Identification of CD44 as a Reliable Biomarker for Glioblastoma Invasion: Based on Magnetic Resonance Imaging and Spectroscopic Analysis of 5-Aminolevulinic Acid Fluorescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Imaging Studies and Analysis
2.3. Image-Guided Navigation Surgery and Selective Tissue Sampling
2.4. Immunohistochemistry
2.5. RNA Isolation and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Quantitative Evaluation of 5-ALA Fluorescence Intensity by Spectroscopic Analysis
2.7. Statistical Methods
3. Results
3.1. Patient Characteristics
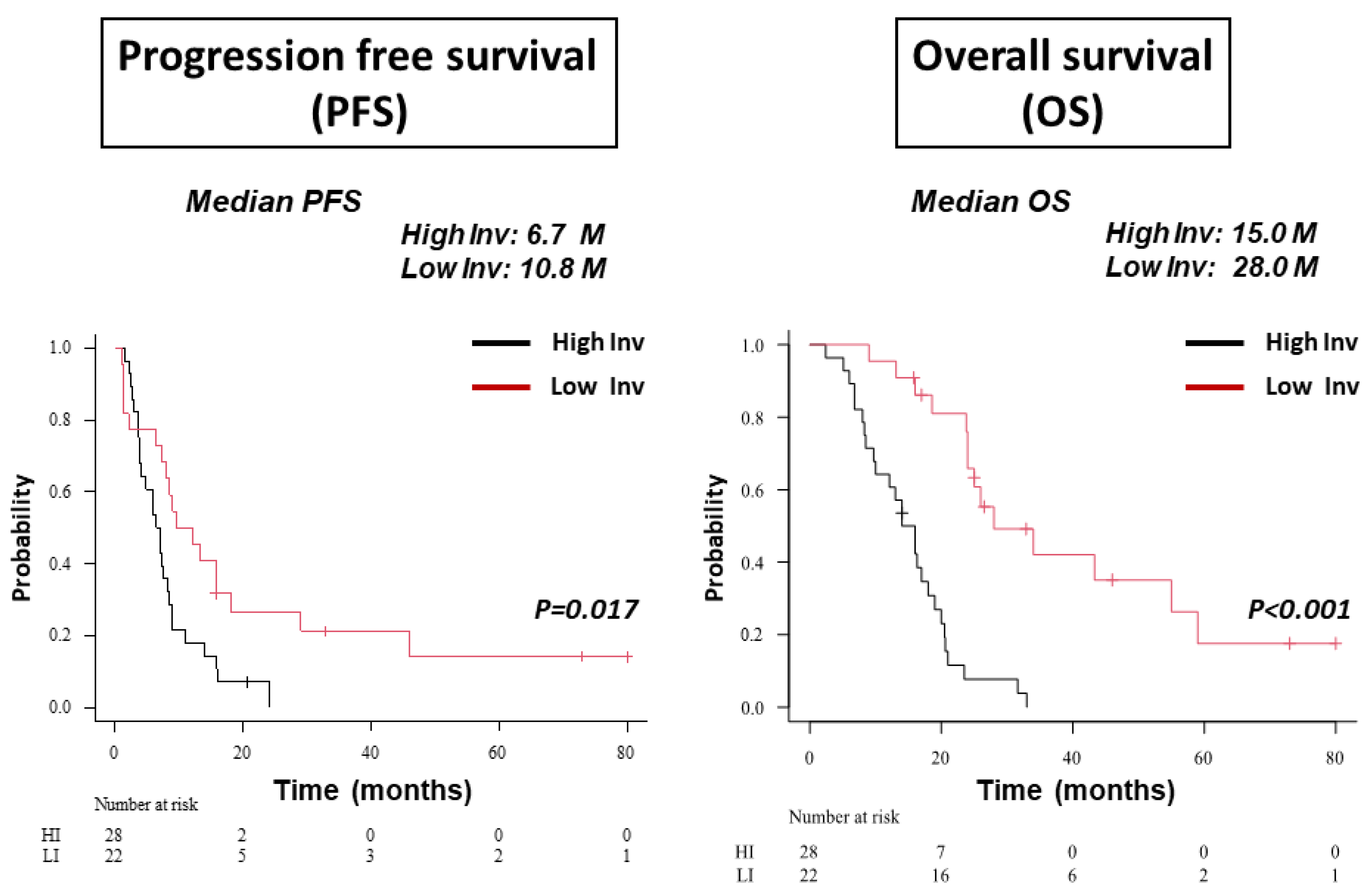
3.2. Classification of GBM Patients to HI and LI Types on MRI
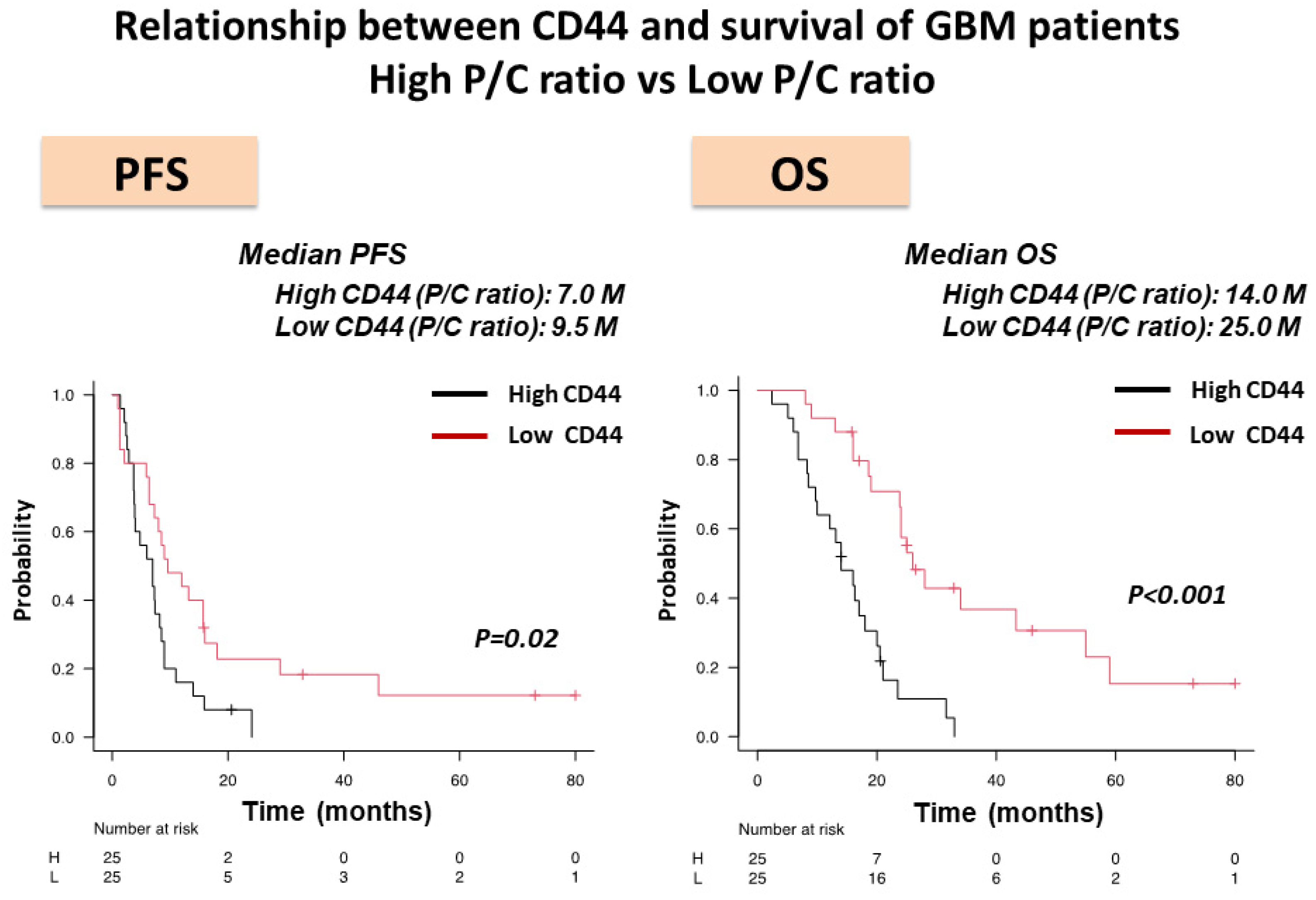
3.3. Expression of CD44 mRNA in the Core and Periphery of GBM and CD44 Expression as Evaluated by P/C ratio in HI- and LI-Type GBM
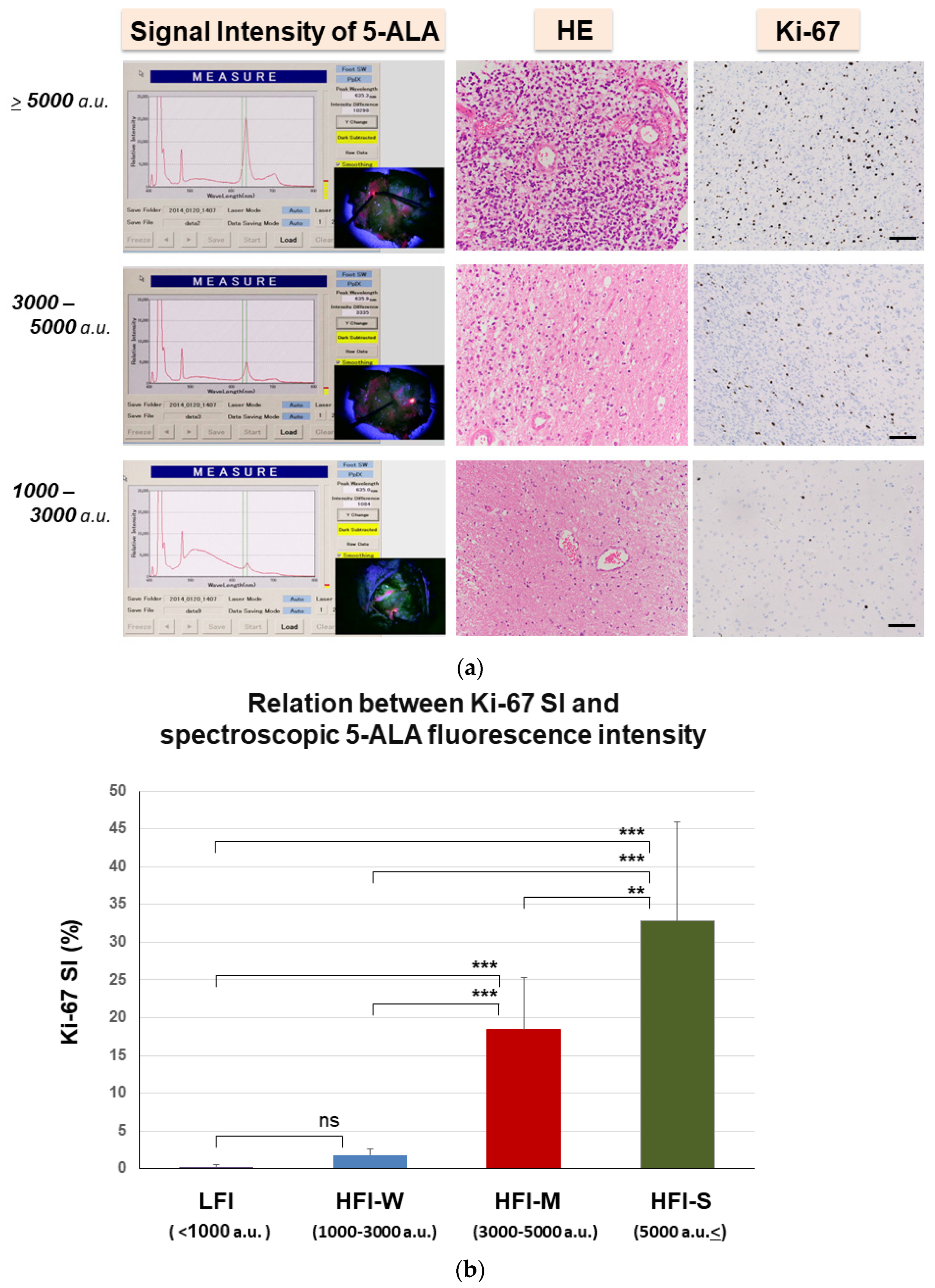
3.4. Quantitative Analysis of Spectroscopic 5-ALA Fluorescence Intensity in the Invasion Area
3.5. Evaluation of Invasiveness of GBM by Spectroscopic Analysis of 5-ALA Fluorescence Intensity
