Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation
Abstract
:1. Introduction
2. Underlying Disease Mechanisms of AFib
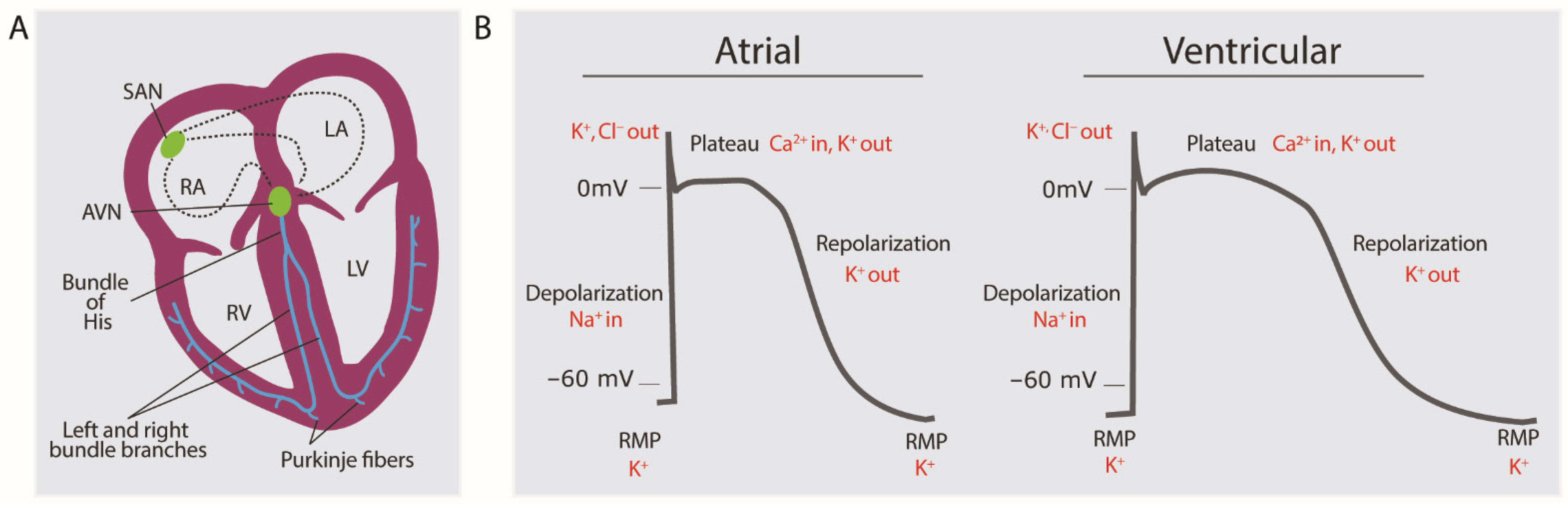
2.1. Brief Overview of Normal Electrophysiology of Cardiomyocytes
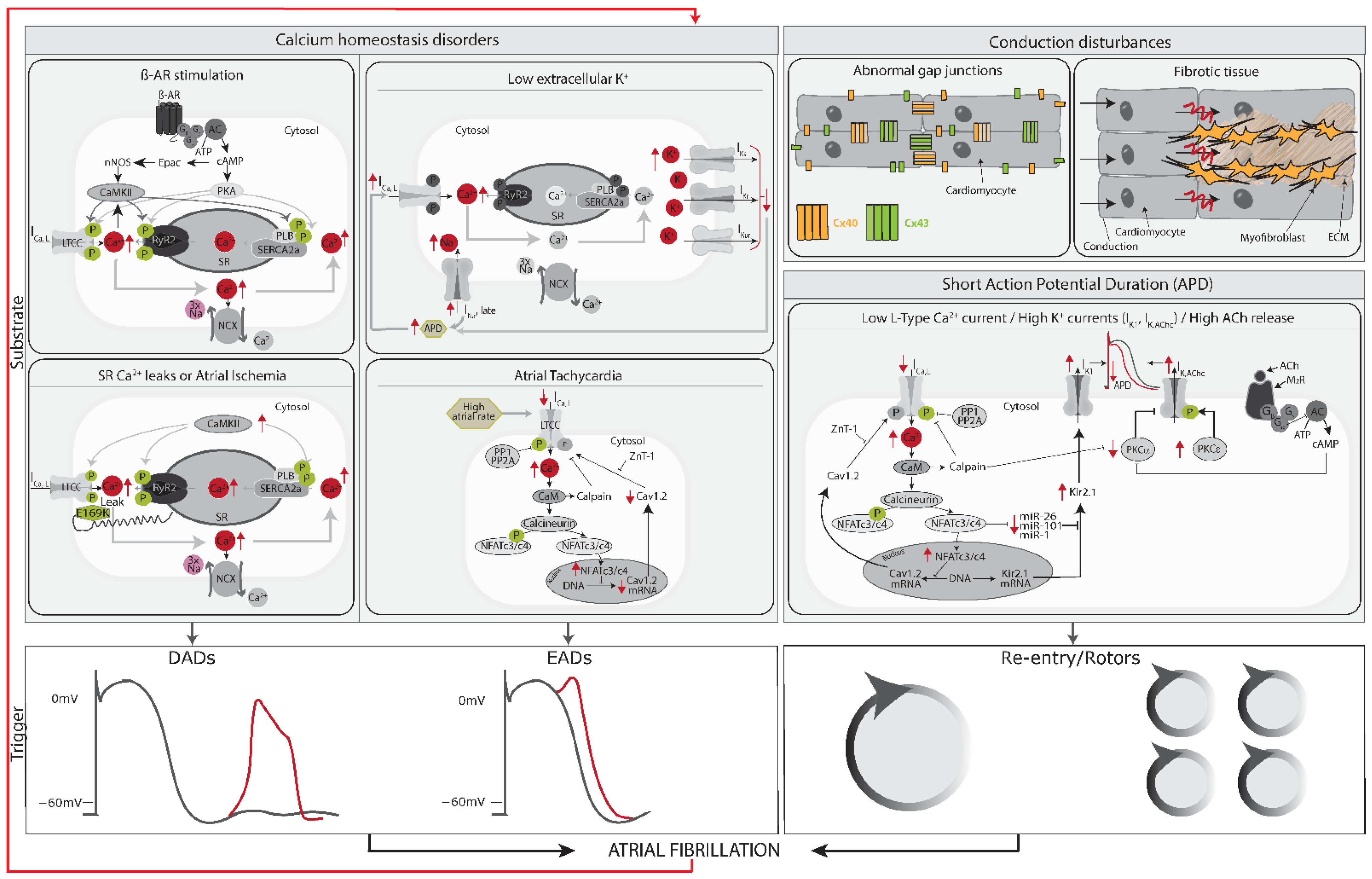
2.2. Trigger and Substrate Cause AFib
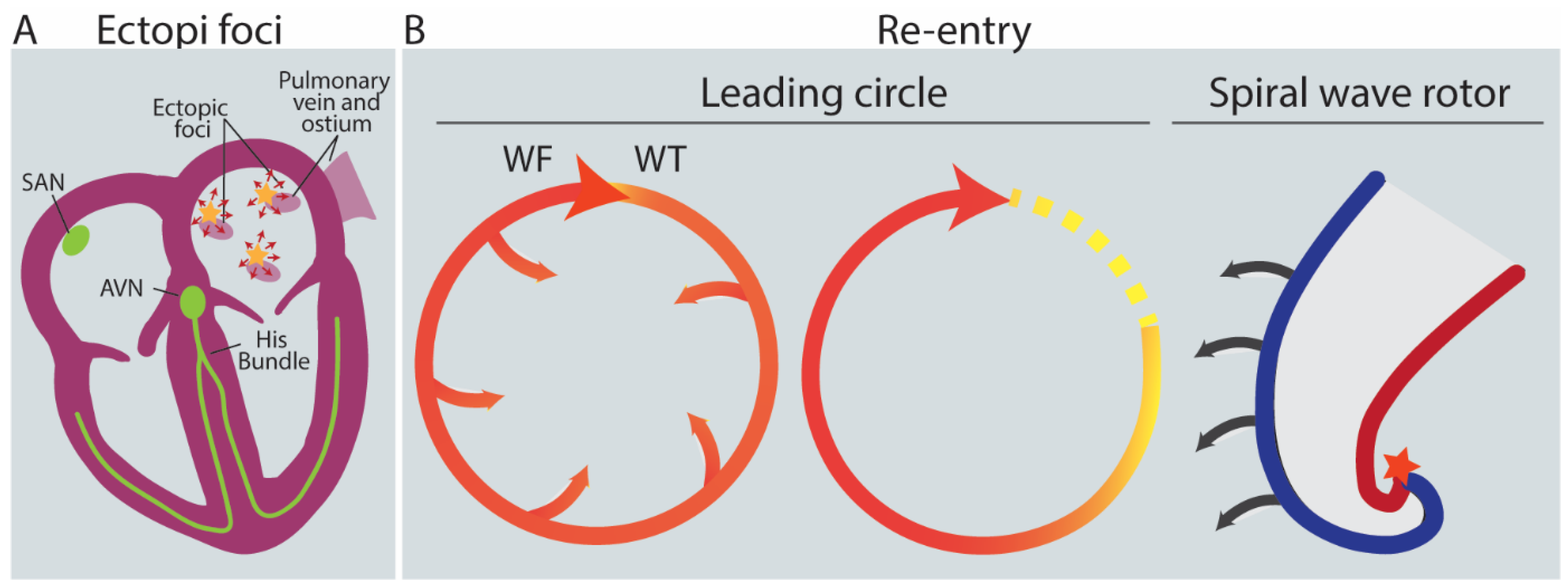
2.3. Triggers: AFib Is Induced by Ectopic Foci, Re-Entry and Rotors
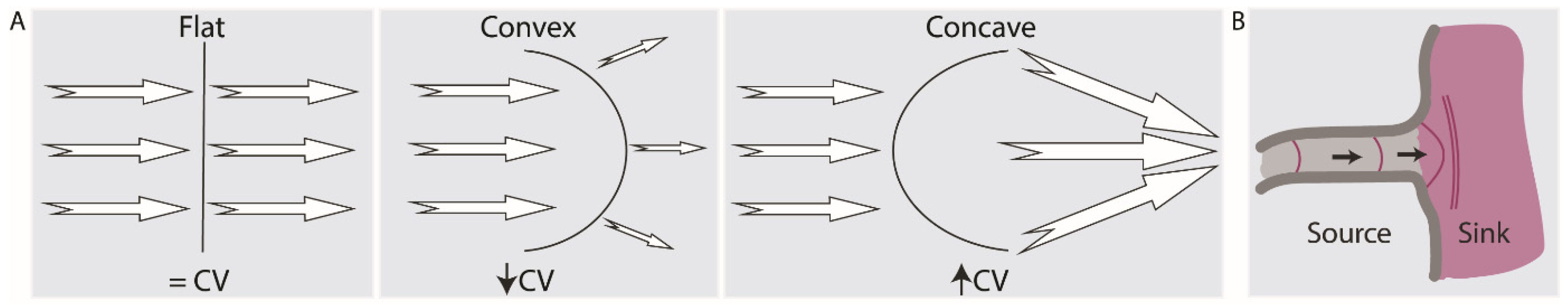
2.4. Current-to-Load (Source-Sink) Mismatches Affect Conduction Velocity
2.5. Substrate: AFib Is Maintained via Electrical, Structural, or Autonomic Remodeling of Atrial Tissue
2.6. AFib Begets AFib
3. Treatment of AFib
4. Models of AFib
4.1. In Vivo Modeling: Animal Models
| Animal Model | AFib Model | AFib Promotion | Clinical Causes of AFib |
|---|---|---|---|
| Dog [50,53,87,88] | Paroxysmal and Persistent AFib models | Electrical, structural and autonomic remodeling | Sterile pericarditis, atrial tachycardia remodeling, CHF-related AFib, acute atrial ischemia, atrial volume overload, mitral regurgitation, cesium infusion |
| Goat [53,88,93,95] | Paroxysmal and Persistent AFib models | Electrical and structural remodeling | Atrial tachycardia remodeling |
| Pig [53,88,89] | Paroxysmal and Persistent AFib models | Structural remodeling | Atrial tachycardia remodeling |
