Distinct Cerebrospinal Fluid Lipid Signature in Patients with Subarachnoid Hemorrhage-Induced Hydrocephalus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Liquid Chromatography/Mass Spectrometry (LC-MS) Analysis
2.3. Bioinformatics and Statistical Analysis
3. Results
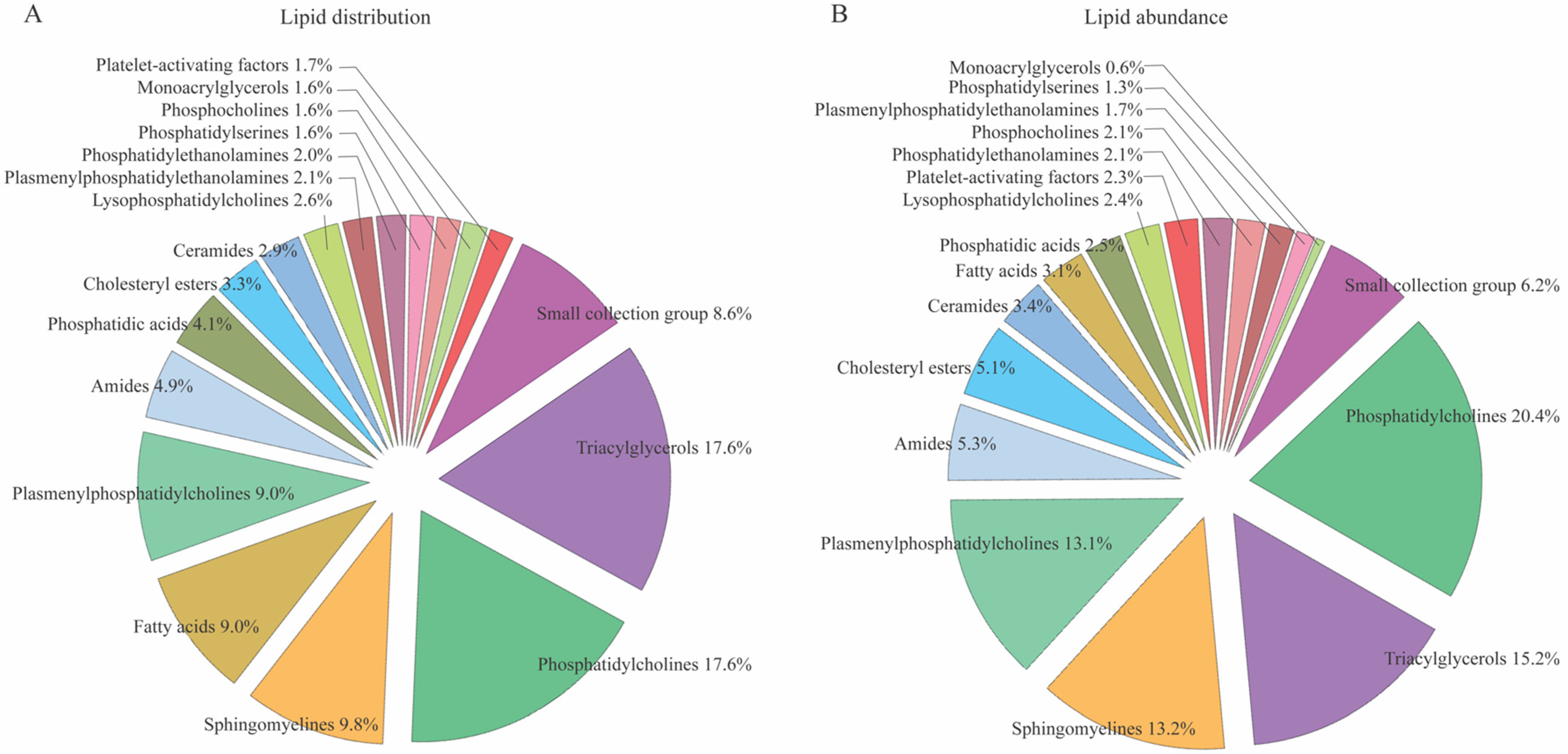
3.1. CSF Lipid Profiles in Control Subjects
3.2. No Age- and Sex-Dependent CSF Lipid Distribution in Control Subjects
3.3. CSF Lipid Profile in Patients with SAH
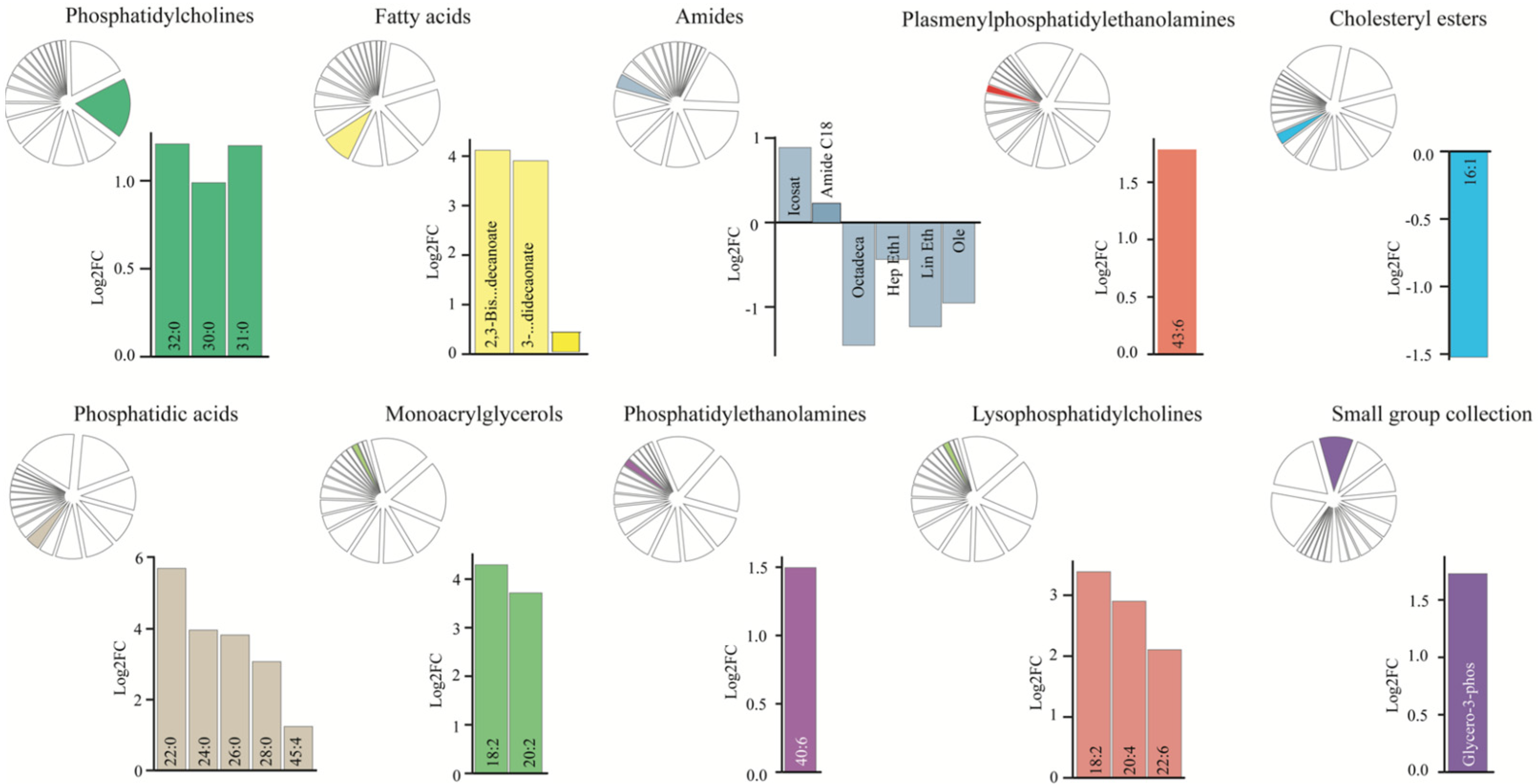
3.4. Dysregulated CSF Lipid Levels in Patients with SAH
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G.; European Stroke, O. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hanggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalik, P.A.; Ziai, W.C. Spontaneous Intraventricular Hemorrhage: When Should Intraventricular tPA Be Considered? Semin. Respir. Crit. Care Med. 2017, 38, 745–759. [Google Scholar] [CrossRef]
- Hokari, M.; Kuroda, S.; Yasuda, H.; Iwasaki, M.; Abe, S.; Saitoh, H. Ruptured aneurysms of the choroidal branches of the posterior inferior cerebellar artery: A review of the literature and a case report. Acta Neurochir. 2010, 152, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Naff, N.J.; Tuhrim, S. Intraventricular hemorrhage in adults: Complications and treatment. New Horiz. 1997, 5, 359–363. [Google Scholar] [PubMed]
- Chen, Q.; Feng, Z.; Tan, Q.; Guo, J.; Tang, J.; Tan, L.; Feng, H.; Chen, Z. Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. J. Neurol. Sci. 2017, 375, 220–230. [Google Scholar] [CrossRef]
- Garton, T.; Keep, R.F.; Wilkinson, D.A.; Strahle, J.M.; Hua, Y.; Garton, H.J.; Xi, G. Intraventricular Hemorrhage: The Role of Blood Components in Secondary Injury and Hydrocephalus. Transl. Stroke Res. 2016, 7, 447–451. [Google Scholar] [CrossRef]
- Cheng, C.; Wan, H.; Cong, P.; Huang, X.; Wu, T.; He, M.; Zhang, Q.; Xiong, L.; Tian, L. Targeting neuroinflammation as a preventive and therapeutic approach for perioperative neurocognitive disorders. J. Neuroinflamm. 2022, 19, 297. [Google Scholar] [CrossRef]
- Hill, A.; Shackelford, G.D.; Volpe, J.J. A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics 1984, 73, 19–21. [Google Scholar] [CrossRef]
- Klebe, D.; McBride, D.; Krafft, P.R.; Flores, J.J.; Tang, J.; Zhang, J.H. Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: Established mechanisms and proposed pathways. J. Neurosci. Res. 2020, 98, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Slavoaca, D.; Muresanu, D.; Birle, C.; Rosu, O.V.; Chirila, I.; Dobra, I.; Jemna, N.; Strilciuc, S.; Vos, P. Biomarkers in traumatic brain injury: New concepts. Neurol. Sci. 2020, 41, 2033–2044. [Google Scholar] [CrossRef]
