Abstract
Accumulation of 4-hydroxynonenal (4-HNE), a marker of lipid peroxidation, has various favorable and unfavorable effects on cancer cells; however, the clinicopathological significance of its accumulation in hepatocellular carcinoma (HCC) and its metabolic pathway remain unknown. This study analyzed 4-HNE accumulation and its clinicopathological significance in HCC. Of the 221 cases, 160 showed relatively low accumulation of 4-HNE in HCC tissues, which was an independent prognostic predictor. No correlation was found between 4-HNE accumulation and the expression of the antioxidant enzymes glutathione peroxidase 4, ferroptosis suppressor protein 1, and guanosine triphosphate cyclohydrolase 1. Therefore, we hypothesized that 4-HNE metabolism is up-regulated in HCC. A database search was focused on the transcriptional regulation of aldo-keto reductases, alcohol dehydrogenases, and glutathione-S-transferases, which are the metabolic enzymes of 4-HNE, and seven candidate transcription factor genes were selected. Among the candidate genes, the knockdown of SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4) increased 4-HNE accumulation. Immunohistochemical analysis revealed an inverse correlation between 4-HNE accumulation and SMARCA4 expression. These results suggest that SMARCA4 regulates 4-HNE metabolism in HCC. Therefore, targeting SMARCA4 provides a basis for a new therapeutic strategy for HCC via 4-HNE accumulation and increased cytotoxicity.
1. Introduction
Hepatocellular carcinoma (HCC) is a major malignancy, accounting for approximately 90% of primary liver cancer cases, and its carcinogenesis has been reported to be associated with hepatitis C virus (HCV) and hepatitis B virus (HBV) infection, alcohol, cirrhosis, and nonalcoholic steatohepatitis. It is the fourth leading cause of cancer-related death worldwide, and, therefore, new cancer therapies are critical to improve the prognosis of patients with HCC [1].
Metabolic changes in cancer cells or metabolic reprogramming occurs during carcinogenesis and cancer progression [2,3], resulting in various abnormalities in metabolites that accumulate in cancer cells [4]. 4-hydroxynonenal (4-HNE) is a lipid peroxide, a marker reflecting the lipid oxidation state, as well as a metabolite with various effects on cells [5]. For instance, 4-HNE reacts with nucleic acids to induce genetic mutations by inhibiting nucleic acid excision repair [6], inducing DNA damage and chromosomal aberrations [7]. It also forms an adduct with p53, a tumor-suppressor gene, and is involved in cancer malignancy [8]. Although 4-HNE inhibits apoptosis [9], its excessive accumulation induces apoptosis [10,11]. It has also been reported that, when nuclear factor erythroid 2–related factor 2 is activated by 4-HNE, it induces the expression of various genes and triggers changes in intracellular signaling [12]. As mentioned above, HNE is cytotoxic, and its accumulation is involved in carcinogenesis [13]. Abnormal accumulation of 4-HNE has been observed in various cancers and other pathological conditions. A higher accumulation of 4-HNE has been reported in nonalcoholic fatty liver disease cases than in controls and is also reported as a prognostic factor in HCV-mediated HCC, where its accumulation is associated with poor prognosis [14,15]. According to previous reports, 4-HNE accumulation is increased in esophageal squamous cell carcinoma, colon adenocarcinoma, lung cancer, and thyroid cancer [16,17,18,19]. Conversely, other studies reported decreased 4-HNE accumulation in colorectal cancer [20] and in clear-cell renal-cell carcinoma and urothelial carcinoma [21]. Changes in the level of 4-HNE accumulation vary depending on the pathological condition and the cancer type.
The metabolic pathway of 4-HNE can be broadly classified into two categories. First, enzymes of antioxidant genes, such as glutathione peroxidase 4 (GPX4), ferroptosis suppressor protein 1 (FSP1), and guanosine triphosphate cyclohydrolase 1 (GCH1) inhibit lipid peroxidation through independent pathways [22,23,24,25,26], resulting in reduced 4-HNE accumulation. Hence, their expression levels influence cancer development, progression, and resistance to chemotherapy [25,27,28]. In HCC, GPX4 overexpression has been reported to be associated with a poor prognosis [29,30]. In contrast, reduced expression of GPX4 and/or FSP1 is associated with poor prognosis in lung squamous cell carcinoma, and the depletion of both enzymes can induce nonapoptotic cell death [31]. Second, the accumulated 4-HNE is metabolized by metabolic enzymes, such as aldo-keto reductase (AKR), alcohol dehydrogenase (ALDH), and glutathione-S-transferase (GST) [10,32], which have multiple isoforms and are regulated by various transcription factors. Exposure to 4-HNE has also been reported to increase the expression of AKR1C in the human neuroblastoma cell line SH-SY5Y [33] and that of glutathione S-transferase alpha 4 (GSTA4), one of the isoforms of GSTs, in colorectal cancer [17]. In various cancers, including HCC, elucidation of the mechanisms of 4-HNE metabolism is important as it contributes to the development of new therapeutic strategies promoting cell death. Although a previous study reported that GSTA4 is overexpressed in HCC, which indicates poor prognosis [34], the correlation of 4-HNE accumulation with clinicopathological factors in cancers, including HCC, and the factors that regulate 4-HNE remain unclear.
In this study, we used immunohistochemical staining to elucidate the clinicopathological significance of 4-HNE accumulation in HCC, and examined the metabolic pathway of 4-HNE using database analysis and in vitro experiments. Our study findings suggest that SMARCA4 is a key regulator of 4-HNE metabolism and has potential as a novel therapeutic target.
2. Materials and Methods
2.1. Samples
A total of 221 formalin-fixed paraffin-embedded (FFPE) samples of HCC that were resected and diagnosed at the Tokyo Medical and Dental University Hospital between 2008 and 2014 were used in this study. Each FFPE sample comprised of a section containing tumor and non-tumor areas. This study was approved by the ethics committee of Tokyo Medical and Dental University (approval no. M2015-548).
2.2. Immunohistochemistry (IHC)
FFPE tissues were sliced into 4-micrometer sections, which were then placed on silane-coated slides. IHC of 4-HNE, GPX4, FSP1, GCH1, and SMARCA4 was performed using the avidin and biotinylated enzyme (ABC) method (VECTASTAIN ABC kit; Vector Laboratories, Newark, CA, USA) or the polymer method (ImmPRESS Reagent kit; Vector Laboratories), and the staining was visualized using Vector 3,3′-Diaminobenzidine Substrate (Vector Laboratories). The details of the staining conditions are presented in Table S1.
The cytoplasmic expression levels of 4-HNE, GPX4, FSP1, and GST1 were evaluated, compared with those in non-tumor tissues, and scored as follows: 0, weak; 1, comparable; and 2, strong. For SMARCA4, nuclear expression was scored as follows: 1, comparable; 2, partially strong; and 3, strongly positive in whole tumor tissue. All tumor and non-tumor boundaries were identified, and viable tumor cells and non-tumor hepatocyte components of the borderline were compared. The most dominant staining attitude of both areas was used as the result, and the necrotic area was excluded from the evaluation.
2.3. Clinicopathological Analysis
Twelve clinicopathological factors were analyzed as follows: age (<70 vs. ≥70 years), sex (male vs. female), alanine transaminase (≤50 vs. >50 IU/L), cirrhosis (yes vs. no), tumor size (≤5 cm vs. >5 cm), number of lesions (single vs. multiple), vascular invasion (yes vs. no), HCV infection (yes vs. no), HBV infection (yes vs. no), differentiation (well vs. moderate vs. poor), stage (stage I, II vs. III, IV), and Ki-67 index (<30% vs. ≥30%).
For univariate and multivariate analyses, 4-HNE accumulation and the expression of GPX4, FSP1, and GCH1 were analyzed.
