Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Pharmacological Treatment
- -
- Control (n = 12): rats submitted to graded endurance exercise test (GEET) as described below, and placed on a stationary treadmill daily for 5 min. Six animals received normal drinking water (C−) and the remaining six animals received Met (metformin hydrochloride, 98%—Bosche Scientific) dissolved in drinking water at a dose of 250 mg/kg body weight until sacrifice (C+).
- -
- Exercised (n = 12): rats submitted to training on treadmill daily for 8 weeks and to GEET. Six animals were given normal drinking water (EX−) and the remaining six rats received Met dissolved in drinking water at a dose of 250 mg/kg body weight until sacrifice (EX+).
- -
- Sedentary (n = 6): rats not submitted to any type of exercise and placed at rest on the treadmill daily for 5 min (SED). This group was included in the study design to check for possible effects of GEET. We showed the results obtained in this group only when they differed significantly from C−.
2.2. Exercise Training Protocol
2.3. GEET
2.4. Haematochemical Parameters
2.5. Myokines Assay
2.6. Total Muscle Lysate and Mitochondrial Fraction
2.7. Western Blot (WB)
2.8. Immunohistochemical (IHC) Analysis
2.9. Statistical Analysis
3. Results
3.1. Food Consumption, Body Weight Increase and Muscle Weight/Body Weight Ratio
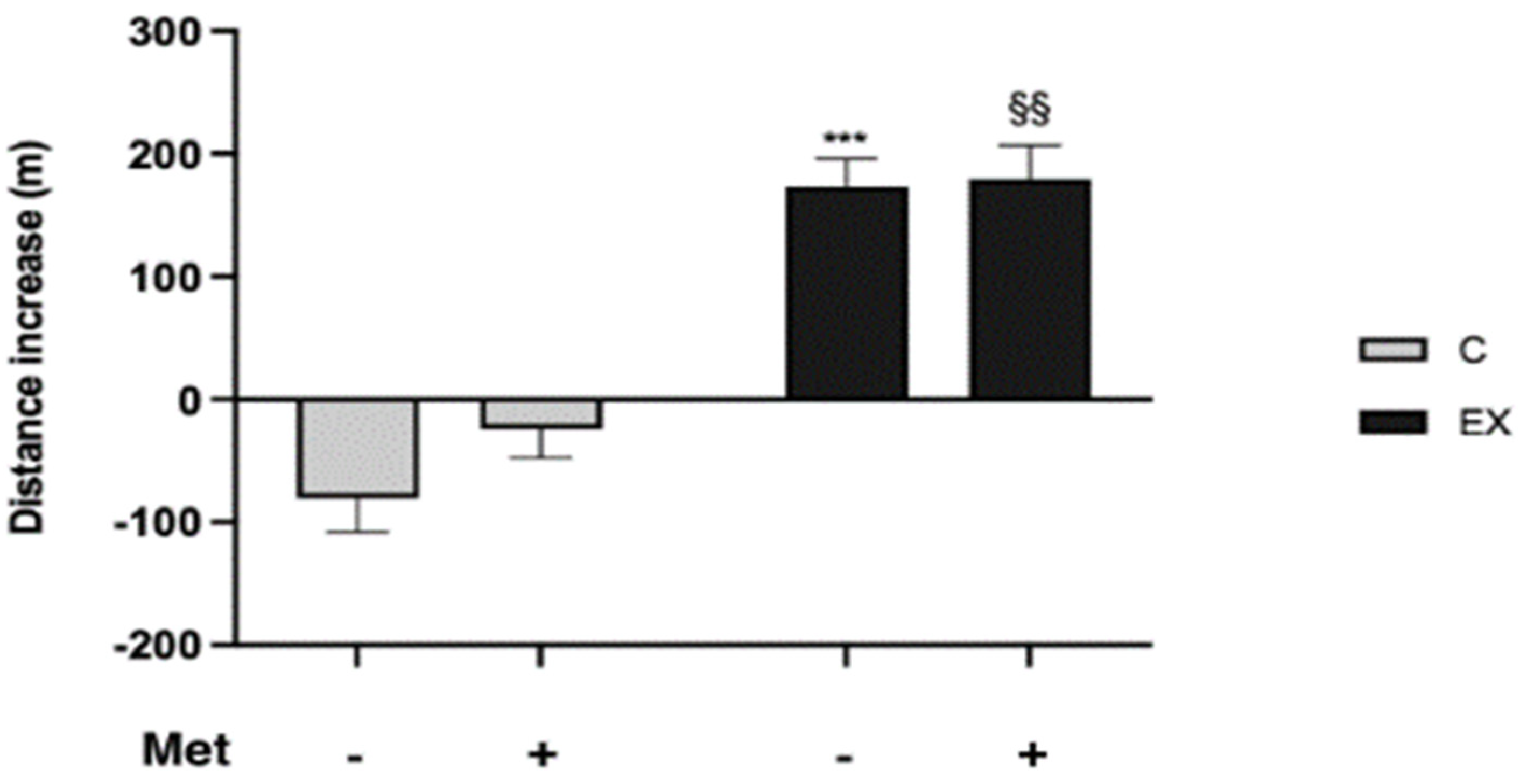
3.2. Evaluation of PP
3.3. Haematochemical Parameters
3.4. Myokines in Serum and in Total Muscle Lysate
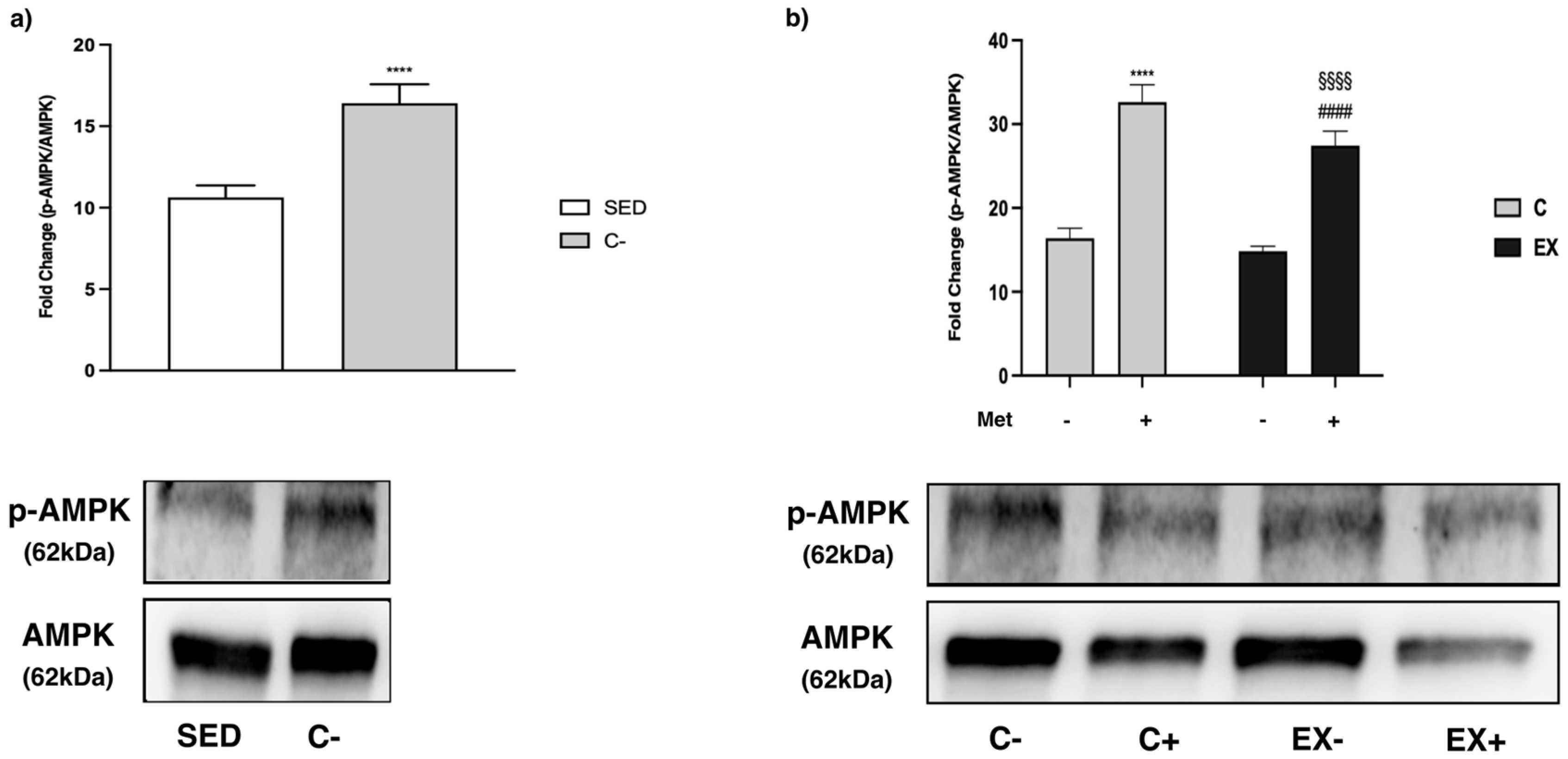
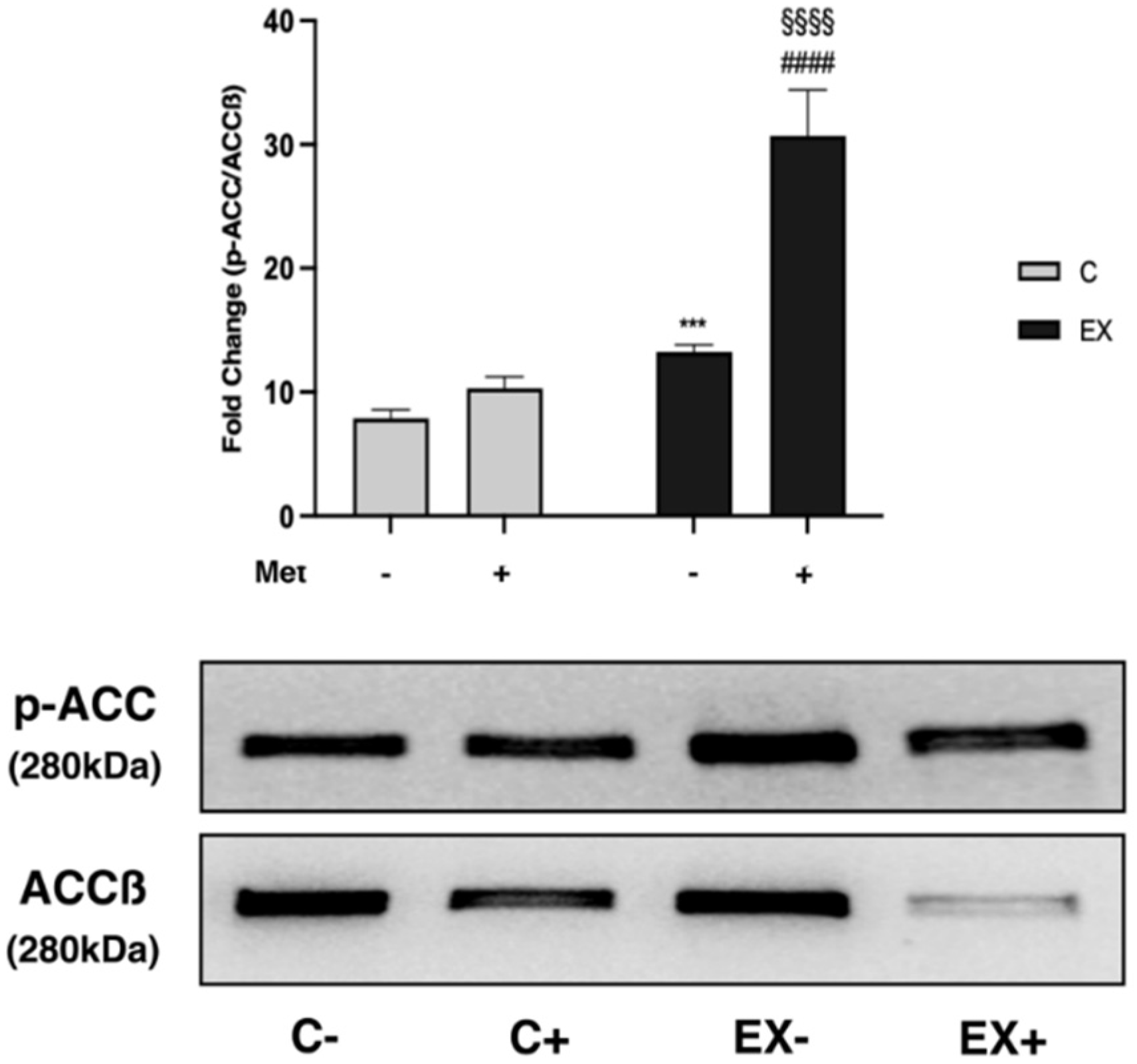
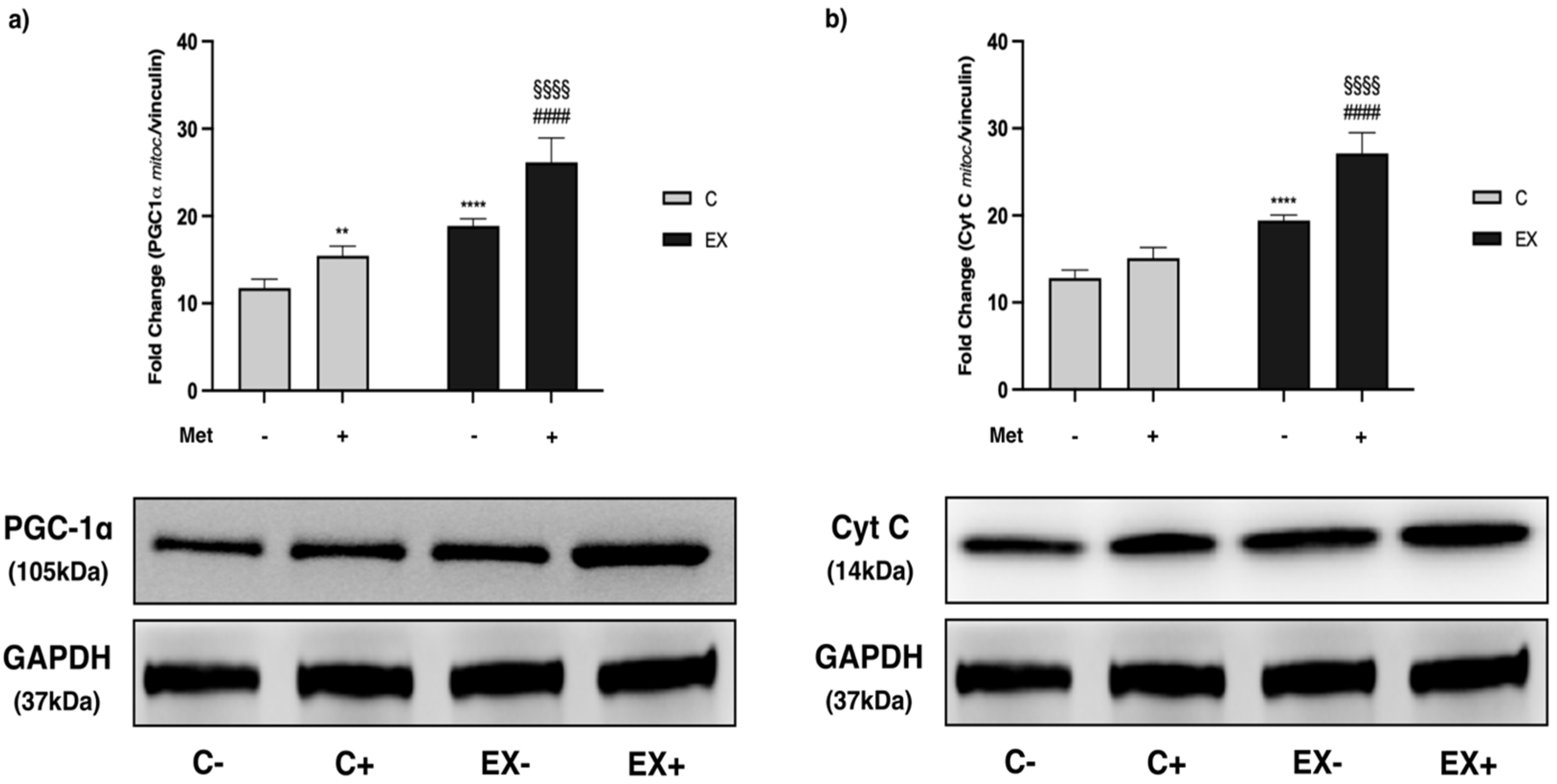
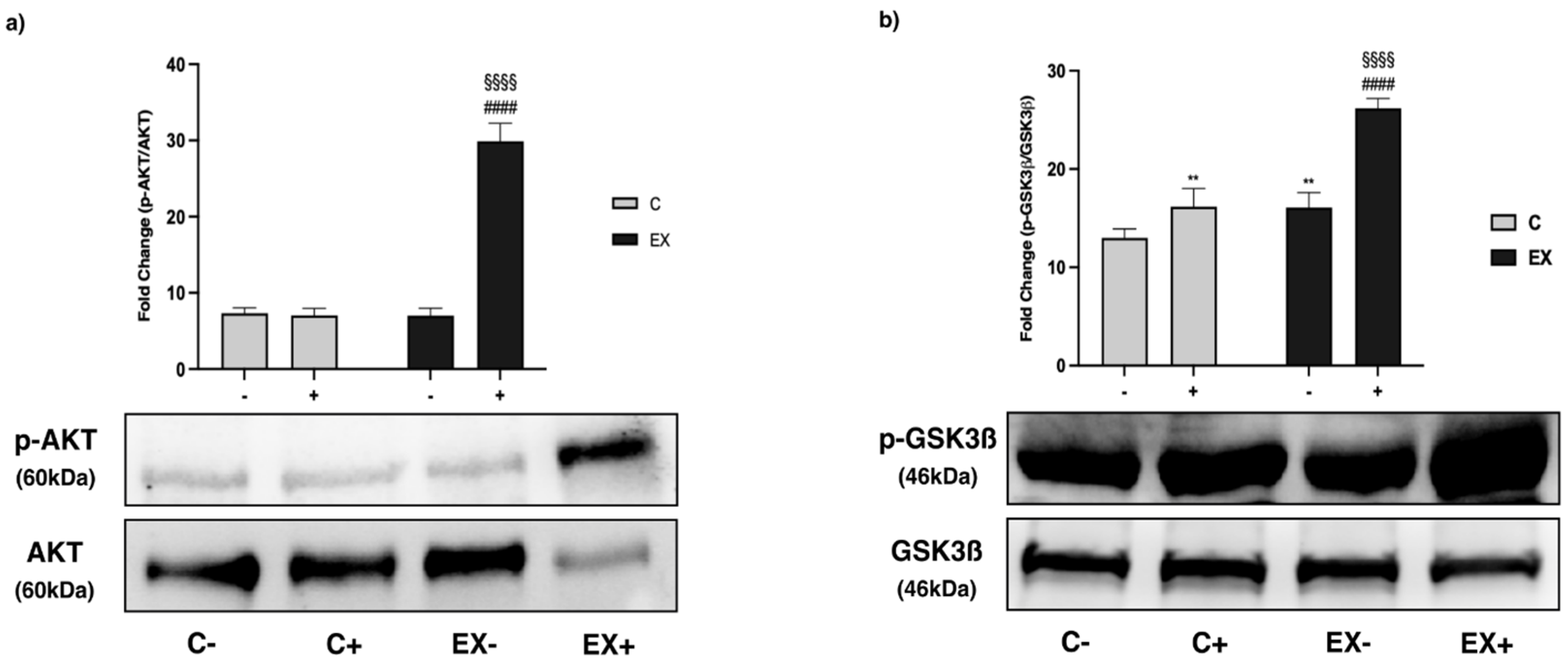
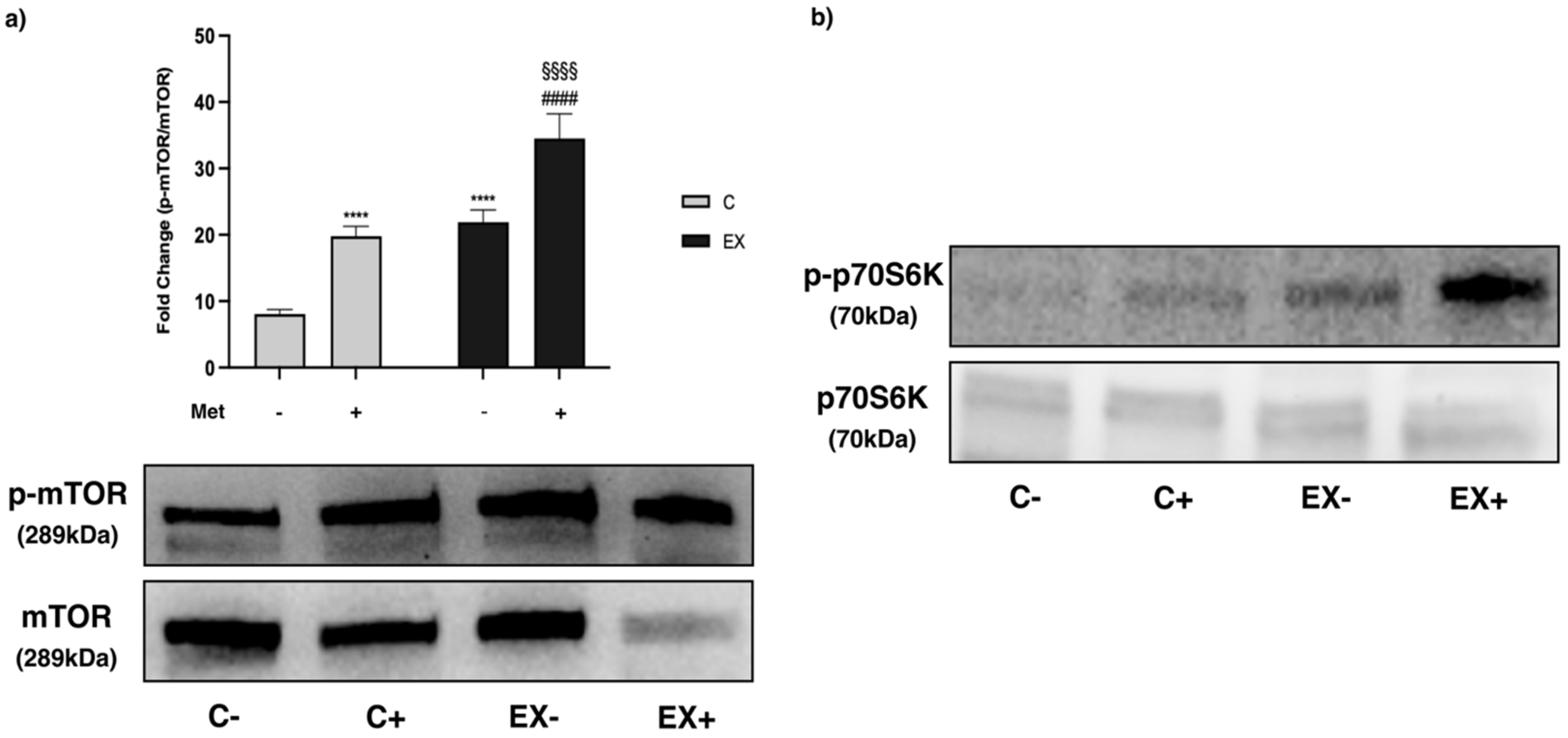
3.5. WB Analysis
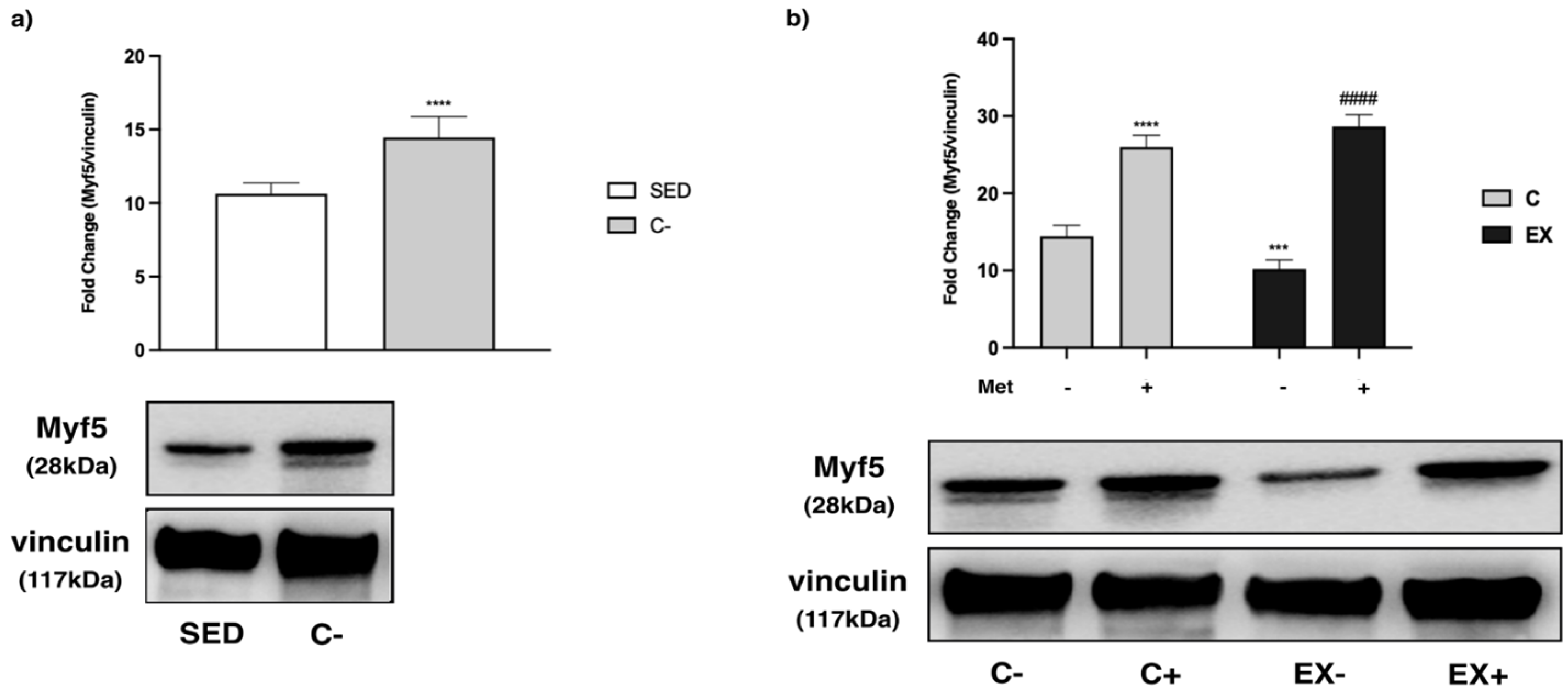