- Martinez, B.I.; Stabenfeldt, S.E. Current trends in biomarker discovery and analysis tools for traumatic brain injury. J. Biol. Eng. 2019, 13, 16. [Google Scholar] [CrossRef]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Dorai, Z.; Hynan, L.S.; Kopitnik, T.A.; Samson, D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 2003, 52, 763–769; discussion 769–771. [Google Scholar] [CrossRef] [PubMed]
- Brisman, J.L.; Berenstein, A. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 2004, 54, 1031. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Alaraj, A.; Calderon, M.; Herrera, S.R.; Gao, W.; Ruland, S.; Roitberg, B.Z. Prediction of ventriculoperitoneal shunt dependency in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2009, 110, 44–49. [Google Scholar] [CrossRef]
- Rincon, F.; Gordon, E.; Starke, R.M.; Buitrago, M.M.; Fernandez, A.; Schmidt, J.M.; Claassen, J.; Wartenberg, K.E.; Frontera, J.; Seder, D.B.; et al. Predictors of long-term shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Clinical article. J. Neurosurg. 2010, 113, 774–780. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.D.; Day, S.P.; Kerr, M.; Nicoll, J.A.; Packard, C.J.; Caslake, M.J. Remodeling of cerebrospinal fluid lipoprotein particles after human traumatic brain injury. J. Neurotrauma 2003, 20, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Toft-Bertelsen, T.L.; Barbuskaite, D.; Heerfordt, E.K.; Lolansen, S.D.; Andreassen, S.N.; Rostgaard, N.; Olsen, M.H.; Norager, N.H.; Capion, T.; Rath, M.F.; et al. Lysophosphatidic acid as a CSF lipid in posthemorrhagic hydrocephalus that drives CSF accumulation via TRPV4-induced hyperactivation of NKCC1. Fluids Barriers CNS 2022, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Crack, P.J.; Zhang, M.; Morganti-Kossmann, M.C.; Morris, A.J.; Wojciak, J.M.; Fleming, J.K.; Karve, I.; Wright, D.; Sashindranath, M.; Goldshmit, Y.; et al. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflamm. 2014, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Pasvogel, A.E.; Miketova, P.; Moore, I.M. Cerebrospinal fluid phospholipid changes following traumatic brain injury. Biol. Res. Nurs. 2008, 10, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pasvogel, A.E.; Miketova, P.; Moore, I.M. Differences in CSF phospholipid concentration by traumatic brain injury outcome. Biol. Res. Nurs. 2010, 11, 325–331. [Google Scholar] [CrossRef]