2.4. Database Analysis
For the estimation of the metabolic cascade of 4-HNE, the following online databases were used: Oncomine (https://www.oncomine.org/ (accessed on 15 November 2020)), The Human Protein Atlas (https://www.proteinatlas.org/ (accessed on 31 September 2021) [35]), and Chromatin Immunoprecipitation (ChIP)-Atlas (http://chip-atlas.org/ (accessed on 31 September 2021) [36]). The search criteria for Oncomine were mRNA levels in cancer tissue that were higher than normal (p < 0.05), while those for the ChIP-Atlas were fold enrichment ≥ 1, log p-value < −1.3, threshold for significance = 50, and distance range from transcription start site (TSS) of −50 ≤ TSS ≤ +50.
2.5. Cell Line and Culture
The HCC cell line HepG2 was purchased from the Japanese Collection of Research Bioresources Cell Bank and incubated in Dulbecco’s Modified Eagle’s Medium (Wako Chemicals, Richmond, VA, USA) with 10% fetal bovine serum (Gibco, Waltham, MA, USA) and 1% penicillin and streptomycin (Gibco). The cells were passaged at a ratio of 1:5 every 2–3 days.
2.6. Small Interfering RNA (siRNA) Transfection
siRNAs targeting FOXP1, MCRS1, PROX1, RUVBL1, SMARCA4, SP1, and ZMYM3 were obtained from Thermo Fisher Scientific (Waltham, MA, USA; Table S2). Non-target sequence siRNA (Thermo Fisher Scientific, 4390843) was used as a control. Using 1 × 105 cells per sample, the cells were transfected via Lipofectamine 3000 (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions, and Opti-modified Eagle’s Medium (Gibco) was used. After 72 h, the cells were collected for enzyme-linked immunosorbent assay (ELISA) analysis.
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction
RNA was extracted from siRNA-transfected cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and was reverse transcribed to complementary DNA (cDNA) using TaqMan reverse transcription reagents (Applied Biosystems, Woburn, MA, USA). Messenger RNA (mRNA) expression was quantified on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) using the THUNDERBIRD SYBR qPCR Mix (Toyobo, Tokyo, Japan). In this study, β-actin, FOXP1, MCRS1, PROX1, RUVBL1, SMARCA4, SP1, and ZMYM3 mRNA levels were measured, with β-actin as a normalization control. The primer sequences are shown in Table S3.
2.8. ELISA
For sample preparation, 2 × 10⁵ siRNA-transfected cells were suspended in 300 µL radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 0.1% w/v sodium dodecyl sulfate, 1.0% w/v NP40, and 0.5% w/v deoxycholic acid) and allowed to rest on ice for 30 min, after which they were sonicated and centrifuged at 9000× g for 15 min. The supernatant was collected, and 4-HNE concentration was quantified using the Lipid Peroxidation (4-HNE) Assay Kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. This experiment was repeated two times.
2.9. Western Blotting
The efficiency of SMARCA4 knockdown by siRNA in HepG2 cells was confirmed using Western blotting. Methods previously described [37] were applied to HepG2 in this study. The primary antibodies used in this study were SMARCA4/BRG1 (21634-1-AP) (Proteintech, Chicago, IL, USA) and b-Actin (13E5) (Cell Signaling Technologies, Danvers, MA, USA) at the dilution of 1:1000. Anti-Rabbit IgG, HRP-Linked Whole Ab Donkey (Cytiva, Tokyo, Japan), was used as a secondary antibody at the dilution rate 1:5000.
2.10. Statistical Analysis
Correlations between two groups were determined using Fisher’s exact test. Overall survival duration was calculated from the date of diagnosis to the date of death or last follow-up. Kaplan–Meier survival curves were used to estimate overall survival rates, and log-rank tests were used to assess differences in survival between groups. Univariate and multivariate analyses were performed using the log-rank test and the Cox proportional-hazard regression model, respectively. One-way analysis of variance with Dunnett’s test was used for ELISA analysis. All statistical analyses were performed using EZR software (version 4.0.3; Saitama Medical Center, Jichi Medical University, Saitama, Japan). The error bars represent the standard deviation in this study.
3. Results
3.1. Immunohistochemical Analysis of 4-HNE and Antioxidant Enzymes
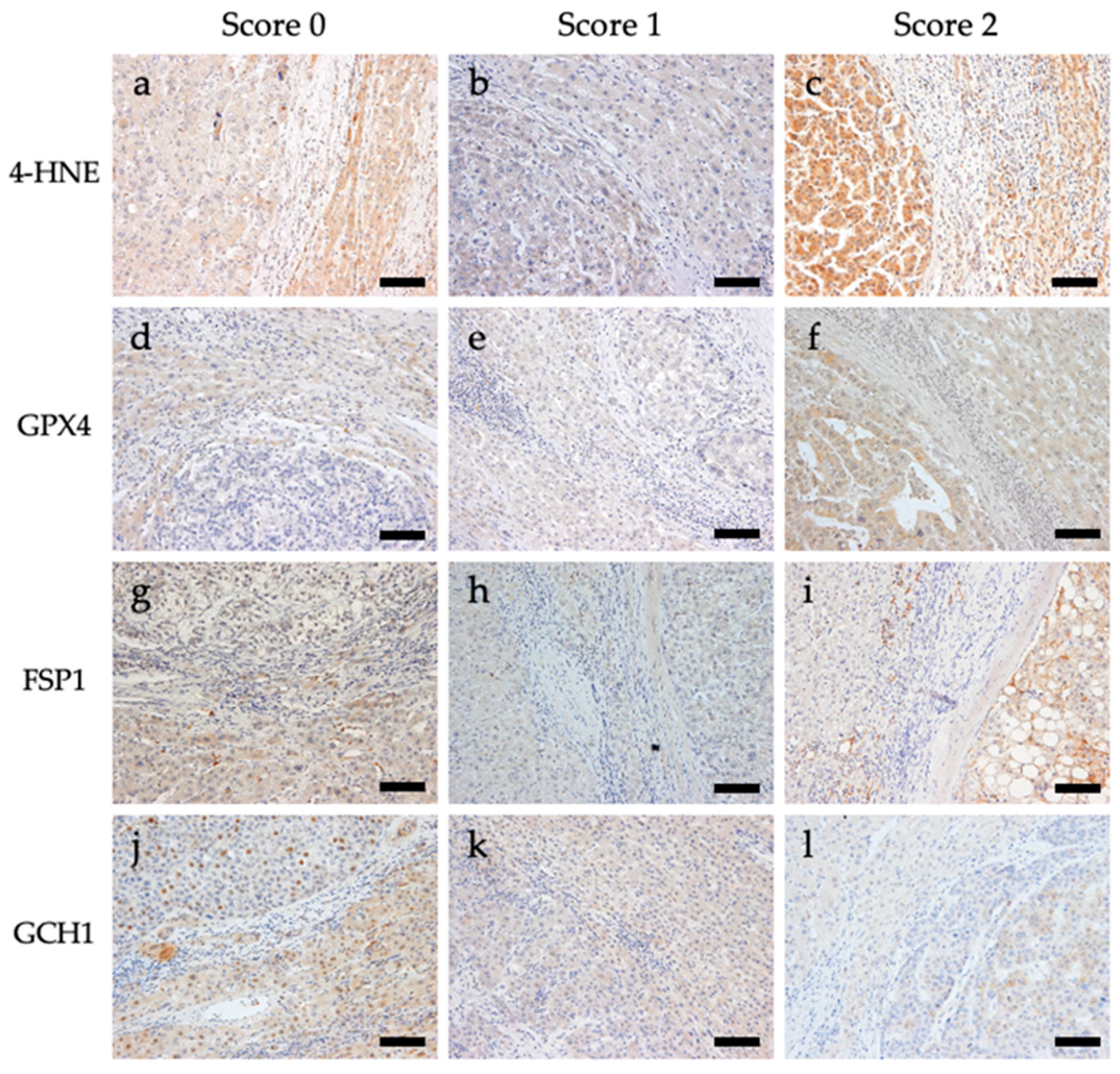
Immunostaining for 4-HNE and the antioxidant enzymes GPX4, FSP1, and GCH1 was performed. Representative images of the immunostaining are shown in Figure 1. For 4-HNE, the numbers of cases with scores of 0 (staining intensity of tumor area was weaker than that of the non-tumoral area), 1 (staining intensity of tumor and non-tumoral area were comparable), and 2 (staining intensity of tumor area was stronger than that of the non-tumoral area) were 160 (72.4%), 34 (15.4%), and 27 (12.2%) out of 221 cases, respectively. For GPX4, the numbers of cases with scores of 0, 1, and 2 were 34 (15.4%), 110 (50.0%), and 77 (34.8%), respectively. For FSP1, the numbers of cases with scores of 0, 1, and 2 were 94 (42.5%), 92 (41.6%), and 35 (15.8%), respectively. For GCH1, the numbers of cases with scores of 0, 1, and 2 were 81 (36.7%), 65 (29.4%), and 75 (33.9%), respectively (Table S4).
