterials. Env. Res. 2023, 229, 115672. [Google Scholar] [CrossRef]
- Breuninger, S.; Stangl, S.; Werner, C.; Sievert, W.; Lobinger, D.; Foulds, G.A.; Wagner, S.; Pickhard, A.; Piontek, G.; Kokowski, K.; et al. Membrane hsp70-a novel target for the isolation of circulating tumor cells after epithelial-to-mesenchymal transition. Front Oncol. 2018, 8, 497. [Google Scholar] [CrossRef]
- Romano, G. Modalities to enumerate circulating tumor cells in the bloodstream for cancer prognosis and to monitor the response to the therapy. Drugs Today 2017, 53, 501–514. [Google Scholar] [CrossRef]
- Grover, P.K.; Cummins, A.G.; Price, T.J.; Roberts-Thomson, I.C.; Hardingham, J.E. Circulating tumour cells: The evolving concept and the inadequacy of their enrichment by epcam-based methodology for basic and clinical cancer research. Ann. Oncol. 2014, 25, 1506–1516. [Google Scholar] [CrossRef]
- Dirix, L.; Buys, A.; Oeyen, S.; Peeters, D.; Liegeois, V.; Prove, A.; Rondas, D.; Vervoort, L.; Marien, V.; Laere, S.V.; et al. Circulating tumor cell detection: A prospective comparison between cellsearch(r) and rarecyte(r) platforms in patients with progressive metastatic breast cancer. Breast Cancer Res Treat. 2022, 193, 437–444. [Google Scholar] [CrossRef]
- Konigsberg, R.; Obermayr, E.; Bises, G.; Pfeiler, G.; Gneist, M.; Wrba, F.; De Santis, M.; Zeillinger, R.; Hudec, M.; Dittrich, C. Detection of epcam positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011, 50, 700–710. [Google Scholar] [CrossRef]
- De Wit, S.; Manicone, M.; Rossi, E.; Lampignano, R.; Yang, L.; Zill, B.; Rengel-Puertas, A.; Ouhlen, M.; Crespo, M.; Berghuis, A.M.S.; et al. Epcam(high) and epcam(low) circulating tumor cells in metastatic prostate and breast cancer patients. Oncotarget 2018, 9, 35705–35716. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.R.; Leversha, M.A.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Gu, B.; Anand, A.; Smith, K.; Maslak, P.; Doyle, G.V.; et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007, 13, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Seigneuric, R.; Mjahed, H.; Gobbo, J.; Joly, A.L.; Berthenet, K.; Shirley, S.; Garrido, C. Heat shock proteins as danger signals for cancer detection. Front. Oncol. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, E.H.; Kim, S.H.; Roh, M.S.; Jung, S.B.; Choi, Y.C. Heat shock protein 70 (hsp70) expression is associated with poor prognosis in intestinal type gastric cancer. Virchows Arch. 2013, 463, 489–495. [Google Scholar] [CrossRef]
- Mano, R.; Zilber, S.; Di Natale, R.G.; Kedar, D.; Lifshitz, D.A.; Yossepowitch, O.; Bankiel, J.; Margel, D. Heat shock proteins 60 and 70 are associated with long-term outcome of t1-stage high-grade urothelial tumors of the bladder treated with intravesical bacillus calmette-guerin immunotherapyk. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 531.e9–531.e17. [Google Scholar]
- Tavassol, F.; Starke, O.F.; Kokemuller, H.; Wegener, G.; Muller-Tavassol, C.C.; Gellrich, N.C.; Eckardt, A. Prognostic significance of heat shock protein 70 (hsp70) in patients with oral cancer. Head Neck Oncol. 2011, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Mouawad, N.; Capasso, G.; Ruggeri, E.; Martinello, L.; Severin, F.; Visentin, A.; Facco, M.; Trentin, L.; Frezzato, F. Is it still possible to think about hsp70 as a therapeutic target in onco-hematological diseases? Biomolecules 2023, 13, 604. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jaattela, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef]
- Graf, L.; Barabas, L.; Madaras, B.; Garam, N.; Malati, E.; Horvath, L.; Prohaszka, Z.; Horvath, Z.; Kocsis, J. High serum hsp70 level predicts poor survival in colorectal cancer: Results obtained in an independent validation cohort. Cancer Biomark. 2018, 23, 539–547. [Google Scholar] [CrossRef]
- De Maio, A.; Vazquez, D. Extracellular heat shock proteins: A new location, a new function. Shock 2013, 40, 239–246. [Google Scholar] [CrossRef]
- Lindner, M.; Pogge Von Strandmann, E.P. The role of extracellular hsp70 in the function of tumor-associated immune cells. Cancers 2021, 13, 4721. [Google Scholar] [CrossRef]