3.6. Relationship between P/C Ratio of CD44 Expression and Spectroscopic Signal Intensity of 5-ALA Fluorescence in the Tumor Periphery of GBM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Munthe, S.; Petterson, S.A.; Dahlrot, R.H.; Poulsen, F.R.; Hansen, S.; Kristensen, B.W. Glioma cells in the tumor periphery have a stem cell phenotype. PLoS ONE 2016, 11, e0155106. [Google Scholar]
- Angelucci, C.; D’Alessio, A.; Lama, G.; Binda, E.; Mangiola, A.; Vescovi, A.L.; Proietti, G.; Masuelli, L.; Bei, R.; Fazi, B.; et al. Cancer stem cells from peritumoral tissue of glioblastoma multiforme: The possible missing link between tumor development and progression. Oncotarget 2018, 9, 28116–28130. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ohnishi, T.; Kohno, S.; Ohue, S.; Nishikawa, M.; Suehiro, S.; Matsumoto, S.; Ozaki, S.; Fukushima, M.; Kurata, M.; et al. Met-PET uptake index for total tumor resection: Identification of 11C-methionine uptake index as a goal for total tumor resection including infiltrating tumor cells in glioblastoma. Neurosurg. Rev. 2021, 44, 587–597. [Google Scholar] [CrossRef]