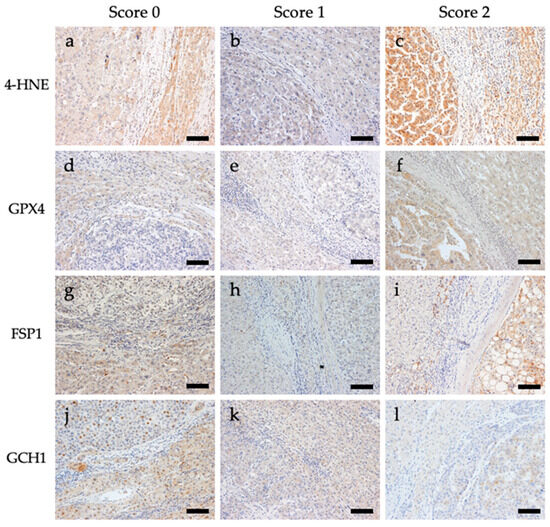
Figure 1.
Representative images of immunohistochemical staining of 4-hydroxynonenal (4-HNE), glutathione peroxidase 4 (GPX4), ferroptosis suppressor protein 1 (FSP1), and guanosine triphosphate cyclohydrolase 1 (GCH1) in tissues from patients with hepatocellular carcinoma. All images are shown at a magnification of 200×, and the scale bars indicate 50 µm. (a) 4-HNE score 0, (b) 4-HNE score 1, (c) 4-HNE score 2, (d) GPX4 score 0, (e) GPX4 score 1, (f) GPX4 score 2, (g) FSP1 score 0, (h) FSP1 score 1, (i) FSP1 score 2, (j) GCH1 score 0, (k) GCH1 score 1, and (l) GCH1 score 2.
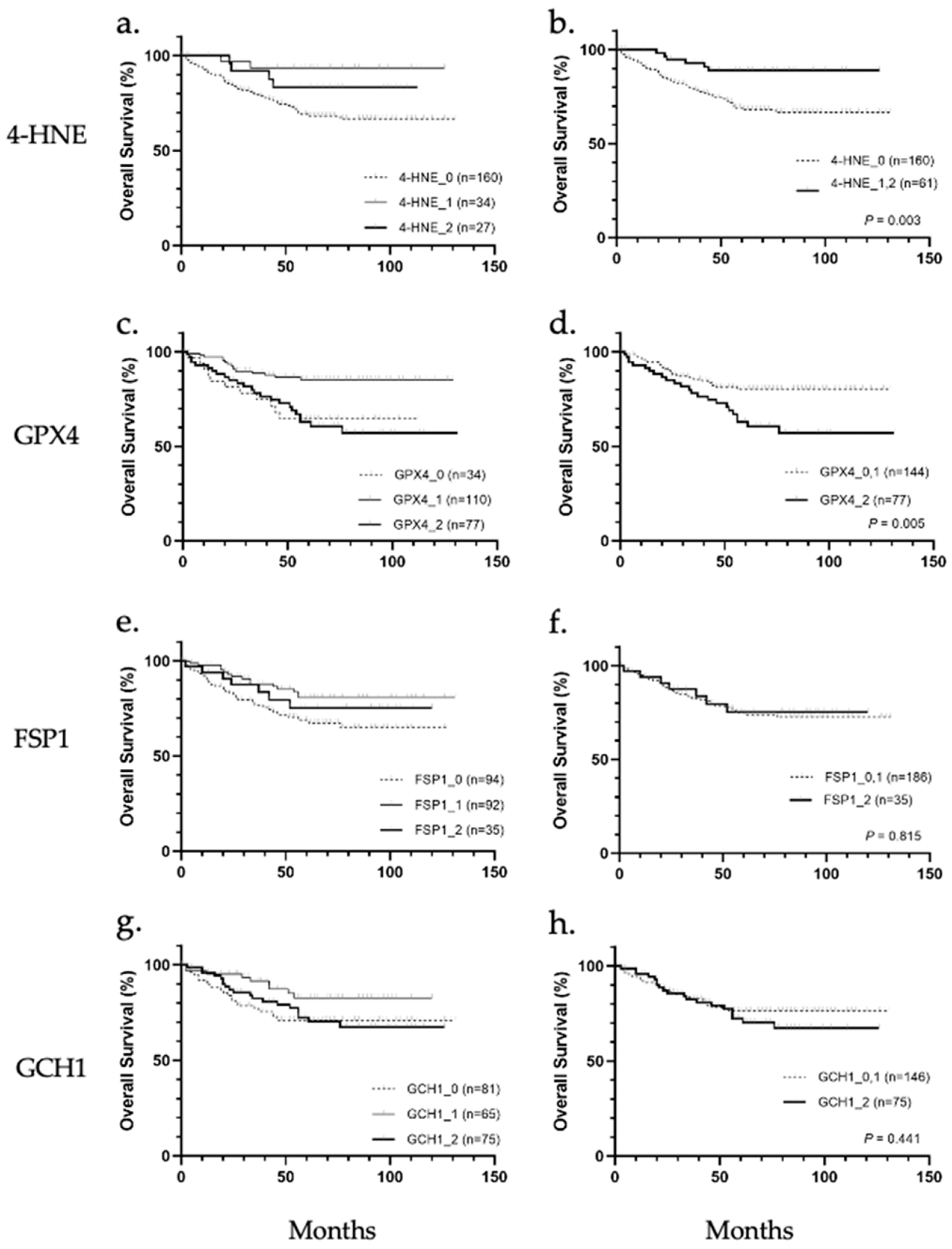
3.2. Survival and Clinicopathological Analyses with 4-HNE Accumulation and Antioxidant Enzymes
Patients were stratified according to 4-HNE accumulation and the expression levels of GPX4, FSP1, and GCH1, and the correlation between the accumulation or expression levels and overall survival was analyzed (Figure 2a–h). Results showed that 4-HNE accumulation and GPX4 protein expression were correlated with overall survival. In the comparison among the three groups (scores 0, 1, and 2), the prognosis tended to be worse in cases of cancer tissues with less 4-HNE accumulation than in noncancerous tissues (Figure 2a). In contrast, a worse prognosis was observed in cases with higher or less GPX4 expression in cancer tissues than in noncancerous tissues (Figure 2c). The prognosis based on 4-HNE was significantly worse in the score 0 group (p = 0.034; Figure 2b), while that based on GPX4 was worse in the score 2 group (p = 0.005, Figure 2d). There were no significant differences in the prognosis classified according to the expression of FSP1 or GCH1 (Figure 2e–h).
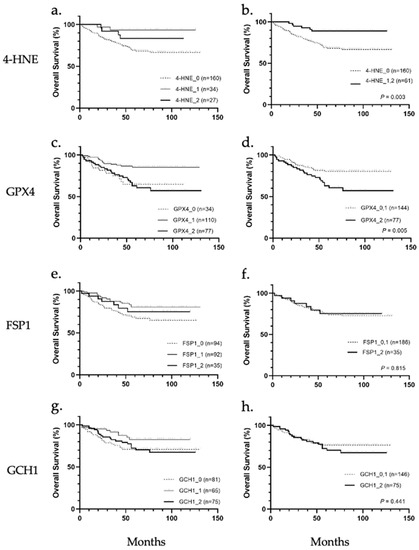
Figure 2.