| Sheep [88,96,97] | Paroxysmal and Persistent AFib models | Structural and autonomic remodeling | Atrial volume overload, aortopulmonary shunt, atrial tachycardia remodeling |
| Rabbit [50,88] | Paroxysmal AFib model | Electrical, structural and autonomic remodeling | Atrial volume overload |
| Transgenic mice [88,98,99] | Atrial conduction abnormalities models | - | Dilated cardiomyopathy, hypertrophic cardiomyopathy, atrial pathology in CHF, atrial tachycardia remodeling |
4.2. Alternatives to Animal Models
4.2.1. In Silico Models
4.2.2. In Vitro Modeling Using hPSC-Derived CMs
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFib | Atrial fibrillation |
| Hpsc | Human pluripotent stem cells |
| NCX | Rheogenic Na+/Ca2+-exchanger |
| SERCA2a | Sarco/endoplasmic reticulum Ca2+-ATPase |
| RyR2 | Ryanodine receptor 2 |
| SR | Sarcoplasmic reticulum |
| EAD | Early afterdepolarizations |
| DAD | Diastolic delayed afterdepolarizations |
| APs | Action potentials |
| AADs | Anti-arrhythmic drugs |
| EHTs | Engineered Heart Tissues |
| ECM | extracellular matrix |
| IKUR | ultrarapid outward current I. |
| Kv1.5 | Channel responsible for IKUR. |
| KCNA5 | Gene coding for Kv1.5 |
| IKACh | Acetylcholine-activated inward rectifier potassium current |
| Kir3.1 | Inward-rectifier potassium ion channel mediating IKACh. Also called GIRK1. |
| Kir3.4 | Inward-rectifier potassium ion channel mediating IKACh. Also called GIRK4. |