- Croci, D.; Nevzati, E.; Muroi, C.; Schopf, S.; Hornemann, T.; Widmer, H.R.; Danura, H.; Fandino, J.; Marbacher, S. Changes in the cerebrospinal fluid lipid profile following subarachnoid hemorrhage in a closed cranium model: Correlations to cerebral vasospasm, neuronal cell death and Interleukin-6 synthesis. A pilot study. J. Stroke Cerebrovasc. Dis. 2020, 29, 105054. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, T.; Yanaka, K.; Nagase, S.; Fujita, K.; Kato, N.; Nose, T. Increased levels of lipid peroxides as predictive of symptomatic vasospasm and poor outcome after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2002, 97, 1302–1305. [Google Scholar] [CrossRef]
- Testai, F.D.; Hillmann, M.; Amin-Hanjani, S.; Gorshkova, I.; Berdyshev, E.; Gorelick, P.B.; Dawson, G. Changes in the cerebrospinal fluid ceramide profile after subarachnoid hemorrhage. Stroke 2012, 43, 2066–2070. [Google Scholar] [CrossRef]
- Testai, F.D.; Xu, H.L.; Kilkus, J.; Suryadevara, V.; Gorshkova, I.; Berdyshev, E.; Pelligrino, D.A.; Dawson, G. Changes in the metabolism of sphingolipids after subarachnoid hemorrhage. J. Neurosci. Res. 2015, 93, 796–805. [Google Scholar] [CrossRef]
- Rostgaard, N.; Olsen, M.H.; Capion, T.; MacAulay, N.; Juhler, M. Inflammatory Markers as Predictors of Shunt Dependency and Functional Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage. Biomedicines 2023, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Marcoz, P.; Nemoz, G.; Prigent, A.F.; Lagarde, M. Phosphatidic acid stimulates the rolipram-sensitive cyclic nucleotide phosphodiesterase from rat thymocytes. Biochem. Biophys. Acta 1993, 1176, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Slomka, A.; Korbal, P.; Piekus, N.; Zekanowska, E. The use of cluster and principal component analysis in the estimation of iron status in term newborns. J. Matern.-Fetal Neonatal. Med. 2013, 26, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Thissen, D.; Steinberg, L.; Kuang, D. Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. J. Educ. Behav. Stat. 2002, 27, 77–83. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 2018, 57, 289–300. [Google Scholar] [CrossRef]
- Saito, K.; Hattori, K.; Hidese, S.; Sasayama, D.; Miyakawa, T.; Matsumura, R.; Tatsumi, M.; Yokota, Y.; Ota, M.; Hori, H.; et al. Profiling of Cerebrospinal Fluid Lipids and Their Relationship with Plasma Lipids in Healthy Humans. Metabolites 2021, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, H.; Wang, Z.; Wang, Y.; Gao, A.; Cui, Y.; Liu, Y.; Chen, G. Evidence for the role of phosphatidylcholine-specific phospholipase in experimental subarachnoid hemorrhage in rats. Exp. Neurol. 2015, 272, 145–151. [Google Scholar] [CrossRef]
- Kuroki, M.; Kanamaru, K.; Suzuki, H.; Waga, S.; Semba, R. Effect of vasospasm on heme oxygenases in a rat model of subarachnoid hemorrhage. Stroke 1998, 29, 683–688; Discussion 688–689. [Google Scholar] [CrossRef]