Overall survival analysis of hepatocellular carcinoma stratified according to immunohistochemical staining of 4-hydroxynonenal (4-HNE), glutathione peroxidase 4 (GPX4), ferroptosis suppressor protein 1 (FSP1), and guanosine triphosphate cyclohydrolase 1 (GCH1). (a) Classification into three groups based on 4-HNE staining intensity showed no significant association with overall survival. (b) 4-HNE score 0 group showed significantly worse prognosis than 4-HNE score 1 and score 2 groups (p = 0.003). (c) Classification into three groups based on GPX4 staining intensity showed no significant association with overall survival. (d) GPX4 score 2 group showed significantly worse prognosis than the GPX4 score 0 and score 1 group (p = 0.005). (e) Classification into three groups based on FSP1 staining intensity. (f) None of the groups (FSP1 score 0, 1, and 2) were significantly associated with overall survival. (g) Classification into three groups based on GCH1 staining intensity. (h) None of the groups (GCH1 score 0, 1, and 2) were significantly associated with overall survival.
Next, we analyzed the correlations of 4-HNE accumulation or the expression levels of GPX4, FSP1, and GCH1 with clinicopathological factors in HCC and found that 4-HNE accumulation significantly correlated with the number of lesions (p = 0.049), vascular invasion (p = 0.002), differentiation (p = 0.002), stage (p = 0.004), and Ki-67 score (p = 0.001), whereas GPX4 expression levels significantly correlated with the presence of cirrhosis (p = 0.004) and HCV infection (p = 0.016). Meanwhile, FSP1 and GCH1 expression did not show a marked correlation with clinicopathological factors (Tables S5–S9). In addition, univariate analysis of clinicopathological factors, 4-HNE accumulation, and GPX4, FSP1, and GCH1 expression, was performed, and 4-HNE accumulation (p = 0.003), GPX4 expression (p = 0.005), tumor size (p = 0.001), number of lesions (p = 0.006), and vascular invasion (p = 0.004) were significantly associated with prognosis (Table 1). Multivariate analysis of these factors revealed that tumor size (hazard ratio (HR): 2.386, 95% confidence interval (CI): 1.328–4.286, p = 0.004), 4-HNE score 0 (HR: 2.726, 95% CI: 1.127–6.596, p = 0.026), and relatively high GPX4 expression (HR: 2.418, 95% CI: 1.379–4.242, p = 0.002) were independent predictive factors for poor prognosis. Decreased 4-HNE accumulation may be advantageous for tumor cells to survive; therefore, we conducted a detailed analysis of the factors that contribute to the decrease in 4-HNE.

Table 1.
Univariate and multivariate analyses.
3.3. Correlation between 4-HNE Accumulation and Antioxidant Enzyme Expression
The estimated mechanism for the reduction in 4-HNE in HCC is that antioxidant enzymes are overexpressed and cancel lipid peroxidation, thereby avoiding the consequent generation of 4-HNE and its associated cytotoxicity. Therefore, we examined the relationship between 4-HNE accumulation and the expression of antioxidant enzymes; however, no significant correlation was found between them (Table 2). Next, the relationship between the expression of GPX4, FSP1, and GCH1 with score 2 in each case and the amount of 4-HNE accumulated was analyzed. The percentage of cases with score 2 for any one of the antioxidant enzymes was 60.0% for 4-HNE with score 0 and 60.7% for 4-HNE with score 1 and score 2, with no significant difference (Table 3). These findings suggest that 4-HNE accumulation is reduced in HCC through mechanisms other than the elimination of lipid peroxidation by GPX, FSP1, and GCH1.

Table 2.
Correlation between 4-hydroxynonenal accumulation and the expression of antioxidants against lipid peroxidation.

Table 3.
Correlation between 4-hydroxynonenal accumulation and the number of the high expression of antioxidants for lipid peroxidation.
3.4. Database Search for Upstream Factors of 4-HNE Metabolism
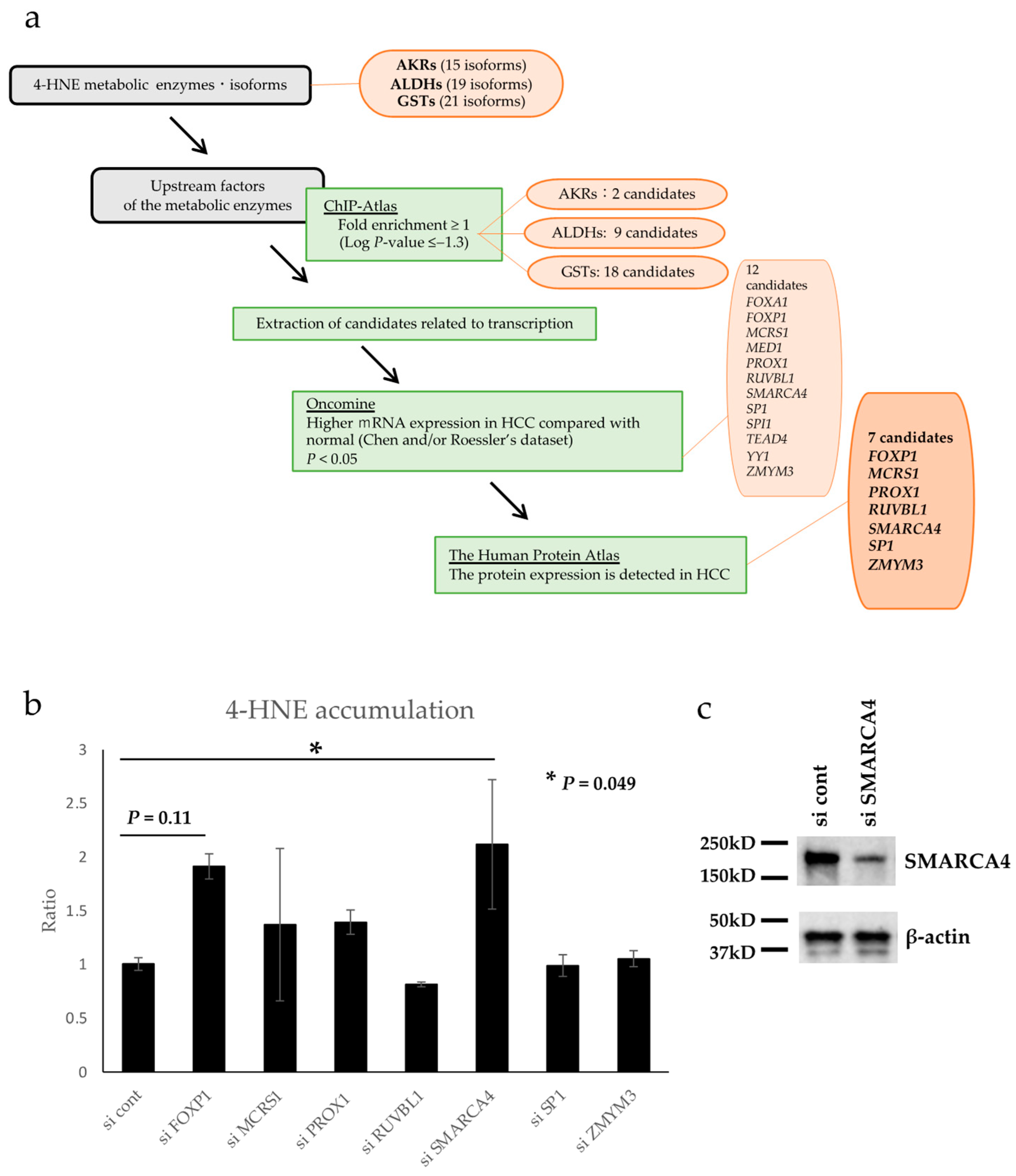
A possible mechanism of 4-HNE metabolism may be involved in the relative reduction in 4-HNE accumulation in HCC. Therefore, the factors that regulate the expression of 4-HNE metabolic enzymes were formulated under several conditions, as shown in the flow chart in Figure 3a. There are 15, 19, and 21 AKR, ALDH, and GST isoforms, respectively, that contribute to 4-HNE metabolism [38,39,40]. Using the chromatin precipitation database ChiP-Atlas, we formulated transcription-related factors that bind specifically to the genes of these metabolic enzymes. Forty candidate genes were extracted as follows: two candidates for AKRs (ARID3A and FOXA2), nine genes for ALDHs (FOXA1, FOXA2, HNF4A, HNF4G, MAFF, MAFK, SNRNP70, USF2, and ZMYM3), and eighteen genes for GSTs (CCAR2, FOXA1, FOXP1, GFP, HNF4A, MAFF, MAFK, MBD4, MCRS1, MED1, PROX1, RXRA, RUVBL1, SMARCA4, SP1, TEAD4, TWIST1, and YY1). In addition, using Oncomine, a gene expression comparison database, 12 of the above 40 genes were selected based on the condition that they are related to transcription and have higher mRNA expression in HCC than in normal hepatocytes (FOXA1, FOXP1, MCRS1, MED1, PROX1, RUVBL1, SMARCA4, SP1, SPI1, TEAD4, YY1, and ZMYM3). Using The Human Protein Atlas, a comparative database of protein expression, 12 genes that had protein expression in HCC tissues were selected. Finally, seven candidate genes, namely, FOXP1, MCRS1, PROX1, RUVBL1, SMARCA4, SP1, and ZMYM3, were identified as transcription factor-related genes associated with 4-HNE metabolism.