| KCNJ3 | Gene coding for Kir3.1 |
| KCNJ5 | Gene coding for Kir3.4 |
| IKr | Delayed rectifier K+ current |
| Ito | Transient outward K+ current |
| INa | Late sodium current |
| Cav1.2 | subunit of L-type Ca2+ channels, mediating L-type calcium current (ICa,L) |
| IK1 | inward rectifying K+ current |
| KIR2 | channelprotein of IK1 current |
References
- WHO. Cardiovascular Diseases WHO, 2017. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 11 June 2020).
- Lip, G.Y.H.; Fauchier, L.; Freedman, S.B.; Van Gelder, I.; Natale, A.; Gianni, C.; Nattel, S.; Potpara, T.; Rienstra, M.; Tse, H.-F.; et al. Atrial fibrillation. Nat. Rev. Dis. Prim. 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.C.; Belew, K.; Beckman, K.; Vidaillet, H.; Kron, J.; Safford, R.; Mickel, M.; Barrell, P. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005, 149, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.J.; et al. Worldwide Epidemiology of Atrial Fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Padanilam, B.J.; Prystowsky, E.N. Epidemiology of Atrial Fibrillation The Rising Prevalence. In Atrial Fibrillation; Humana: Totowa, NJ, USA, 2008; pp. 3–11. [Google Scholar] [CrossRef]
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global epidemiology of atrial fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 4 July 2023).