- Shimizu, K.; Tamura, K.; Hara, S.; Inaji, M.; Tanaka, Y.; Kobayashi, D.; Sugawara, T.; Wakimoto, H.; Nariai, T.; Ishii, K.; et al. Correlation of Intraoperative 5-ALA-Induced Fluorescence Intensity and Preoperative 11C-Methionine PET Uptake in Glioma Surgery. Cancers 2022, 14, 1449. [Google Scholar] [CrossRef]
- Nishikawa, M.; Inoue, A.; Ohnishi, T.; Kohno, S.; Ohue, S.; Matsumoto, S.; Suehiro, S.; Yamashita, D.; Ozaki, S.; Watanabe, H.; et al. Significance of glioma stem-like cells in the tumor periphery that express high levels of CD44 in tumor invasion, early progression, and poor prognosis in glioblastoma. Stem Cells Int. 2018, 23, 5387041. [Google Scholar] [CrossRef]
- Nishikawa, M.; Inoue, A.; Ohnishi, T.; Yano, H.; Ozaki, S.; Kanemura, Y.; Suehiro, S.; Ohtsuka, Y.; Kohno, S.; Ohue, S.; et al. hypoxia-induced phenotypic transition from highly invasive to less invasive tumors in glioma stem-like cells: Significance of CD44 and osteopontin as therapeutic targets in glioblastoma. Trans. Oncol. 2021, 14, 101137. [Google Scholar] [CrossRef]