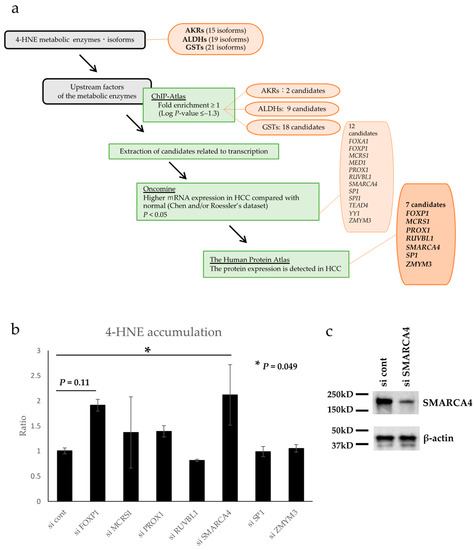
Figure 3.
Flowchart for database analysis and 4-hydroxynonenal (4-HNE) quantification by enzyme-linked immunosorbent assay (ELISA). (a) The database was analyzed as illustrated in the flow chart. Seven candidate genes were finally identified. (b) Intracellular 4-HNE accumulation was quantified by ELISA, and the knockdown of SMARCA4 significantly increased 4-HNE accumulation (p < 0.05). The error bars represent standard deviation. Abbreviations: AKR, aldo-keto reductase; ALDH, alcohol dehydrogenase; GST, glutathione-S-transferase; HCC, Hepatocellular carcinoma. (c) Western blot analysis of SMARCA4 confirmed down-regulation of its expression with si SMARCA4.
3.5. In Vitro Validation of Genes Selected via Database Analysis
Seven candidate genes identified by database analysis were examined for their association with 4-HNE accumulation in vitro. Each gene was knocked down using siRNA (Figure S1), and the intracellular accumulation of 4-HNE was evaluated using ELISA. The results showed a significant increase in 4-HNE accumulation following SMARCA4 knockdown (2.12-fold, p = 0.049, Figure 3b). Western blot analysis confirmed SMARCA4 protein expression was down regulated in cells transfected with si SMARCA4 (Figure 3c).
3.6. IHC of SMARCA4 and the Relation between Its Expression and 4-HNE Accumulation
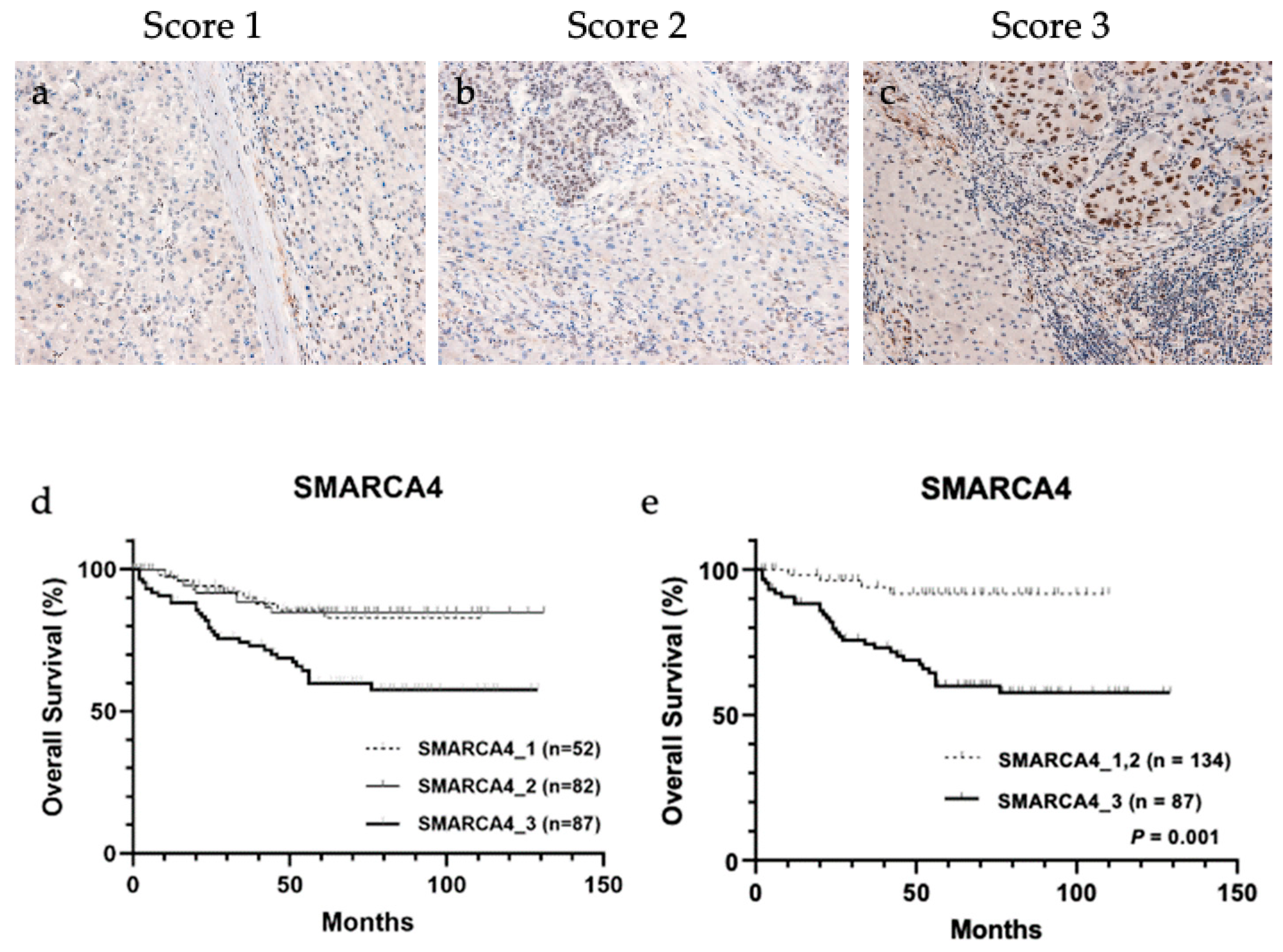
Considering ELISA results, SMARCA4 is a candidate transcription factor that regulates the expression of a group of metabolic enzymes; therefore, immunohistochemical analysis of the association between SMARCA4 expression and 4-HNE accumulation in HCC tissues was performed (Figure 4a–c and Table 4). The result showed a significant correlation between the increased expression of SMARCA4 and decreased accumulation of 4-HNE (p = 0.041). In addition, patients were stratified according to SMARCA4 expression levels, and the result of their association with the outcome is shown in Figure 4d. When classified into three groups for each score, a trend toward worse prognosis was observed in the group with score 3. The results were divided into two groups: (a) group with score 1 and score 2 and (b) group with score 3. The group with score 3, which showed higher expression of SMARCA4 in whole tumor tissue than in normal hepatocytes, had a significantly poorer prognosis than the group with score 1 and score 2 (p < 0.001).