- Li, H.; Song, X.; Liang, Y.; Bai, X.; Liu-Huo, W.S.; Tang, C.; Chen, W.; Zhao, L. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990–2019: Results from a global burden of disease study, 2019. BMC Public Health 2022, 22, 2015. [Google Scholar] [CrossRef]
- Christophersen, I.E.; Ellinor, P.T. Genetics of atrial fibrillation: From families to genomes. J. Hum. Genet. 2016, 61, 61–70. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.-J.; Bendahhou, S.; Wang, X.-L.; Wang, Y.; Xu, W.-Y.; Jin, H.; Sun, H.; Su, X.; Zhuang, Q.; et al. KCNQ1 Gain-of-Function Mutation in Familial Atrial Fibrillation. Science 2003, 299, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M.; Alekseev, A.E.; Liu, X.K.; Park, S.; Zingman, L.V.; Bienengraeber, M.; Sattiraju, S.; Ballew, J.D.; Jahangir, A.; Terzic, A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006, 15, 2185–2191. [Google Scholar] [CrossRef]
- Oldgren, J.; Healey, J.S.; Ezekowitz, M.; Commerford, P.; Avezum, A.; Pais, P.; Zhu, J.; Jansky, P.; Sigamani, A.; Morillo, C.A.; et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15 400 emergency department patients in 46 countries: The RE-LY atrial fibrillation registry. Circulation 2014, 129, 1568–1576. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef]
- Li, G.; Brumback, B.D.; Huang, L.; Zhang, D.M.; Yin, T.; Lipovsky, C.E.; Hicks, S.C.; Jimenez, J.; Boyle, P.M.; Rentschler, S.L. Acute Glycogen Synthase Kinase-3 Inhibition Modulates Human Cardiac Conduction. JACC Basic Transl. Sci. 2022, 7, 1001–1017. [Google Scholar] [CrossRef]
- Veldhuis, M.G. A Little Too Much: Cardiac Electrophysiological Effects of Elevated Inward Rectifying Current Carried by the K_(IR)2.1 Ion Channel Protein. Adapt. Med. 2015, 7, 1–8. [Google Scholar] [CrossRef]
- Makiyama, T.; Akao, M.; Shizuta, S.; Doi, T.; Nishiyama, K.; Oka, Y.; Ohno, S.; Nishio, Y.; Tsuji, K.; Itoh, H.; et al. A Novel SCN5A Gain-of-Function Mutation M1875T Associated With Familial Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 52, 1326–1334. [Google Scholar] [CrossRef]
- Roberts, J.D.; Gollob, M.H. A Contemporary Review on the Genetic Basis of Atrial Fibrillation. Methodist Debakey Cardiovasc. J. 2014, 10, 18–24. [Google Scholar] [CrossRef]
- Fatkin, D.; Santiago, C.F.; Huttner, I.G.; Lubitz, S.A.; Ellinor, P.T. Genetics of Atrial Fibrillation: State of the Art in 2017. Heart Lung Circ. 2017, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dong, J.; Ma, C. Is Atrial Fibrillation a Preventable Disease? J. Am. Coll. Cardiol. 2017, 69, 1968–1982. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef]
- Imazio, M.; Lazaros, G.; Picardi, E.; Vasileiou, P.; Orlando, F.; Carraro, M.; Tsiachris, D.; Vlachopoulos, C.; Georgiopoulos, G.; Tousoulis, D.; et al. Incidence and prognostic significance of new onset atrial fibrillation/flutter in acute pericarditis. Heart 2015, 101, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.E.; DiMarco, J.P. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012, 125, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-S.; Lin, C.-L. Risk of Atrial Fibrillation in Patients with Congenital Heart Disease: Results of a Propensity Score-Matched, Nationwide Cohort Study. J. Atheroscler. Thromb. 2019, 26, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Heist, E.K.; Ruskin, J.N. Atrial Fibrillation and Congestive Heart Failure: Risk Factors, Mechanisms, and Treatment. Prog. Cardiovasc. Dis. 2006, 48, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Barbisan, J.N.; Fuchs, F.D.; Schaan, B.D. Prevalence of thyroid dysfunction in patients with acute atrial fibrillation attended at a cardiology emergency room. Sao Paulo Med. J. 2003, 121, 159–162. [Google Scholar] [CrossRef]
- Knollmann, B.C.; Roden, D.M. A genetic framework for improving arrhythmia therapy. Nature 2008, 451, 929–936. [Google Scholar] [CrossRef]
- Wilhelms, M.; Hettmann, H.; Maleckar, M.M.; Koivumäki, J.T.; Dössel, O.; Seemann, G. Benchmarking electrophysiological models of human atrial myocytes. Front. Physiol. 2013, 3, 487. [Google Scholar] [CrossRef]
- Kaese, S.; Verheule, S. Cardiac electrophysiology in mice: A matter of size. Front. Physiol. 2012, 3, 345. [Google Scholar] [CrossRef]
- De Sousa Lopes, S.M.C.; Hassink, R.J.; Feijen, A.; Van Rooijen, M.A.; Doevendans, P.A.; Tertoolen, L.; De La Rivière, A.B.; Mummery, C.L. Patterning the heart, a template for human cardiomyocyte development. Dev. Dyn. 2006, 235, 1994–2002. [Google Scholar] [CrossRef]
- Zanella, F.; Lyon, R.C.; Sheikh, F. Modeling heart disease in a dish: From somatic cells to disease-relevant cardiomyocytes. Trends Cardiovasc. Med. 2014, 24, 32–44. [Google Scholar] [CrossRef]
- Anderson, R. The Gross Physiology of the Cardiovascular System; Racquet Press: Tucson, AZ, USA, 2008; ISBN 9780961752811. [Google Scholar]