- Jahromi, B.S.; Komuro, T.; Macdonald, R.L.; Marton, L.S.; Weir, B.K. Phosphatidylcholine peroxidized by hemoglobin increases intracellular calcium in dog basilar artery smooth muscle cells. Acta Neuro. Suppl. 2001, 77, 45–47. [Google Scholar] [CrossRef]
- Innis, S.M. Fatty acids and early human development. Early Hum. Dev. 2007, 83, 761–766. [Google Scholar] [CrossRef]
- Pilitsis, J.G.; Coplin, W.M.; O’Regan, M.H.; Wellwood, J.M.; Diaz, F.G.; Fairfax, M.R.; Michael, D.B.; Phillis, J.W. Free fatty acids in human cerebrospinal fluid following subarachnoid hemorrhage and their potential role in vasospasm: A preliminary observation. J. Neurosurg. 2002, 97, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Purdon, A.D.; Rosenberger, T.A.; Shetty, H.U.; Rapoport, S.I. Energy consumption by phospholipid metabolism in mammalian brain. Neurochem. Res. 2002, 27, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Glycerophospholipids in brain: Their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Barnette, B.L.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatr. 2015, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Yamada, T.; Asada, T.; Tsuboi, Y.; Wakana, C.; Mawatari, S.; Kono, S. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. eBioMedicine 2017, 17, 199–205. [Google Scholar] [CrossRef]
- Ogawa, S.; Hattori, K.; Ota, M.; Hidese, S.; Miyakawa, T.; Matsumura, R.; Yokota, Y.; Ishida, I.; Matsuo, J.; Yoshida, S.; et al. Altered ethanolamine plasmalogen and phosphatidylethanolamine levels in blood plasma of patients with bipolar disorder. Psychiatry Clin. Neurosci. 2020, 74, 204–210. [Google Scholar] [CrossRef]
- Cappa, M.; Bizzarri, C.; Petroni, A.; Carta, G.; Cordeddu, L.; Valeriani, M.; Vollono, C.; De Pasquale, L.; Blasevich, M.; Banni, S. A mixture of oleic, erucic and conjugated linoleic acids modulates cerebrospinal fluid inflammatory markers and improve somatosensorial evoked potential in X-linked adrenoleukodystrophy female carriers. J. Inherit. Metab. Dis. 2012, 35, 899–907. [Google Scholar] [CrossRef]
- Gauster, M.; Rechberger, G.; Sovic, A.; Horl, G.; Steyrer, E.; Sattler, W.; Frank, S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 2005, 46, 1517–1525. [Google Scholar] [CrossRef]
- Dullaart, R.P.; Gansevoort, R.T.; Dikkeschei, B.D.; de Zeeuw, D.; de Jong, P.E.; Van Tol, A. Role of elevated lecithin: Cholesterol acyltransferase and cholesteryl ester transfer protein activities in abnormal lipoproteins from proteinuric patients. Kidney Int. 1993, 44, 91–97. [Google Scholar] [CrossRef]