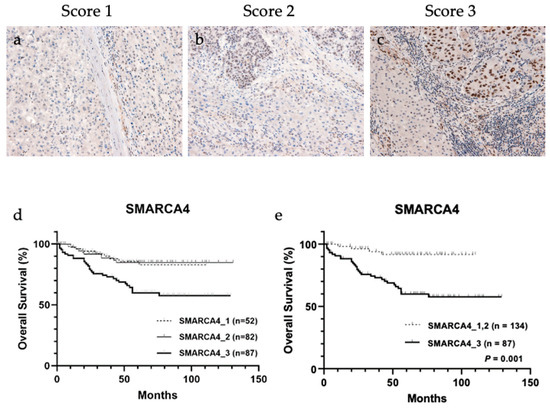
Figure 4.
Representative images of immunohistochemical staining of SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4) in tissues from patients with hepatocellular carcinoma (HCC). (a) Score 1, (b) score 2, and (c) score 3. Overall survival analysis of HCC based on SMARCA4 staining intensity. (d) Classification into three groups based on SMARCA4 staining intensity showed no significant association with overall survival. (e) SMARCA4 score 3 group showed significantly worse prognosis than SMARCA4 score 1 and score 2 groups (p < 0.001).

Table 4.
Correlation between 4-hydroxynonenal accumulation and the expression of SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4.
4. Discussion
4-HNE is the final metabolite of lipid peroxidation, resulting from the peroxidation of ω6 polyunsaturated fatty acids such as linolenic and arachidonic acids. It is associated with carcinogenesis and cancer progression, has highly reactive C=C and C=O, and forms adducts with intracellular substances such as proteins, nucleic acids, and phospholipids, leading to impaired intracellular signal transduction, genetic mutations, and cytotoxicity [5,41]. This study showed that the accumulation of 4-HNE is relatively low in cancer tissues compared with that in noncancerous tissues, which is associated with a poor prognosis. It has been reported that exposure to low concentrations of 4-HNE promotes cell proliferation, whereas exposure to high concentrations of 4-HNE inhibits proliferation. Moreover, higher concentrations of 4-HNE induce cell death, such as apoptosis and necrosis [6,42]. In cases with lower 4-HNE accumulation in HCC, 4-HNE may be less cytotoxic and favor proliferation and survival. On the other hand, a high accumulation of 4-HNE also has a poor prognosis, but in these cases, it may have acquired some form of viability that exceeds the cytotoxicity of 4-HNE itself, which may include the influence of other factors not examined in this study.
An inverse correlation between GPX4 expression and accumulation of 8-hydroxy-2′-deoxyguanosine, the end-product of oxidation, has been observed in diffuse large B-cell lymphoma (DLBCL) [43], whereas GPX4 expression is upregulated, and oxidative stress is reduced in colon cancer [44]. However, no significant inverse correlation was observed between 4-HNE accumulation and antioxidant enzyme expression in HCC, which indicates that 4-HNE accumulation in HCC may be reduced by means other than the high expression of antioxidant enzymes and elimination of lipid peroxidation in cases with relatively low 4-HNE accumulation. Among the candidate factors regulating the 4-HNE-metabolizing enzymes AKRs, ALDHs, and GSTs, SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4) is suggested to regulate the metabolism of 4-HNE in HCC. SMARCA4 is involved in chromatin remodeling and promotes or inhibits the expression of several genes by forming the BRM-associated factor complex [45]. Since SMARCA4-deficient tumors in thoracic malignancies were reported in 2015 [46], SMARCA4 expression and its genetic mutations have received attention in various cancers [47,48,49,50]. SMARCA4 acts as a tumor suppressor in hypercalcemic small cell carcinoma of the ovary, and its deficiency has been reported to be involved in carcinogenesis [47]. SMARCA4 not only acts as a tumor suppressor but also cooperates with p53 loss and Kras activation in mice, and SMARCA4 enhances oxidative phosphorylation [48]. In contrast, upregulation of SMARCA4 is observed in HCC, in which it promotes cell proliferation and progression, and a worse clinical outcome was observed in the group with higher SMARCA4 expression [49,50]. Furthermore, SMARCA4 expression, in concert with c-MET and NRASv12 mutations, is involved in HCC carcinogenesis [49], whereas its inhibition reduces cell migration [51]. A database search (http://chip-atlas.org/, accessed on 31 September 2021) showed that SMARCA4 regulates GSTA4, a 4-HNE metabolic enzyme. Of the GSTs, GSTA4 is known to have a particularly high metabolic capacity for 4-HNE [52]. GSTs contribute to the metabolism of 4-HNE either by forming a complex with reduced glutathione and being excreted through efflux transporters (MRP and RLIP76) or via further metabolism of this complex by AKR and ALDH [10].
In this study, SMARCA4 negatively regulated 4-HNE accumulation in HCC, suggesting that it positively regulates the expression of 4-HNE metabolic enzymes and contributes to 4-HNE metabolism and its decrease in cancer tissues. This reduces the cytotoxicity of 4-HNE and may favor the survival of HCC cells. Furthermore, SMARCA4 upregulated the expression of HO-1, an antioxidant enzyme [53], which may reduce intracellular oxidative stress. Therefore, targeting SMARCA4 and its downstream metabolic enzymes is expected to lead to the development of novel therapeutic strategies due to 4-HNE accumulation and its associated cytotoxicity and oxidative stress accumulation.
In addition, a poor prognosis was observed in the group with high expression of the antioxidant enzyme GPX4, suggesting that lipid peroxidation was suppressed in this group. In turn, this suggests that low 4-HNE expression may be partly due to the overexpression of GPX4, which is a key negative regulator of ferroptosis (nonapoptotic cell death related to lipid peroxidation) that has recently attracted attention [23]. The overexpression of GPX4 may suppress ferroptosis, thus favoring cancer cell survival. It is advantageous to establish the molecular basis for a novel antitumor strategy by inducing ferroptosis in HCC with high GPX4 expression.
Despite these findings, this study has several limitations. The expression levels of GPX4, FSP1, and GCH1 in HCC differed from case to case; however, we were unable to investigate the molecular mechanisms that regulate this in the present study. Overexpression of GPX4 in DLBCL has been shown to upregulate SECISBP2, which regulates GPX4 expression, and its expression may be controlled by a similar mechanism in HCC [54]. Moreover, we found that 4-HNE reduction serves as a poor prognostic factor in HCC tissues and is detected in SMARCA4 knockdown cells in vitro; however, the current study lacks in vivo support. Therefore, additional in vivo experiments are necessary to support our claim. In addition, the mechanism of SMARCA4 overexpression, which is a poor prognostic predictor of HCC, remains unclear. Furthermore, the metabolic enzymes related to 4-HNE that are regulated by SMARCA4 have not been fully investigated, and further studies are needed to determine whether SMARCA4 regulates GSTA4 and other metabolic enzymes. In addition to SMARCA4, the possibility that FOXP1 also regulates 4-HNE is undeniable, although ELISA experiments showed no statistical differences. The standard deviation was large due to the small number of repeated experiments in this study. Therefore, further studies are needed to obtain more reliable results.