3.6. IHC Analysis of MRFs and MYH1/2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiernsperger, N.F.; Bailey, C.J. The antihyperglycaemic effect of metformin: Therapeutic and cellular mechanisms. Drugs 1999, 58 (Suppl. S1), 31–39; discussion 75–82. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Wessels, B.; Ciapaite, J.; van den Broek, N.M.; Nicolay, K.; Prompers, J.J. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS ONE 2014, 9, e100525. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Kane, D.A.; Anderson, E.J.; Price, J.W., 3rd; Woodlief, T.L.; Lin, C.T.; Bikman, B.T.; Cortright, R.N.; Neufer, P.D. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic. Biol. Med. 2010, 49, 1082–1087. [Google Scholar] [CrossRef]
- World Anti-Doping Agency. The Prohibited List. Available online: https://www.wada-ama.org/en/prohibited-list (accessed on 1 January 2019).
- Spaulding, H.R.; Yan, Z. AMPK and the Adaptation to Exercise. Annu. Rev. Physiol. 2022, 84, 209–227. [Google Scholar] [CrossRef]
- Petrocelli, J.J.; Mahmassani, Z.S.; Fix, D.K.; Montgomery, J.A.; Reidy, P.T.; McKenzie, A.I.; de Hart, N.M.; Ferrara, P.J.; Kelley, J.J.; Eshima, H.; et al. Metformin and leucine increase satellite cells and collagen remodeling during disuse and recovery in aged muscle. FASEB J. 2021, 35, e21862. [Google Scholar] [CrossRef]
- Kurosaka, M.; Naito, H.; Ogura, Y.; Machida, S.; Katamoto, S. Satellite cell pool enhancement in rat plantaris muscle by endurance training depends on intensity rather than duration. Acta Physiol. 2012, 205, 159–166. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Hällsten, K.; Virtanen, K.A.; Lönnqvist, F.; Sipilä, H.; Oksanen, A.; Viljanen, T.; Rönnemaa, T.; Viikari, J.; Knuuti, J.; Nuutila, P. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 2002, 51, 3479–3485. [Google Scholar] [CrossRef]
- Smith, A.C.; Mullen, K.L.; Junkin, K.A.; Nickerson, J.; Chabowski, A.; Bonen, A.; Dyck, D.J. Metformin and exercise reduce muscle FAT/CD36 and lipid accumulation and blunt the progression of high-fat diet-induced hyperglycemia. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E172–E181. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Reed, M.J. Exercise adds to metformin and acarbose efficacy in db/db mice. Metabolism 2001, 50, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, S.D.; Meininger, G.E.; Lareau, M.T.; Dolan, S.E.; Killilea, K.M.; Hadigan, C.M.; Lloyd-Jones, D.M.; Klibanski, A.; Frontera, W.R.; Grinspoon, S.K. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. Aids 2004, 18, 465–473. [Google Scholar] [CrossRef]
- Malin, S.K.; Braun, B. Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Appl. Physiol. Nutr. Metab. 2013, 38, 427–430. [Google Scholar] [CrossRef]
- Cadeddu, C.; Nocco, S.; Cugusi, L.; Deidda, M.; Bina, A.; Fabio, O.; Bandinu, S.; Cossu, E.; Baroni, M.G.; Mercuro, G. Effects of metformin and exercise training, alone or in association, on cardio-pulmonary performance and quality of life in insulin resistance patients. Cardiovasc. Diabetol. 2014, 13, 93. [Google Scholar] [CrossRef]
- Konopka, A.R.; Laurin, J.L.; Schoenberg, H.M.; Reid, J.J.; Castor, W.M.; Wolff, C.A.; Musci, R.V.; Safairad, O.D.; Linden, M.A.; Biela, L.M.; et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 2019, 18, e12880. [Google Scholar] [CrossRef]
- Senesi, P.; Montesano, A.; Luzi, L.; Codella, R.; Benedini, S.; Terruzzi, I. Metformin Treatment Prevents Sedentariness Related Damages in Mice. J. Diabetes Res. 2016, 2016, 8274689. [Google Scholar] [CrossRef]
- Soya, H.; Nakamura, T.; Deocaris, C.C.; Kimpara, A.; Iimura, M.; Fujikawa, T.; Chang, H.; McEwen, B.S.; Nishijima, T. BDNF induction with mild exercise in the rat hippocampus. Biochem. Biophys. Res. Commun. 2007, 358, 961–967. [Google Scholar] [CrossRef]
- Inoue, K.; Okamoto, M.; Shibato, J.; Lee, M.C.; Matsui, T.; Rakwal, R.; Soya, H. Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PLoS ONE 2015, 10, e0128720. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Matsui, T.; Jesmin, S.; Okamoto, M.; Soya, M.; Inoue, K.; Liu, Y.F.; Torres-Aleman, I.; McEwen, B.S.; Soya, H. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 2017, 60, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Liu, Y.F.; Soya, M.; Shima, T.; Soya, H. Tyrosine as a Mechanistic-Based Biomarker for Brain Glycogen Decrease and Supercompensation With Endurance Exercise in Rats: A Metabolomics Study of Plasma. Front. Neurosci. 