- Dobrev, D.; Wehrens, X.H.T. Calcium-mediated cellular triggered activity in atrial fibrillation. J. Physiol. 2017, 595, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Priest, B.T.; McDermott, J.S. Cardiac ion channels. Channels 2015, 9, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 2002, 415, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Krummen, D.E.; Hebsur, S.; Salcedo, J.; Narayan, S.M.; Lalani, G.G.; Schricker, A.A. Mechanisms Underlying AF: Triggers, Rotors, Other? Curr. Treat. Options Cardiovasc. Med. 2015, 17, 14. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, S.A.; Chen, Y.C.; Yeh, H.I.; Chan, P.; Chang, M.S.; Lin, C.I. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation 2001, 104, 2849–2854. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced Sarcoplasmic Reticulum Ca2+ Leak and Increased Na+-Ca2+ Exchanger Function Underlie Delayed Afterdepolarizations in Patients With Chronic Atrial Fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Trausch, A.; Mintert-Jancke, E.; Pott, L.; Ravens, U.; Dobrev, D. Impaired Na+-dependent regulation of acetylcholine-activated inward-rectifier K+ current modulates action potential rate dependence in patients with chronic atrial fibrillation. J. Mol. Cell. Cardiol. 2013, 61, 142–152. [Google Scholar] [CrossRef]
- Burashnikov, A.; Antzelevitch, C. Reinduction of Atrial Fibrillation Immediately After Termination of the Arrhythmia Is Mediated by Late Phase 3 Early Afterdepolarization–Induced Triggered Activity. Circulation 2003, 107, 2355–2360. [Google Scholar] [CrossRef]
- Weiss, J.N.; Garfinkel, A.; Karagueuzian, H.S.; Chen, P.-S.; Qu, Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 2010, 7, 1891–1899. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Wakili, R.; Qi, X.Y.; Chartier, D.; Boknik, P.; Kääb, S.; Ravens, U.; Coutu, P.; Dobrev, D.; Nattel, S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ. Arrhythm. Electrophysiol. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and Molecular Mechanisms of Atrial Arrhythmogenesis in Patients With Paroxysmal Atrial Fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Beavers, D.L.; Wang, W.; Ather, S.; Voigt, N.; Garbino, A.; Dixit, S.S.; Landstrom, A.P.; Li, N.; Wang, Q.; Olivotto, I.; et al. Mutation E169K in Junctophilin-2 Causes Atrial Fibrillation Due to Impaired RyR2 Stabilization. J. Am. Coll. Cardiol. 2013, 62, 2010–2019. [Google Scholar] [CrossRef]
- Waks, J.W.; Josephson, M.E. Mechanisms of Atrial Fibrillation—Reentry, Rotors and Reality. Arrhythm. Electrophysiol. Rev. 2014, 3, 90. [Google Scholar] [CrossRef]
- Nattel, S.; Shiroshita-Takeshita, A.; Brundel, B.J.J.M.; Rivard, L. Mechanisms of Atrial Fibrillation: Lessons From Animal Models. Prog. Cardiovasc. Dis. 2005, 48, 9–28. [Google Scholar] [CrossRef]
- Cheniti, G.; Vlachos, K.; Pambrun, T.; Hooks, D.; Frontera, A.; Takigawa, M.; Bourier, F.; Kitamura, T.; Lam, A.; Martin, C.; et al. Atrial fibrillation mechanisms and implications for catheter ablation. Front. Physiol. 2018, 9, 1458. [Google Scholar] [CrossRef]
- Antoons, G.; Sipido, K.R. Targeting calcium handling in arrhythmias. Europace 2008, 10, 1364–1369. [Google Scholar] [CrossRef]
- Nishida, K.; Michael, G.; Dobrev, D.; Nattel, S. Animal models for atrial fibrillation: Clinical insights and scientific opportunities. EP Eur. 2010, 12, 160–172. [Google Scholar] [CrossRef]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef]
- Kato, T.; Iwasaki, Y.K.; Nattel, S. Connexins and atrial fibrillation: Filling in the gaps. Circulation 2012, 125, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.M. An Introduction to Cardiovascular Disease. In Social Support and Cardiovascular Disease; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Fast, V.G.; Kléber, A.G. Role of wavefront curvature in propagation of cardiac impulse. Cardiovasc. Res. 1997, 33, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, E.J.; Coromilas, J.; Wit, A.L.; Peters, N.S.; Garan, H. Source-Sink Mismatch Causing Functional Conduction Block in Re-Entrant Ventricular Tachycardia. JACC Clin. Electrophysiol. 2018, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Spector, P. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ. Arrhythm. Electrophysiol. 2013, 6, 655–661. [Google Scholar] [CrossRef]
- Rohr, S.; Kucera, J.P.; Fast, V.G.; Kleber, A.G. Paradoxical Improvement of Impulse Conduction in Cardiac Tissue by Partial Cellular Uncoupling. Science 1997, 275, 841–844. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent Advances and Translational Perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Qi, X.Y.; Yeh, Y.H.; Xiao, L.; Burstein, B.; Maguy, A.; Chartier, D.; Villeneuve, L.R.; Brundel, B.J.J.M.; Dobrev, D.; Nattel, S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ. Res. 2008, 103, 845–854. [Google Scholar] [CrossRef]
- Chi, X.; Chatterjee, P.K.; Wilson, W.; Zhang, S.-X.; Demayo, F.J.; Schwartz, R.J. Complex cardiac Nkx2-5 gene expression activated by noggin-sensitive enhancers followed by chamber-specific modules. Proc. Natl. Acad. Sci. USA 2005, 102, 13490–13495. [Google Scholar] [CrossRef]
- Qi, X.Y.; Diness, J.G.; Brundel, B.J.J.M.; Zhou, X.B.; Naud, P.; Wu, C.T.; Huang, H.; Harada, M.; Aflaki, M.; Dobrev, D.; et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 2014, 129, 430–440. [Google Scholar] [CrossRef]
- Özgen, N.; Dun, W.; Sosunov, E.A.; Anyukhovsky, E.P.; Hirose, M.; Duffy, H.S.; Boyden, P.A.; Rosen, M.R. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc. Res. 2007, 75, 758–769. [Google Scholar] [CrossRef]