5. Conclusions
Collectively, our findings elucidate the clinicopathological significance of 4-HNE in HCC and the molecular mechanisms underlying the 4-HNE metabolic pathway. Future studies on the details of SMARCA4-mediated metabolism of 4-HNE and examination of preclinical models may help establish evidence for SMARCA4 as a therapeutic target and improve the prognosis of patients with HCC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11082278/s1, Figure S1: mRNA levels of each gene downregulated using small interfering RNA (siRNA) were measured, and knockdown efficiency was detected by RT-qPCR. All mRNA levels were significantly decreased on knockdown by siRNA transfection. a. FOXP1 (p < 0.001), b. MCRS1 (p < 0.01), c. PROX1 (p < 0.05), d. RUVBL1 (p < 0.01), e. SMARCA4 (p < 0.001), f. SP1 (p < 0.05), and g. ZMYM3 (p < 0.05); Table S1: Primary antibodies and conditions for immunostaining; Table S2: Small interfering RNA sequences for knockdown; Table S3: Primer sequences for quantitative PCR; Table S4: The number of cases of each score in each antibody; Table S5: Relationship between background liver status and 4-hydroxynonenal accumulation; Table S6: Correlation between 4-HNE and clinicopathological features; Table S7: Correlation between GPX4 expression and clinicopathological features; Table S8: Correlation between FSP1 expression and clinicopathological features; Table S9: Correlation between GCH1 expression and clinicopathological features.
Author Contributions
Conceptualization, K.Y.; validation, S.W., Y.A., R.K., G.K., Y.K. (Yuki Kubo), A.T., I.O., Y.K. (Yuko Kinowaki), S.I., M.I., Y.F. and M.K. (Morito Kurata); formal analysis, S.W., Y.A., R.K., G.K., Y.K. (Yuki Kubo) and S.I.; investigation, S.W., Y.A., R.K., G.K., Y.K. (Yuki Kubo), A.T., I.O., Y.K. (Yuko Kinowaki), S.I., M.I., Y.F. and M.K. (Morito Kurata); resources, K.A. and M.T.; writing—original draft preparation, S.W.; writing—review and editing, K.Y.; visualization, S.W. and S.I.; supervision, M.K. (Masanobu Kitagawa) and K.O.; project administration, K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, grant number 18K07063.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Tokyo Medical and Dental University (approval number M2015-548, (date of approval: 25 March 2016)).
Informed Consent Statement
Patient consent was waived as the used material was historical pathological material.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, S. Metabolic Changes in Cancer: Beyond the Warburg Effect. Acta Biochim. Biophys. Sin. 2013, 45, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Ahmed, K.B.; Garcia-Prieto, C.; Huang, P. Metabolic Alterations in Cancer Cells and Therapeutic Implications. Chin. J. Cancer 2011, 30, 508–525. [Google Scholar] [CrossRef]
- Schaur, R.J. Basic Aspects of the Biochemical Reactivity of 4-Hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Tang, M.S. Trans-4-hydroxy-2-nonenal Inhibits Nucleotide Excision Repair in Human Cells: A Possible Mechanism for Lipid Peroxidation-Induced Carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 8598–8602. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Moore, D.R.; Nimmo, S.L.; Lightfoot, S.A.; Huycke, M.M. 4-Hydroxy-2-Nonenal Mediates Genotoxicity and Bystander Effects Caused by Enterococcus faecalis-Infected Macrophages. Gastroenterology 2012, 142, 543–551.e7. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Eveleigh, J.; Iyer, G.; Pan, J.; Amin, S.; Chung, F.L.; Tang, M.S. The Major Lipid Peroxidation Product, trans-4-hydroxy-2-nonenal, Preferentially Forms DNA Adducts at Codon 249 of Human p53 Gene, a Unique Mutational Hotspot in Hepatocellular Carcinoma. Carcinogenesis 2002, 23, 1781–1789. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell Death and Diseases Related to Oxidative Stress: 4-Hydroxynonenal (HNE) in the Balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Dwivedi, S.; Zimniak, P.; Awasthi, S.; Awasthi, Y.C. 4-Hydroxynonenal Self-Limits fas-Mediated DISC-Independent Apoptosis by Promoting Export of Daxx from the Nucleus to the Cytosol and Its Binding to Fas. Biochemistry 2008, 47, 143–156. [Google Scholar] [CrossRef]
- Awasthi, Y.C.; Sharma, R.; Cheng, J.Z.; Yang, Y.; Sharma, A.; Singhal, S.S.; Awasthi, S. Role of 4-Hydroxynonenal in Stress-Mediated Apoptosis Signaling. Mol. Asp. Med. 2003, 24, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 Talks, Who’s Listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer Growth Regulation by 4-Hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Podszun, M.C.; Chung, J.Y.; Ylaya, K.; Kleiner, D.E.; Hewitt, S.M.; Rotman, Y. 4-HNE Immunohistochemistry and Image Analysis for Detection of Lipid Peroxidation in Human Liver Samples Using Vitamin E Treatment in NAFLD as a Proof of Concept. J. Histochem. Cytochem. 2020, 68, 635–643. [Google Scholar] [CrossRef]
- Maki, A.; Kono, H.; Gupta, M.; Asakawa, M.; Suzuki, T.; Matsuda, M.; Fujii, H.; Rusyn, I. Predictive Power of Biomarkers of Oxidative Stress and Inflammation in Patients with Hepatitis C Virus-Associated Hepatocellular Carcinoma. Ann. Surg. Oncol. 2007, 14, 1182–1190. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wu, K.; Liu, Z. Expression of 4-Hydroxynonenal in Esophageal Squamous Cell Carcinoma. Oncol. Lett. 2017, 14, 35–40. [Google Scholar] [CrossRef]
- Yang, Y.; Huycke, M.M.; Herman, T.S.; Wang, X. Glutathione S-transferase Alpha 4 Induction by Activator Protein 1 in Colorectal Cancer. Oncogene 2016, 35, 5795–5806. [Google Scholar] [CrossRef]
- Gęgotek, A.; Nikliński, J.; Žarković, N.; Žarković, K.; Waeg, G.; Łuczaj, W.; Charkiewicz, R.; Skrzydlewska, E. Lipid Mediators Involved in the Oxidative Stress and Antioxidant Defence of Human Lung Cancer Cells. Redox Biol. 2016, 9, 210–219. [Google Scholar] [CrossRef]
- Young, O.; Crotty, T.; O’Connell, R.; O’Sullivan, J.; Curran, A.J. Levels of Oxidative Damage and Lipid Peroxidation in Thyroid Neoplasia. Head Neck 2010, 32, 750–756. [Google Scholar] [CrossRef]
- Biasi, F.; Tessitore, L.; Zanetti, D.; Cutrin, J.C.; Zingaro, B.; Chiarpotto, E.; Zarkovic, N.; Serviddio, G.; Poli, G. Associated Changes of Lipid Peroxidation and Transforming Growth Factor beta1 Levels in Human Colon Cancer During Tumour Progression. Gut 2002, 50, 361–367. [Google Scholar] [CrossRef]