2019, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Hokayem, M.; Thomas, C.; Fabre, O.; Cassan, C.; Bourret, A.; Bernex, F.; Feuillet-Coudray, C.; Notarnicola, C.; Mercier, J.; et al. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci. Rep. 2018, 8, 2885. [Google Scholar] [CrossRef]
- Penna, C.; Perrelli, M.G.; Raimondo, S.; Tullio, F.; Merlino, A.; Moro, F.; Geuna, S.; Mancardi, D.; Pagliaro, P. Postconditioning induces an anti-apoptotic effect and preserves mitochondrial integrity in isolated rat hearts. Biochim. Biophys. Acta 2009, 1787, 794–801. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Berta, G.N.; Di Scipio, F.; Yang, Z.; Oberto, A.; Abbadessa, G.; Romano, F.; Carere, M.E.; Ceccarelli, A.; Hirsch, E.; Mognetti, B. Chemical Oral Cancerogenesis Is Impaired in PI3Kγ Knockout and Kinase-Dead Mice. Cancers 2021, 13, 4211. [Google Scholar] [CrossRef]
- Biolo, G.; Williams, B.D.; Fleming, R.Y.; Wolfe, R.R. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999, 48, 949–957. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Nathwani, R.A.; Pais, S.; Reynolds, T.B.; Kaplowitz, N. Serum alanine aminotransferase in skeletal muscle diseases. Hepatology 2005, 41, 380–382. [Google Scholar] [CrossRef]
- Cabaniss, C.D. Creatine Kinase. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; A Division of Reed Publishing, Butterworth Publishers: Boston, MA, USA, 1990. [Google Scholar]
- Siegel, A.J.; Silverman, L.M.; Holman, B.L. Elevated creatine kinase MB isoenzyme levels in marathon runners. Normal myocardial scintigrams suggest noncardiac source. JAMA 1981, 246, 2049–2051. [Google Scholar] [CrossRef] [PubMed]