- Tzekov, R.; Dawson, C.; Orlando, M.; Mouzon, B.; Reed, J.; Evans, J.; Crynen, G.; Mullan, M.; Crawford, F. Sub-Chronic Neuropathological and Biochemical Changes in Mouse Visual System after Repetitive Mild Traumatic Brain Injury. PLoS ONE 2016, 11, e0153608. [Google Scholar] [CrossRef] [PubMed]
- Lummis, N.C.; Sanchez-Pavon, P.; Kennedy, G.; Frantz, A.J.; Kihara, Y.; Blaho, V.A.; Chun, J. LPA(1/3) overactivation induces neonatal posthemorrhagic hydrocephalus through ependymal loss and ciliary dysfunction. Sci. Adv. 2019, 5, eaax2011. [Google Scholar] [CrossRef]
- Yung, Y.C.; Mutoh, T.; Lin, M.E.; Noguchi, K.; Rivera, R.R.; Choi, J.W.; Kingsbury, M.A.; Chun, J. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med. 2011, 3, 99ra87. [Google Scholar] [CrossRef]
- Benitez-Angeles, M.; Romero, A.E.L.; Llorente, I.; Hernandez-Araiza, I.; Vergara-Jaque, A.; Real, F.H.; Gutierrez Castaneda, O.E.; Arciniega, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F.; et al. Modes of action of lysophospholipids as endogenous activators of the TRPV4 ion channel. J. Physiol. 2023, 601, 1655–1673. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, A.B.; Oernbo, E.K.; Stoica, A.; Gerkau, N.J.; Barbuskaite, D.; Tritsaris, K.; Rose, C.R.; MacAulay, N. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 2018, 9, 2167. [Google Scholar] [CrossRef] [PubMed]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef]



| Study Cohort | SAH | Control (Unruptured Aneurysm) | Statistics |
|---|---|---|---|
| n | 13 | 11 | |
| Age (years), median (range) | 62 (44–71) | 64 (40–77) | p = 0.66 |
| Sex (F/M) | 7F/6M | 7F/4M | |
| BMI (kg/m2), median (range) | 26 (21.8–32.1) | 25 (18.9–35.6) | p = 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toft-Bertelsen, T.L.; Andreassen, S.N.; Rostgaard, N.; Olsen, M.H.; Norager, N.H.; Capion, T.; Juhler, M.; MacAulay, N. Distinct Cerebrospinal Fluid Lipid Signature in Patients with Subarachnoid Hemorrhage-Induced Hydrocephalus. Biomedicines 2023, 11, 2360. https://doi.org/10.3390/biomedicines11092360
Toft-Bertelsen TL, Andreassen SN, Rostgaard N, Olsen MH, Norager NH, Capion T, Juhler M, MacAulay N. Distinct Cerebrospinal Fluid Lipid Signature in Patients with Subarachnoid Hemorrhage-Induced Hydrocephalus. Biomedicines. 2023; 11(9):2360. https://doi.org/10.3390/biomedicines11092360
Chicago/Turabian StyleToft-Bertelsen, Trine L., Søren Norge Andreassen, Nina Rostgaard, Markus Harboe Olsen, Nicolas H. Norager, Tenna Capion, Marianne Juhler, and Nanna MacAulay. 2023. "Distinct Cerebrospinal Fluid Lipid Signature in Patients with Subarachnoid Hemorrhage-Induced Hydrocephalus" Biomedicines 11, no. 9: 2360. https://doi.org/10.3390/biomedicines11092360