- Jijiwa, M.; Demir, H.; Gupta, S.; Leung, C.; Joshi, K.; Orozco, N.; Huang, T.; Yildiz, V.O.; Shibahara, I.; de Jesus, J.A.; et al. CD44v6 regulates growth of brain tumor stem cells partially through the AKT-mediated pathway. PLoS ONE 2011, 6, e242217. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Kamad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar]
- Hassn, M.M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A multifunctional mediator of cancer progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Ewelt, C.; Nemes, A.; Senner, V.; Wolfer, J.; Brokinkel, B.; Stummer, W.; Holling, M. Fluorescence in neurosurgery: Its diagnostic and therapeutic use. Review of the literature. J. Photochem. Photobiol. B 2015, 148, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Ilic, I.; Potthoff, A.L.; Hamed, M.; Schafer, N.; Velten, M.; Guresir, E.; Herrlinger, U.; Borger, V.; Vatter, H.; et al. Safety metric profiling in surgery for temporal glioblastoma: Lobectomy as a supra-total resection regime preserves perioperative standard quality rates. J. Neuro-Oncol. 2020, 149, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kim, S.Y.; Na, Y.C.; Cho, J.M. Supratotal resection of glioblastoma: Better survival outcome than gross total resection. J. Pers. Med. 2023, 13, 383. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Ferraro, N.; Barbarite, E.; Albert, T.R.; Berchmans, E.; Shah, A.H.; Bregy, A.; Ivan, M.E.; Brown, T.; Komotar, R.J. The role of 5-aminolevulinic acid in brain tumor surgery: A systematic review. Neurosurg. Rev. 2016, 39, 545–555. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ala fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Ohue, S.; Kohno, S.; Inoue, A.; Yamashita, D.; Matsumoto, S.; Suehiro, S.; Kumon, Y.; Kikuchi, K.; Ohnishi, T. Surgical results of tumor resection using tractography-integrated navigation-guided fence-post catheter techniques and motor-evoked potentials for preservation of motor function in patients with glioblastomas near the pyramidal tracts. Neurosurg. Rev. 2015, 38, 293–306. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Okita, Y.; Nonaka, M.; Shofuda, T.; Kanematsu, D.; Yoshioka, E.; Kodama, Y.; Mano, M.; Nakajima, S.; Kanemura, Y. (11)C-methinine uptake correlates with MGMT promoter methylation in nonenhancing gliomas. Clin. Neurol. Neurosurg. 2014, 125, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Mooney, K.L.; Choy, W.; Sidhu, S.; Pelargos, P.; Bui, T.T.; Voth, B.; Barnette, N.; Yang, I. The role of CD44 in glioblastoma multiforme. J. Clin. Neurosci. 2016, 34, 1–5. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hemandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef]
- Chen, J.E.; Wu, Q.; Guryanova, O.A.; Huang, Z.; Hung, Q.; Ricch, J.N. Elevated invasive potential of glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2011, 406, 643–648. [Google Scholar] [CrossRef]
- Miwa, K.; Shinoda, J.; Yano, H.; Okumura, A.; Iwama, T.; Nakashima, T.; Sakai, N. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: Insight from a PET and MR fusion image study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1457–1462. [Google Scholar] [CrossRef]
- Kracht, L.W.; Miletic, H.; Busch, S.; Jacobs, A.H.; Voges, J.; Hoevels, M.; Klein, J.C.; Herholz, K.; Heiss, W.D. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: Local comparison with stereotactic histopathology. Clin. Cancer Res. 2004, 10, 7163–7170. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.T., Jr.; Serrano, N.; Kim, H.; Li, X.; Snyder, S.E.; Hwang, S.; Li, Y.; Hua, C.H.; Broniscer, A.; Merchant, T.E.; et al. 11C-Methionine positron emission tomography delineates non-contrast enhancing tumor regions at high risk for recurrence in pediatric high-grade glioma. J. Neurooncol. 2017, 132, 163–170. [Google Scholar] [CrossRef]
- Mirza, A.B.; Christodoulides, I.; Lavradort, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Noman, I.; Murphy, C.; Devi, S.; et al. 5-aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma-a comparative cohort study of 343 patients. Neuro-Oncol. Adv. 2021, 3, 1–11. [Google Scholar]
- Taniguchi, D.; Saeki, H.; Nakashima, Y.; Kudou, K.; Nakanishi, R.; Kubo, N.; Ando, K.; Oki, E.; Oda, Y.; Maehara, Y. CD44v9 is associated with epithelial-mesenchymal transition and poor outcomes in esophageal squamous cell carcinoma. Cancer Med. 2018, 7, 6258–6268. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef] [PubMed]