- Cialoni, D.; Brizzolari, A.; Sponsiello, N.; Lancellotti, V.; Lori, C.; Bosco, G.; Marroni, A.; Barassi, A. Serum Cardiac and Skeletal Muscle Marker Changes in Repetitive Breath-hold Diving. Sports Med. Open 2021, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- So, B.; Kim, H.J.; Kim, J.; Song, W. Exercise-induced myokines in health and metabolic diseases. Integr. Med. Res. 2014, 3, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Zumárraga, F.J.; Argüelles Castilla, S.; Ayala Gómez, A.; Cano Rodríguez, M. Physical exercise and myokines. Approaches Aging Control. 2019, 23, 7–16. [Google Scholar]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, Y.T.; Kim, S.H.; Jung, H.S.; Park, K.S.; Lee, H.Y.; Lee, M.S. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem. Biophys. Res. Commun. 2013, 440, 76–81. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, N.; Sun, P.; Xia, J.; Zhang, C.; Pang, S. The effects of metformin on fibroblast growth factor 19, 21 and fibroblast growth factor receptor 1 in high-fat diet and streptozotocin induced diabetic rats. Endocr. J. 2017, 64, 543–552. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Chen, J.Q.; Huang, Y.Y.; Gusdon, A.M.; Qu, S. Irisin: A new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015, 14, 2. [Google Scholar] [CrossRef]
- Yano, N.; Zhao, Y.T.; Zhao, T.C. The Physiological Role of Irisin in the Regulation of Muscle Glucose Homeostasis. Endocrines 2021, 2, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Horii, N.; Fujie, S.; Inoue, K.; Hasegawa, N.; Iemitsu, K.; Uchida, M.; Iemitsu, M. Decreased muscle-derived musclin by chronic resistance exercise is associated with improved insulin resistance in rats with type 2 diabetes. Physiol. Rep. 2021, 9, e14823. [Google Scholar] [CrossRef]
- Petersson, S.J.; Jørgensen, L.H.; Andersen, D.C.; Nørgaard, R.C.; Jensen, C.H.; Schrøder, H.D. SPARC is up-regulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol. Histopathol. 2013, 28, 1451–1460. [Google Scholar] [CrossRef]