- Broquet, A.H.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the molecular chaperone hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of hsp70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef] [PubMed]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring hsp70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles. 2020, 9, 1766192. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, M.; Chanteloup, G.; Isambert, N.; Seigneuric, R.; Fumoleau, P.; Garrido, C.; Gobbo, J. Exosomes in cancer theranostic: Diamonds in the rough. Cell Adh. Migr. 2017, 11, 151–163. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. The circulating tumor cells: Liquid biopsy of cancer. Klin. Lab. Diagn. 2014, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Patell, K.; Kurian, M.; Garcia, J.A.; Mendiratta, P.; Barata, P.C.; Jia, A.Y.; Spratt, D.E.; Brown, J.R. Lutetium-177 PSMA for the treatment of metastatic castarte resistant prostate cancer: A systematic review. Expert Rev. Anticancer Ther. 2023, 7, 731–744. [Google Scholar] [CrossRef]
- Francini, S.; Duraes, M.; Rathat, G.; Macioce, V.; Mollevi, C.; Pages, L.; Ferrer, C.; Cayrefourcq, L.; Alix-Panabieres, C. Circulating Tumor Cell Detection by Liquid Biopsy during Early-Stage Endometrial Cancer Surgery: A Pilot Study. Biomolecules 2023, 13, 428. [Google Scholar] [CrossRef]







| Breast Cancer Patients 8 Years after Diagnosis | (n = 35) | |
|---|---|---|
| Grade | ||
| T1 (a–c) | 30 | |
| T2 | 5 | |
| N0 | 30 | |
| N1 | 5 | |
| M0 | 35 | |
| G1 | 7 | |
| G2 | 26 | |
| G3 | 2 | |
| Therapy | Yes | No |
| Surgery | 35 | 0 |
| Radiotherapy | 33 | 2 |
| 40 Gy | 3 | |
| 60 Gy | 24 | |
| 66 Gy | 6 | |
| Chemotherapy | 7 | 28 |
| Anti-hormone therapy | 12 | 23 |
| Estrogen receptor+ | 35 | 0 |
| Progesterone receptor+ | 35 | 0 |
| Clinical status after 8 years | Recurrence-free | Contralateral recurrence/ Endometrial cancer |
| 33 | 2 |
| Tumor | Number of Patients | Hsp70 Values (ng/mL) |
|---|---|---|
| Metastatic endometrial carcinoma | 1 | 1171 |
| Non-metastatic endometrial carcinoma | 2 | 200.4 |
| Metastatic castration resistant prostate carcinoma (mCRPC) | 16 | 272.8 ± 433.7 |
| N0 | 1 | |
| N1 | 13 | |
| Unknown | 2 | |
| M1a | 2 | |
| M1b | 10 | |
| M1c | 4 | |
| Therapies | ||
| Pretreatments for metastatic prostate cancer | ||
| Next generation hormonal treatment | 16 | |
| Taxane-based chemotherapy | 9 | |
| Best treatment response 3 months after [177Lu]-PSMA- radioligand therapy | ||
| PR | 4 | |
| SD | 3 | |
| PD | 7 | |
| Lost to follow-up | 2 | |
| Tumor | Number of patients | Hsp70 values (ng/mL) |
| Lung carcinoma | 19 | 161.7 ± 253.9 |
| IA | 4 | |
| IB | 2 | |
| IIB | 3 | |
| IIIA | 3 | |
| IVA/B | 4 | |
| Pulmonary metastases | ||
| renal/urothelial | 2 | 127.5 |
| Carcinoid | 1 | 1.23 |
| Head and neck carcinoma | 24 | 196.4 ± 300.8 |
| Oral cavity | 6 | |
| Oropharynx | 8 | |
| Hypopharynx | 3 | |
| Tonsil | 3 | |
| Thyroid | 1 | |
| Uvula | 1 | |
| Tongue | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulos, A.; Samt, A.-K.; Guder, C.; Taylor, N.; Roberts, E.; Herf, H.; Messner, V.; Trill, A.; Holzmann, K.L.K.; Kiechle, M.; et al. Hsp70—A Universal Biomarker for Predicting Therapeutic Failure in Human Female Cancers and a Target for CTC Isolation in Advanced Cancers. Biomedicines 2023, 11, 2276. https://doi.org/10.3390/biomedicines11082276
Xanthopoulos A, Samt A-K, Guder C, Taylor N, Roberts E, Herf H, Messner V, Trill A, Holzmann KLK, Kiechle M, et al. Hsp70—A Universal Biomarker for Predicting Therapeutic Failure in Human Female Cancers and a Target for CTC Isolation in Advanced Cancers. Biomedicines. 2023; 11(8):2276. https://doi.org/10.3390/biomedicines11082276
Chicago/Turabian StyleXanthopoulos, Alexia, Ann-Kathrin Samt, Christiane Guder, Nicholas Taylor, Erika Roberts, Hannah Herf, Verena Messner, Anskar Trill, Katharina Larissa Kreszentia Holzmann, Marion Kiechle, and et al. 2023. "Hsp70—A Universal Biomarker for Predicting Therapeutic Failure in Human Female Cancers and a Target for CTC Isolation in Advanced Cancers" Biomedicines 11, no. 8: 2276. https://doi.org/10.3390/biomedicines11082276
APA StyleXanthopoulos, A., Samt, A.-K., Guder, C., Taylor, N., Roberts, E., Herf, H., Messner, V., Trill, A., Holzmann, K. L. K., Kiechle, M., Seifert-Klauss, V., Zschaeck, S., Schatka, I., Tauber, R., Schmidt, R., Enste, K., Pockley, A. G., Lobinger, D., & Multhoff, G. (2023). Hsp70—A Universal Biomarker for Predicting Therapeutic Failure in Human Female Cancers and a Target for CTC Isolation in Advanced Cancers. Biomedicines, 11(8), 2276. https://doi.org/10.3390/biomedicines11082276