- Igarashi, T.; Finet, J.E.; Takeuchi, A.; Fujino, Y.; Strom, M.; Greener, I.D.; Rosenbaum, D.S.; Donahue, J.K. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 2012, 125, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Pellman, J.; Lyon, R.C.; Sheikh, F. Extracellular matrix remodeling in atrial fibrosis: Mechanisms and implications in atrial fibrillation. J. Mol. Cell. Cardiol. 2010, 48, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.J.; Zhao, J.; Csepe, T.A.; Moore, B.T.; Li, N.; Jayne, L.A.; Kalyanasundaram, A.; Lim, P.; Bratasz, A.; Powell, K.A.; et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur. Heart J. 2015, 36, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, L.J.M.E.; Starreveld, R.; Kharbanda, R.K.; Knops, P.; Kik, C.; Bogers, A.J.J.C.; de Groot, N.M.S. Detection of Endo-epicardial Asynchrony in the Atrial Wall Using One-Sided Unipolar and Bipolar Electrograms. J. Cardiovasc. Transl. Res. 2021, 14, 902–911. [Google Scholar] [CrossRef]
- Van der Does, L.J.M.E.; Kik, C.; Bogers, A.J.J.C.; Allessie, M.A.; de Groot, N.M.S. Dynamics of Endo- and Epicardial Focal Fibrillation Waves at the Right Atrium in a Patient With Advanced Atrial Remodelling. Can. J. Cardiol. 2016, 32, 1260.e19–1260.e21. [Google Scholar] [CrossRef]
- Pellman, J.; Sheikh, F. Atrial fibrillation: Mechanisms, therapeutics, and future directions. Compr. Physiol. 2015, 5, 649–665. [Google Scholar] [CrossRef]
- Youn, J.Y.; Zhang, J.; Zhang, Y.; Chen, H.; Liu, D.; Ping, P.; Weiss, J.N.; Cai, H. Oxidative stress in atrial fibrillation: An emerging role of NADPH oxidase. J. Mol. Cell. Cardiol. 2013, 62, 72–79. [Google Scholar] [CrossRef]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Burashnikov, A.; Antzelevitch, C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE—Pacing Clin. Electrophysiol. 2006, 29, 290–295. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The Clinical Profile and Pathophysiology of Atrial Fibrillation. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Savelieva, I.; Lip, G.Y.H. Rate control in the medical management of atrial fibrillation. BMJ 2007, 93, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.P.; Bernard, M.L.; Madias, C.; Rogers, P.A.; Thihalolipavan, S.; Estes, N.A.M. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin. Proc. 2016, 91, 1778–1810. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef]
- Nattel, S. Experimental evidence for proarrhythmic mechanisms of antiarrhythmic drugs. Cardiovasc. Res. 1998, 37, 567–577. [Google Scholar] [CrossRef]
- Ravens, U.; Poulet, C.; Wettwer, E.; Knaut, M. Atrial selectivity of antiarrhythmic drugs. J. Physiol. 2013, 591, 4087–4097. [Google Scholar] [CrossRef]
- Milnes, J.T.; Madge, D.J.; Ford, J.W. New pharmacological approaches to atrial fibrillation. Drug Discov. Today 2012, 17, 654–659. [Google Scholar] [CrossRef]
- Savelieva, I.; Graydon, R.; Camm, A.J. Pharmacological cardioversion of atrial fibrillation with vernakalant: Evidence in support of the ESC Guidelines. Europace 2014, 16, 162–173. [Google Scholar] [CrossRef]
- Woods, C.E.; Olgin, J. AF Therapy Now and in the Future: Drugs, Biologicals, and Ablation. Circ. Res. 2014, 114, 1532–1546. [Google Scholar] [CrossRef]
- Nattel, S.; Bourne, G.; Talajic, M. Insights into mechanisms of antiarrhythmic drug action from experimental models of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1997, 8, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Melnyk, P.; Gaspo, R.; Wang, Z.; Nattel, S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ. Res. 1999, 84, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, D.; Bapat, A.; Kääb, S.; Lee, K.; Tomsits, P.; Clauss, S.; Hucker, W.J. Animal Models of Atrial Fibrillation. Circ. Res. 2020, 127, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; McDonald, A.D.; Donahue, J.K. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc. Res. 2004, 61, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, J.G.; Alfonso-Almazán, J.M.; Pérez-Castellano, N.; Pandit, S.V.; Jalife, J.; Pérez-Villacastín, J.; Filgueiras-Rama, D. Instantaneous Amplitude and Frequency Modulations Detect the Footprint of Rotational Activity and Reveal Stable Driver Regions as Targets for Persistent Atrial Fibrillation Ablation. Circ. Res. 2020, 125, 609–627. [Google Scholar] [CrossRef]
- Kishihara, J.; Niwano, S.; Niwano, H.; Aoyama, Y.; Satoh, A.; Oikawa, J.; Kiryu, M.; Fukaya, H.; Masaki, Y.; Tamaki, H.; et al. Effect of carvedilol on atrial remodeling in canine model of atrial fibrillation. Cardiovasc. Diagn. Ther. 2014, 4, 28–35. [Google Scholar] [CrossRef]
- Nakatani, Y.; Nishida, K.; Sakabe, M.; Kataoka, N.; Sakamoto, T.; Yamaguchi, Y.; Iwamoto, J.; Mizumaki, K.; Fujiki, A.; Inoue, H. Tranilast Prevents Atrial Remodeling and Development of Atrial Fibrillation in a Canine Model of Atrial Tachycardia and Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2013, 61, 582–588. [Google Scholar] [CrossRef]