- Songsorn, P.; Ruffino, J.; Vollaard, N.B. No effect of acute and chronic supramaximal exercise on circulating levels of the myokine SPARC. Eur. J. Sport. Sci. 2017, 17, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Szewczyk, G.; Pyzlak, M.; Stangret, A.; Bachanek, M.; Trojanowski, S.; Alkhalayla, H.; Wejman, J. Anti-inflammatory Action of Metformin with Respect to CX3CL1/CX3CR1 Signaling in Human Placental Circulation in Normal-Glucose Versus High-Glucose Environments. Inflammation 2018, 41, 2246–2264. [Google Scholar] [CrossRef] [PubMed]
- Rutti, S.; Arous, C.; Schvartz, D.; Timper, K.; Sanchez, J.C.; Dermitzakis, E.; Donath, M.Y.; Halban, P.A.; Bouzakri, K. Fractalkine (CX3CL1), a new factor protecting β-cells against TNFα. Mol. Metab. 2014, 3, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, A.; Olsson, K.; Dijksterhuis, J.P.; Rullman, E.; Schulte, G.; Gustafsson, T. CX3CL1—A macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R297–R304. [Google Scholar] [CrossRef]
- Jessen, N.; Sundelin, E.I.; Møller, A.B. AMP kinase in exercise adaptation of skeletal muscle. Drug Discov. Today 2014, 19, 999–1002. [Google Scholar] [CrossRef]
- Maniscalco, E.; Abbadessa, G.; Giordano, M.; Grasso, L.; Borrione, P.; Racca, S. Metformin regulates myoblast differentiation through an AMPK-dependent mechanism. PLoS ONE 2023, 18, e0281718. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Daugaard, J.R.; Young, M.E.; Saha, A.; Vavvas, D.; Asp, S.; Kiens, B.; Kim, K.H.; Witters, L.; Richter, E.A.; et al. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes 2000, 49, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Mukai, K.; Lally, J.S.; Maher, A.C.; Gurd, B.J.; Heigenhauser, G.J.; Spriet, L.L.; Holloway, G.P. AMP-activated protein kinase is required for exercise-induced peroxisome proliferator-activated receptor co-activator 1 translocation to subsarcolemmal mitochondria in skeletal muscle. J. Physiol. 2013, 591, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.D.; Summers, S.A.; Birnbaum, M.J.; Roth, R.A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 1996, 271, 31372–31378. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Markuns, J.F.; Wojtaszewski, J.F.; Goodyear, L.J. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J. Biol. Chem. 1999, 274, 24896–24900. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Reynolds, T.H.t.; Bodine, S.C.; Lawrence, J.C., Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J. Biol. Chem. 2002, 277, 17657–17662. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Figueiredo, V.C.; Markworth, J.F.; Cameron-Smith, D. Considerations on mTOR regulation at serine 2448: Implications for muscle metabolism studies. Cell Mol. Life Sci. 2017, 74, 2537–2545. [Google Scholar] [CrossRef]
- Hoedt, A.; Christensen, B.; Nellemann, B.; Mikkelsen, U.R.; Hansen, M.; Schjerling, P.; Farup, J. Satellite cell response to erythropoietin treatment and endurance training in healthy young men. J. Physiol. 2016, 594, 727–743. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, D.; Zhao, L.; Li, Y.; Yao, X.; Wang, H.; Zhang, S.; Liu, W.; Cao, H.; Yu, S.; et al. CaMKK2 Suppresses Muscle Regeneration through the Inhibition of Myoblast Proliferation and Differentiation. Int. J. Mol. Sci. 2016, 17, 1695. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhu, M.J.; Dodson, M.V.; Du, M. AMP-activated protein kinase stimulates Warburg-like glycolysis and activation of satellite cells during muscle regeneration. J. Biol. Chem. 2015, 290, 26445–26456. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, M.; Zhang, S.; Foretz, M.; Viollet, B.; Du, M. Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK. Diabetes 2016, 65, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef]
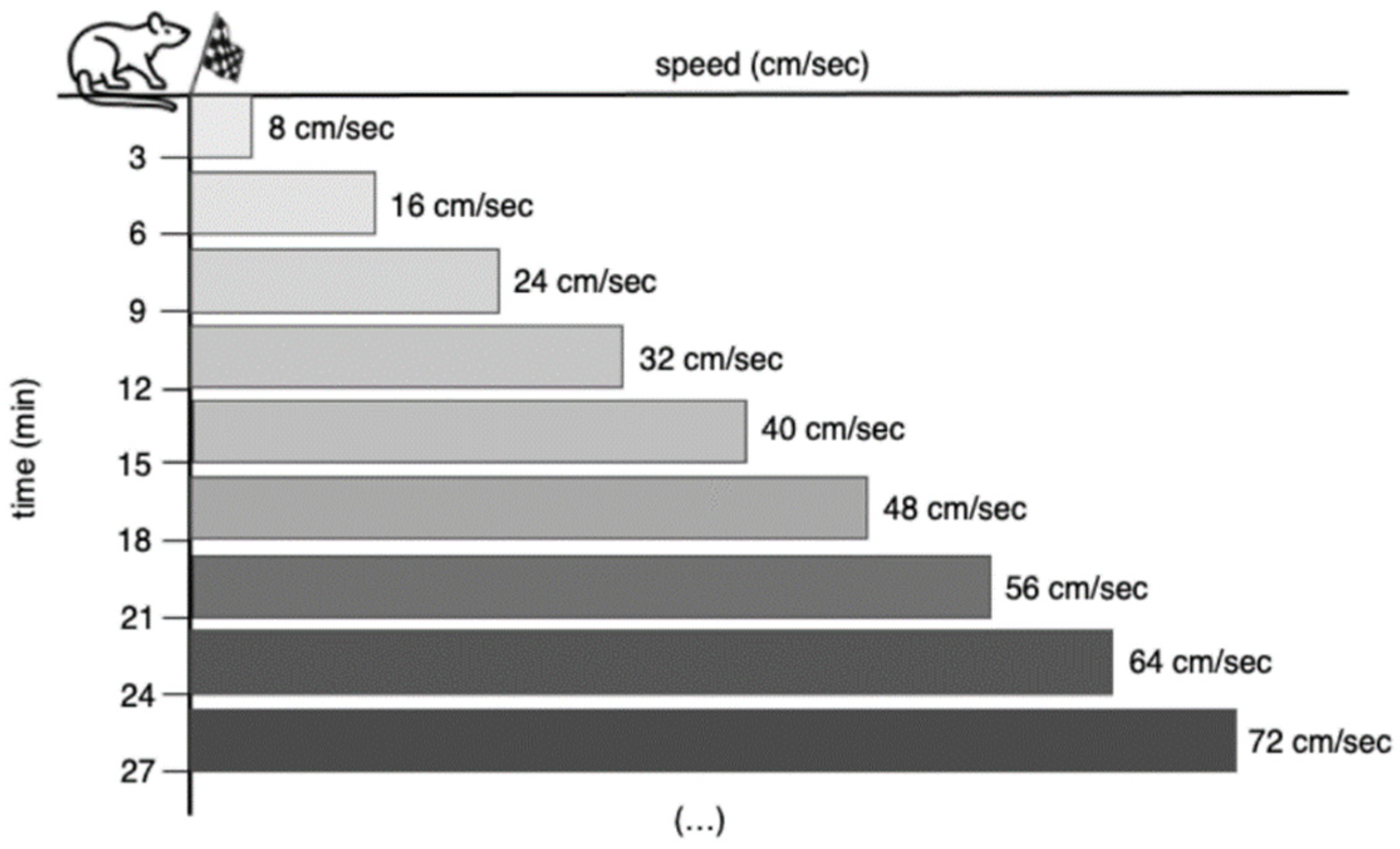







| Week | Belt Speed (cm/s) | Inclination (Degrees) | Total Time (min) |
|---|---|---|---|
| 1 | 25 | 0 | 5 |
| 2 | 27 | 0 | 15 |
| 3 | 30 | 0 | 30 |
| 4 | 32 | 0 | 45 |
| 5 | 34 | 0 | 60 |
| 6 | 34 | 0 | 60 |
| 7 | 34 | 0 | 60 |
| 8 | 34 | 0 | 60 |
| SED | C− | C+ | EX− | EX+ | |
|---|---|---|---|---|---|
| ALT (U/L) | 35.25 ± 8.77 | 53.8 ± 9.88 | 48 ± 7.07 | 38.6 ± 12.36 | 31 ± 4.74 |
| AST (U/L) | 282.75 ± 72.07 | 323.6 ± 75.49 | 249.8 ± 29.91 | 350 ± 89.20 | 263.8 ± 50.95 |