- Oberley, T.D.; Toyokuni, S.; Szweda, L.I. Localization of Hydroxynonenal Protein Adducts in Normal Human Kidney and Selected Human Kidney Cancers. Free Radic. Biol. Med. 1999, 27, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yi, X.; Zhu, X.H.; Jiang, D.S. Posttranslational Modifications in Ferroptosis. Oxid. Med. Cell. Longev. 2020, 2020, 8832043. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis Through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-Tolerant Persister Cancer Cells Are Vulnerable to GPX4 Inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Koppula, P.; Lei, G.; Zhang, Y.; Yan, Y.; Mao, C.; Kondiparthi, L.; Shi, J.; Liu, X.; Horbath, A.; Das, M.; et al. A Targetable CoQ-FSP1 Axis Drives Ferroptosis- and Radiation-Resistance in KEAP1 Inactive Lung Cancers. Nat. Commun. 2022, 13, 2206. [Google Scholar] [CrossRef]
- Guerriero, E.; Capone, F.; Accardo, M.; Sorice, A.; Costantini, M.; Colonna, G.; Castello, G.; Costantini, S. GPX4 and GPX7 Over-Expression in Human Hepatocellular Carcinoma Tissues. Eur. J. Histochem. 2015, 59, 2540. [Google Scholar] [CrossRef]
- Rohr-Udilova, N.; Bauer, E.; Timelthaler, G.; Eferl, R.; Stolze, K.; Pinter, M.; Seif, M.; Hayden, H.; Reiberger, T.; Schulte-Hermann, R.; et al. Impact of Glutathione Peroxidase 4 on Cell Proliferation, Angiogenesis and Cytokine Production in Hepatocellular Carcinoma. Oncotarget 2018, 9, 10054–10068. [Google Scholar] [CrossRef]
- Asakawa, A.; Kawade, G.; Kurata, M.; Fukuda, S.; Onishi, I.; Kinowaki, Y.; Ishibashi, S.; Ikeda, M.; Watabe, S.; Kobayashi, M.; et al. Stratification of Lung Squamous Cell Carcinoma Based on Ferroptosis Regulators: Potential for New Therapeutic Strategies Involving Ferroptosis Induction. Published online ahead of print, January 24 2022. Lung Cancer 2022, 165, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in Redox Homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef]
- Lyon, R.C.; Li, D.; McGarvie, G.; Ellis, E.M. Aldo-Keto Reductases Mediate Constitutive and Inducible Protection Against Aldehyde Toxicity in Human Neuroblastoma SH-SY5Y Cells. Neurochem. Int. 2013, 62, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Yang, J.H.; Huang, F.Z.; Nie, W.P.; Liu, C.P.; Mao, X.H.; Yin, X.M.; Shen, X.B.; Peng, C.; Chen, M.F.; et al. Glutathione-s-Transferase A 4 (GSTA4) Suppresses Tumor Growth and Metastasis of Human Hepatocellular Carcinoma by Targeting AKT Pathway. Am. J. Transl. Res. 2017, 9, 301–315. [Google Scholar] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Oki, S.; Ohta, T.; Shioi, G.; Hatanaka, H.; Ogasawara, O.; Okuda, Y.; Kawaji, H.; Nakaki, R.; Sese, J.; Meno, C. ChIP-Atlas: A Data-Mining Suite Powered by Full Integration of Public ChIP-Seq Data. EMBO Rep. 2018, 19, e46255. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kurata, M.; Yamamoto, K.; Nogawa, D.; Inoue, M.; Ishibashi, S.; Ikeda, M.; Miyasaka, N.; Kitagawa, M. High ampli fication of PVT1 and MYC predict favorable prognosis in early ovarian carcinoma. Pathol. Res. Pract. 2020, 216, 153175. [Google Scholar] [CrossRef]
- Dai, T.; Ye, L.; Yu, H.; Li, K.; Li, J.; Liu, R.; Lu, X.; Deng, M.; Li, R.; Liu, W.; et al. Regulation Network and Prognostic Significance of Aldo-Keto Reductase (AKR) Superfamily Genes in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 997–1021. [Google Scholar] [CrossRef]
- Muzio, G.; Maggiora, M.; Paiuzzi, E.; Oraldi, M.; Canuto, R.A. Aldehyde Dehydrogenases and Cell Proliferation. Free Radic. Biol. Med. 2012, 52, 735–746. [Google Scholar] [CrossRef]
- Josephy, P.D. Genetic Variations in Human Glutathione Transferase Enzymes: Significance for Pharmacology and Toxicology. Hum. Genom. Proteom. 2010, 2010, 876940. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of Lipid Peroxidation Derived 4-Hydroxynonenal (4-HNE) in Cancer: Focusing on Mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Guéraud, F. 4-Hydroxynonenal Metabolites and Adducts in Pre-carcinogenic Conditions and Cancer. Free Radic. Biol. Med. 2017, 111, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione Peroxidase 4 Overexpression Inhibits ROS-Induced Cell Death in Diffuse Large B-Cell Lymphoma. Lab. Investig. 2018, 98, 609–619. [Google Scholar] [CrossRef]
- Yagublu, V.; Arthur, J.R.; Babayeva, S.N.; Nicol, F.; Post, S.; Keese, M. Expression of Selenium-Containing Proteins in Human Colon Carcinoma Tissue. Anticancer Res. 2011, 31, 2693–2698. [Google Scholar]
- Mittal, P.; Roberts, C.W.M. The SWI/SNF Complex in Cancer—Biology, Biomarkers and Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 435–448. [Google Scholar] [CrossRef]
- Le Loarer, F.; Watson, S.; Pierron, G.; de Montpreville, V.T.; Ballet, S.; Firmin, N.; Auguste, A.; Pissaloux, D.; Boyault, S.; Paindavoine, S.; et al. SMARCA4 Inactivation Defines a Group of Undifferentiated Thoracic Malignancies Transcriptionally Related to BAF-Deficient Sarcomas. Nat. Genet. 2015, 47, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Karnezis, A.N.; Craig, D.W.; Sekulic, A.; Russell, M.L.; Hendricks, W.P.; Corneveaux, J.J.; Barrett, M.T.; Shumansky, K.; Yang, Y.; et al. Small Cell Carcinoma of the Ovary, Hypercalcemic Type, Displays Frequent Inactivating Germline and Somatic Mutations in SMARCA4. Nat. Genet. 2014, 46, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Lissanu Deribe, Y.; Sun, Y.; Terranova, C.; Khan, F.; Martinez-Ledesma, J.; Gay, J.; Gao, G.; Mullinax, R.A.; Khor, T.; Feng, N.; et al. Mutations in the SWI/SNF Complex Induce a Targetable Dependence on Oxidative Phosphorylation in Lung Cancer. Nat. Med. 2018, 24, 1047–1057. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Jia, D.; Jing, Y.; Chen, D.; Wang, Q.; Zhao, F.; Li, J.; Yao, M.; Cong, W.; et al. Hepatic SMARCA4 Predicts HCC Recurrence and Promotes Tumour Cell Proliferation by Regulating SMAD6 Expression. Cell Death Dis. 2018, 9, 59. [Google Scholar] [CrossRef]
- Wang, P.; Song, X.; Cao, D.; Cui, K.; Wang, J.; Utpatel, K.; Shang, R.; Wang, H.; Che, L.; Evert, M.; et al. Oncogene-Dependent Function of BRG1 in Hepatocarcinogenesis. Cell Death Dis. 2020, 11, 91. [Google Scholar] [CrossRef]
- Choi, H.I.; An, G.Y.; Baek, M.; Yoo, E.; Chai, J.C.; Lee, Y.S.; Jung, K.H.; Chai, Y.G. BET Inhibitor Suppresses Migration of Human Hepatocellular Carcinoma by Inhibiting SMARCA4. Sci. Rep. 2021, 11, 11799. [Google Scholar] [CrossRef] [PubMed]
- Shireman, L.M.; Kripps, K.A.; Balogh, L.M.; Conner, K.P.; Whittington, D.; Atkins, W.M. Glutathione Transferase A4-4 Resists Adduction by 4-Hydroxynonenal. Arch. Biochem. Biophys. 2010, 504, 182–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- You, S.; Zhang, Y.; Xu, J.; Qian, H.; Wu, S.; Wu, B.; Lu, S.; Sun, Y.; Zhang, N. The Role of BRG1 in Antioxidant and Redox Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 6095673. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Kurata, M.; Onishi, I.; Kinowaki, Y.; Sato, Y.; Shiono, S.; Ishibashi, S.; Ikeda, M.; Yamamoto, M.; Kitagawa, M.; et al. SECISBP2 Is a Novel Prognostic Predictor That Regulates Selenoproteins in Diffuse Large B-Cell Lymphoma. Lab. Investig. 2021, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).