- Remes, J.; van Brakel, T.J.; Bolotin, G.; Garber, C.; de Jong, M.M.; van der Veen, F.H.; Maessen, J.G. Persistent atrial fibrillation in a goat model of chronic left atrial overload. J. Thorac. Cardiovasc. Surg. 2008, 136, 1005–1011. [Google Scholar] [CrossRef]
- Nyns, E.C.A.; Poelma, R.H.; Volkers, L.; Plomp, J.J.; Bart, C.I.; Kip, A.M.; van Brakel, T.J.; Zeppenfeld, K.; Schalij, M.J.; Zhang, G.Q.; et al. An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation. Sci. Transl. Med. 2019, 11, eaau6447. [Google Scholar] [CrossRef]
- Ausma, J.; Van der Velden, H.M.W.; Lenders, M.H.; Van Ankeren, E.P.; Jongsma, H.J.; Ramaekers, F.C.S.; Borgers, M.; Allessie, M.A. Reverse Structural and Gap-Junctional Remodeling After Prolonged Atrial Fibrillation in the Goat. Circulation 2003, 107, 2051–2058. [Google Scholar] [CrossRef]
- Anné, W.; Willems, R.; Holemans, P.; Beckers, F.; Roskams, T.; Lenaerts, I.; Ector, H.; Heidbüchel, H. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J. Mol. Cell. Cardiol. 2007, 43, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, C.; Battaglia, E.; Young, R.; Suzuki, G. Lkb1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J. Am. Heart Assoc. 2015, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.U.; Lewin, G.; Baba, H.A.; Bokník, P.; Fabritz, L.; Kirchhefer, U.; Kirchhof, P.; Loser, K.; Matus, M.; Neumann, J.; et al. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J. Biol. Chem. 2005, 280, 6906–6914. [Google Scholar] [CrossRef]
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am. J. Physiol.-Heart Circ. Physiol. 1998, 275, H301–H321. [Google Scholar] [CrossRef]
- Nygren, A.; Fiset, C.; Firek, L.; Clark, J.W.; Lindblad, D.S.; Clark, R.B.; Giles, W.R. Mathematical model of an adult human atrial cell: The role of K+ currents in repolarization. Circ. Res. 1998, 82, 63–81. [Google Scholar] [CrossRef]
- Moe, G.K.; Rheinboldt, W.C.; Abildskov, J. A computer model of atrial fibrillation. Am. Heart J. 1964, 67, 200–220. [Google Scholar] [CrossRef]
- Majumder, R.; De Coster, T.; Kudryashova, N.; Verkerk, A.O.; Kazbanov, I.V.; Ördög, B.; Harlaar, N.; Wilders, R.; de Vries, A.A.F.; Ypey, D.L.; et al. Self-restoration of cardiac excitation rhythm by anti-arrhythmic ion channel gating. eLife 2020, 9, e55921. [Google Scholar] [CrossRef]
- Boyle, P.M.; Zahid, S.; Trayanova, N.A. Using personalized computer models to custom-tailor ablation procedures for atrial fibrillation patients: Are we there yet? Expert Rev. Cardiovasc. Ther. 2017, 15, 339–341. [Google Scholar] [CrossRef]
- Heijman, J.; Sutanto, H.; Crijns, H.J.G.M.; Nattel, S.; Trayanova, N.A. Computational models of atrial fibrillation: Achievements, challenges, and perspectives for improving clinical care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Protze, S.I.; Laksman, Z.; Backx, P.H.; Keller, G.M. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell 2017, 21, 179–194.e4. [Google Scholar] [CrossRef] [PubMed]
- Schwach, V.; Cofiño-Fabres, C.; ten Den, S.A.; Passier, R. Improved Atrial Differentiation of Human Pluripotent Stem Cells by Activation of Retinoic Acid Receptor Alpha (RARα). J. Pers. Med. 2022, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Laksman, Z.; Wauchop, M.; Lin, E.; Protze, S.; Lee, J.; Yang, W.; Izaddoustdar, F.; Shafaattalab, S.; Gepstein, L.; Tibbits, G.F.; et al. Modeling Atrial Fibrillation using Human Embryonic Stem Cell-Derived Atrial Tissue. Sci. Rep. 2017, 7, 5268. [Google Scholar] [CrossRef]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef]
- Liu, J.; Volkers, L.; Jangsangthong, W.; Bart, C.I.; Engels, M.C.; Zhou, G.; Schalij, M.J.; Ypey, D.L.; Pijnappels, D.A.; de Vries, A.A.F. Generation and primary characterization of iAM-1, a versatile new line of conditionally immortalized atrial myocytes with preserved cardiomyogenic differentiation capacity. Cardiovasc. Res. 2018, 114, 1848–1859. [Google Scholar] [CrossRef]
- Harlaar, N.; Dekker, S.O.; Zhang, J.; Snabel, R.R.; Veldkamp, M.W.; Verkerk, A.O.; Fabres, C.C.; Schwach, V.; Lerink, L.J.S.; Rivaud, M.R.; et al. Conditional immortalization of human atrial myocytes for the generation of in vitro models of atrial fibrillation. Nat. Biomed. Eng. 2022, 6, 389–402. [Google Scholar] [CrossRef]
- Van Gorp, P.R.R.; Trines, S.A.; Pijnappels, D.A.; de Vries, A.A.F. Multicellular In vitro Models of Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 43. [Google Scholar] [CrossRef]
- Hamers, J.; Sen, P.; Merkus, D.; Seidel, T.; Lu, K.; Dendorfer, A. Preparation of Human Myocardial Tissue for Long-Term Cultivation. J. Vis. Exp. 2022, 184, e63964. [Google Scholar] [CrossRef]
- Amesz, J.H.; Zhang, L.; Everts, B.R.; De Groot, N.M.S.; Taverne, Y.J.H.J. Living myocardial slices: Advancing arrhythmia research. Front. Physiol. 2023, 14, 17. [Google Scholar] [CrossRef]
- Meki, M.H.; Miller, J.M.; Mohamed, T.M.A. Heart Slices to Model Cardiac Physiology. Front. Pharmacol. 2021, 12, 617922. [Google Scholar] [CrossRef]
- Amesz, J.H.; de Groot, N.M.S.; Langmuur, S.J.J.; el Azzouzi, H.; Tiggeloven, V.P.C.; van Rooij, M.M.M.M.; Knops, P.; Bogers, A.J.J.C.; Taverne, Y.J.H.J. Biomimetic cultivation of atrial tissue slices as novel platform for in-vitro atrial arrhythmia studies. Sci. Rep. 2023, 13, 3648. [Google Scholar] [CrossRef]