| Cholesterol (mg/dL) | 59.5 ± 5.74 | 54.4 ± 8.79 | 61.8 ± 7.40 | 56 ± 5.24 | 58.6 ± 6.80 |
| Creatinine (mg/dL) | 0.498 ± 0.03 | 0.456 ± 0.02 | 0.450 ± 0.03 | 0.486 ± 0.06 | 0.464 ± 0.07 |
| PCR (mg/dL) | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 |
| Glucose (mg/dL) | 290.5 ± 26.96 | 259 ± 54.11 | 267 ± 47.44 | 308.4 ± 59.50 | 297.4 ± 37.27 |
| Triglycerides (mg/dL) | 118.75 ± 45.21 | 125.8 ± 25.62 | 101.4 ± 39.21 | 122.2 ± 51.09 | 87.4 ± 34.90 |
| HDL (mg/dL) | 25.5 ± 1.29 | 24 ± 2.74 | 28 ± 2.45 | 25.2 ± 0.84 | 25.2 ± 2.86 |
| Urea (mg/dL) | 45.5 ± 1.91 | 48.6 ± 3.13 | 47.8 ± 5.85 | 45.8 ± 5.93 | 50.4 ± 5.59 |
| CK–MB (U/L) | 1560 ± 359.44 | 1794 ± 260.35 | 1670 ± 250.20 | 1606 ± 403.34 | 1208 ± 174.99 |
| LDH (U/L) | 2377.5 ± 450.95 | 2454.2 ± 410.96 | 2174.2 ± 185.66 | 2436.2 ± 681.21 | 1789.4 ± 236.22 |
| SED | C− | C+ | EX− | EX+ | |
|---|---|---|---|---|---|
| Myf5 | +/− | ++ | +++ | + | ++++ |
| MyoD | +/− | + | ++ | ++++ | +++ |
| PAX7 | +/− | +/− | +/− | +/− | +/− |
| Myogenin | +/− | + | +++ | ++ | ++++ |
| MYH1/2 | +/− | + | +++ | ++ | ++++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbadessa, G.; Maniscalco, E.; Grasso, L.; Popara, J.; Di Scipio, F.; Franco, F.; Mancardi, D.; Pigozzi, F.; Borrione, P.; Berta, G.N.; et al. Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury. Biomedicines 2023, 11, 2334. https://doi.org/10.3390/biomedicines11092334
Abbadessa G, Maniscalco E, Grasso L, Popara J, Di Scipio F, Franco F, Mancardi D, Pigozzi F, Borrione P, Berta GN, et al. Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury. Biomedicines. 2023; 11(9):2334. https://doi.org/10.3390/biomedicines11092334
Chicago/Turabian StyleAbbadessa, Giuliana, Eleonora Maniscalco, Loredana Grasso, Jasmin Popara, Federica Di Scipio, Francesco Franco, Daniele Mancardi, Fabio Pigozzi, Paolo Borrione, Giovanni Nicolao Berta, and et al. 2023. "Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury" Biomedicines 11, no. 9: 2334. https://doi.org/10.3390/biomedicines11092334