- Kang, C.; Qiao, Y.; Li, G.; Baechle, K.; Camelliti, P.; Rentschler, S.; Efimov, I.R. Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials. Sci. Rep. 2016, 6, 28798. [Google Scholar] [CrossRef]
- Pertsov, A.M.; Davidenko, J.M.; Salomonsz, R.; Baxter, W.T.; Jalife, J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ. Res. 1993, 72, 631–650. [Google Scholar] [CrossRef]
- Watanabe, M.; Feola, I.; Majumder, R.; Jangsangthong, W.; Teplenin, A.S.; Ypey, D.L.; Schalij, M.J.; Zeppenfeld, K.; de Vries, A.A.F.; Pijnappels, D.A. Optogenetic manipulation of anatomical re-entry by light-guided generation of a reversible local conduction block. Cardiovasc. Res. 2017, 113, 354–366. [Google Scholar] [CrossRef]
- Devalla, H.D.; Passier, R. Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Sci. Transl. Med. 2018, 10, eaah5457. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, J.; Han, P.; Yuan, Q.; Zhang, J.; Zhang, X.; Xu, Y.; Cao, H.; Meng, Q.; Chen, L.; et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011, 21, 579–587. [Google Scholar] [CrossRef]
- Schwach, V.; Verkerk, A.O.; Mol, M.; Monshouwer-Kloots, J.J.; Devalla, H.D.; Orlova, V.V.; Anastassiadis, K.; Mummery, C.L.; Davis, R.P.; Passier, R. A COUP-TFII Human Embryonic Stem Cell Reporter Line to Identify and Select Atrial Cardiomyocytes. Stem Cell Rep. 2017, 9, 1765–1779. [Google Scholar] [CrossRef]
- Veerman, C.C.; Kosmidis, G.; Mummery, C.L.; Casini, S.; Verkerk, A.O.; Bellin, M. Immaturity of human stem-cell-derived cardiomyocytes in culture: Fatal flaw or soluble problem? Stem Cells Dev. 2015, 24, 1035–1052. [Google Scholar] [CrossRef]
- Nakanishi, H.; Lee, J.K.; Miwa, K.; Masuyama, K.; Yasutake, H.; Li, J.; Tomoyama, S.; Honda, Y.; Deguchi, J.; Tsujimoto, S.; et al. Geometrical patterning and constituent cell heterogeneity facilitate electrical conduction disturbances in a human induced pluripotent stem cell-based platform: An in vitro disease model of atrial arrhythmias. Front. Physiol. 2019, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.; Rivera-Arbeláez, J.M.; Cofiño-Fabres, C.; Schwach, V.; Slaats, R.H.; ten Den, S.A.; Vermeul, K.; van den Berg, A.; Pérez-Pomares, J.M.; Segerink, L.I.; et al. A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues. J. Pers. Med. 2022, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef]
- Lemoine, M.D.; Lemme, M.; Ulmer, B.M.; Braren, I.; Krasemann, S.; Hansen, A.; Kirchhof, P.; Meyer, C.; Eschenhagen, T.; Christ, T. Intermittent Optogenetic Tachypacing of Atrial Engineered Heart Tissue Induces Only Limited Electrical Remodelling. J. Cardiovasc. Pharmacol. 2020, 77, 291–299. [Google Scholar] [CrossRef]
- Lemme, M.; Braren, I.; Prondzynski, M.; Aksehirlioglu, B.; Ulmer, B.M.; Schulze, M.L.; Ismaili, D.; Meyer, C.; Hansen, A.; Christ, T.; et al. Chronic intermittent tachypacing by an optogenetic approach induces arrhythmia vulnerability in human engineered heart tissue. Cardiovasc. Res. 2019, 116, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Yi, B.A.; Ellinor, P.T. Genetics of Atrial Fibrillation. Heart Fail. Clin. 2010, 6, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Marczenke, M.; Piccini, I.; Mengarelli, I.; Fell, J.; Röpke, A.; Seebohm, G.; Verkerk, A.O.; Greber, B. Cardiac Subtype-Specific Modeling of Kv1.5 Ion Channel Deficiency Using Human Pluripotent Stem Cells. Front. Physiol. 2017, 8, 469. [Google Scholar] [CrossRef]
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef]
- Ravenscroft, S.M.; Pointon, A.; Williams, A.W.; Cross, M.J.; Sidaway, J.E. Cardiac Non-myocyte Cells Show Enhanced Pharmacological Function Suggestive of Contractile Maturity in Stem Cell Derived Cardiomyocyte Microtissues. Toxicol. Sci. 2016, 152, 99–112. [Google Scholar] [CrossRef]
- Vunjak Novakovic, G.; Eschenhagen, T.; Mummery, C. Myocardial tissue engineering: In vitro models. Cold Spring Harb. Perspect. Med. 2014, 4, a014076. [Google Scholar] [CrossRef]
- Heijman, J.; Algalarrondo, V.; Voigt, N.; Melka, J.; Wehrens, X.H.T.; Dobrev, D.; Nattel, S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: A critical analysis. Cardiovasc. Res. 2016, 109, 467–479. [Google Scholar] [CrossRef] [PubMed]


 In vivo models | 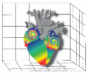 In silico models | 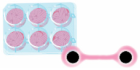 In vitro models | |
| PROS | |||
| CONS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofiño-Fabres, C.; Passier, R.; Schwach, V. Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines 2023, 11, 2355. https://doi.org/10.3390/biomedicines11092355
Cofiño-Fabres C, Passier R, Schwach V. Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines. 2023; 11(9):2355. https://doi.org/10.3390/biomedicines11092355
Chicago/Turabian StyleCofiño-Fabres, Carla, Robert Passier, and Verena Schwach. 2023. "Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation" Biomedicines 11, no. 9: 2355. https://doi.org/10.3390/biomedicines11092355
APA StyleCofiño-Fabres, C., Passier, R., & Schwach, V. (2023). Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines, 11(9), 2355. https://doi.org/10.3390/biomedicines11092355