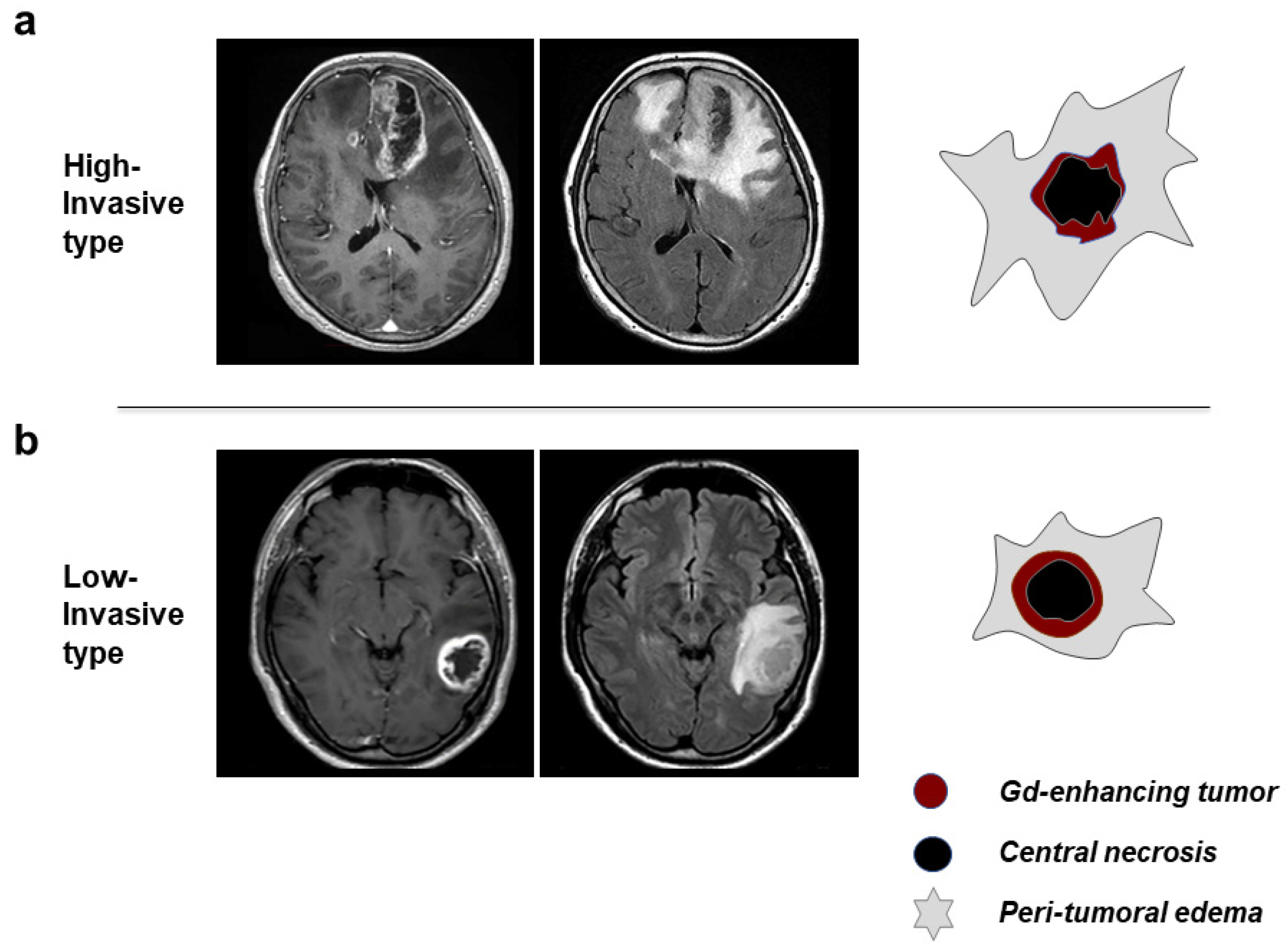

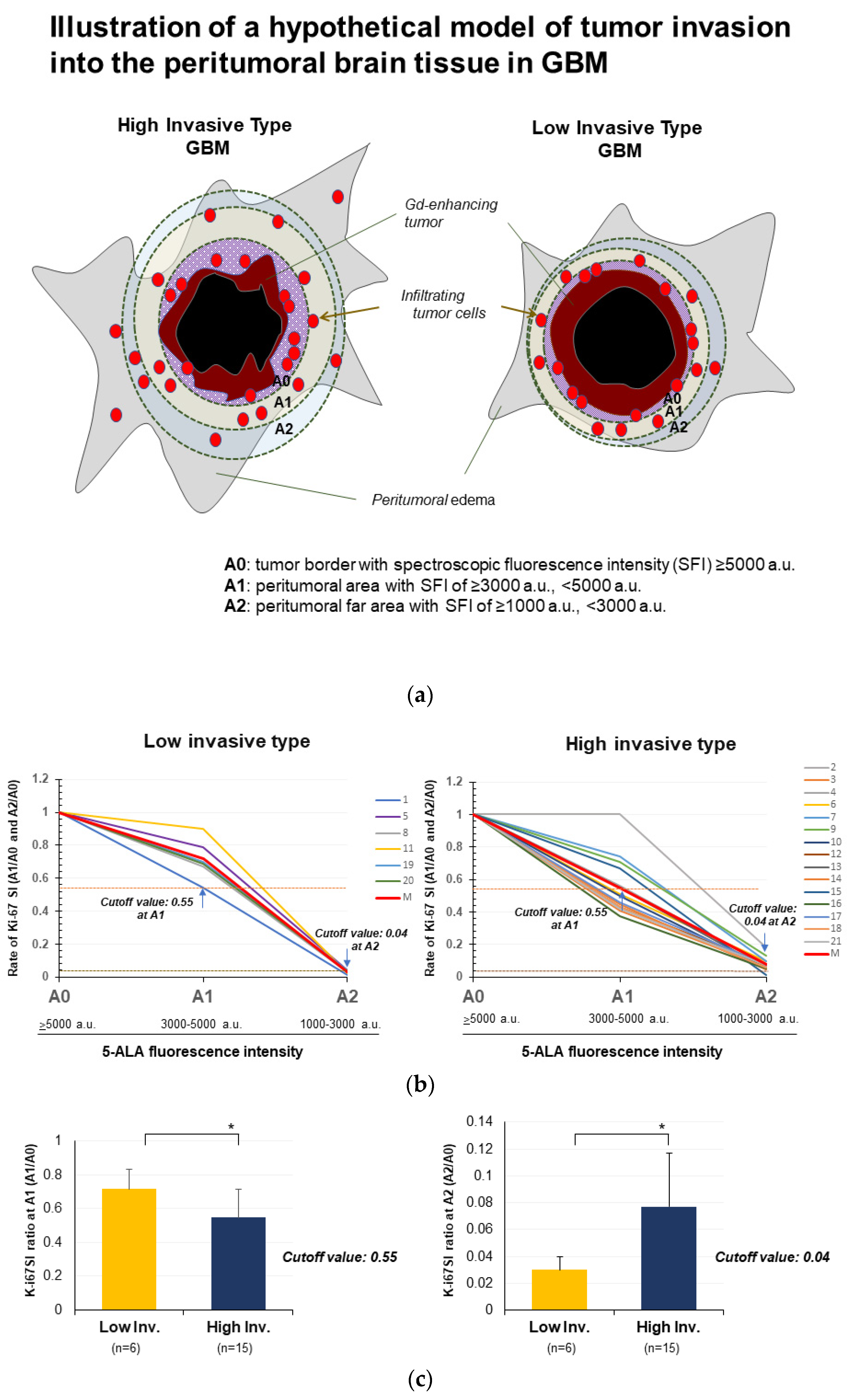
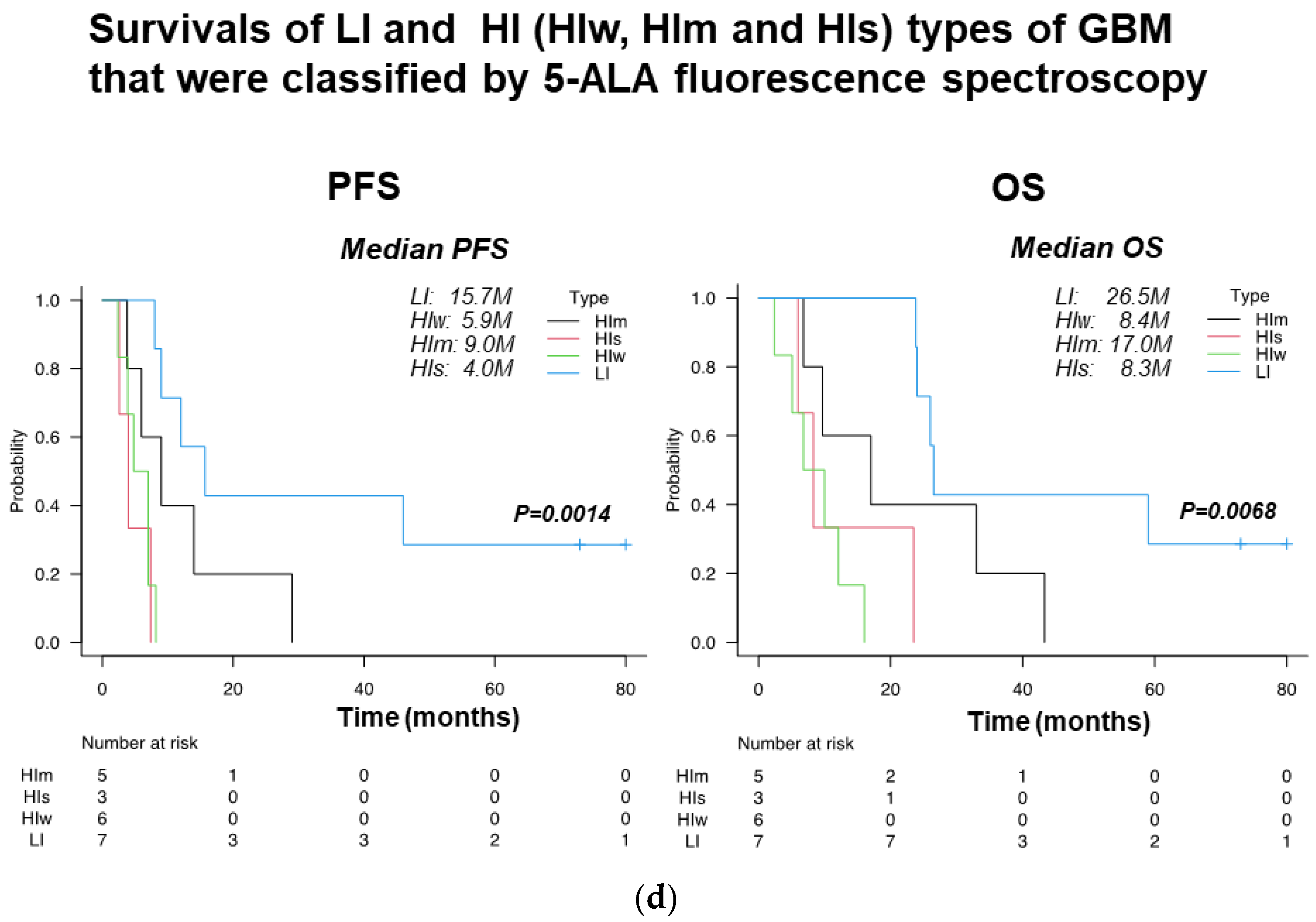
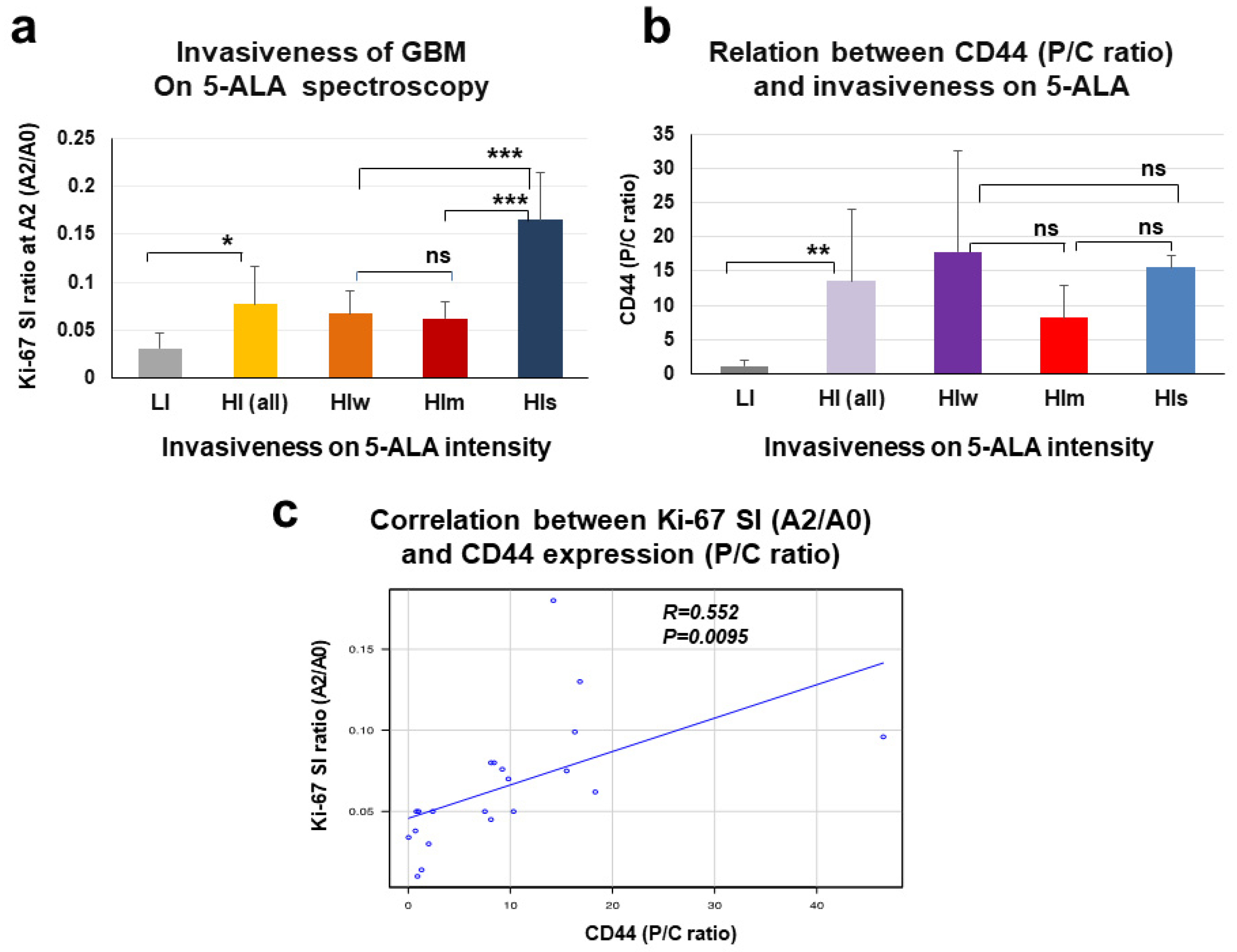
| Features on MRI | High-Invasive Type | Low-Invasive Type |
|---|---|---|
| Morphology of tumor margin | irregular, thin wall | demarcated, thick wall |
| Enhancement of tumor margin wall | heterogeneous, weak-moderate | homogeneous, intense |
| Peritumoral brain edema | diffuse, extensive | focal, localized |
| Morphology of central necrosis | irregular, unevenly located | round, located at or near center |
| Tumor Phenotypes | Age (Years) | Sex | KPS (%) | mMGMT | IDH-1 Mut | Ki-67 SI (%) | EOR | |
|---|---|---|---|---|---|---|---|---|
| (Mean ± SD) | Male/Female | (Median) | +/− | +/− | Mean ± SD | GTR + STR ** (%) | PR (%) | |
| High invasive (28 patients) | 64.5 ± 13.4 | 23/5 | 70 | 15/12 * | 2/26 | 33.5 ± 17.2 | 19 (68) | 9 (32) |
| Low invasive (22 patients) | 66.4 ± 13.2 | 14/8 | 80 | 7/14 * | 3/19 | 31.9 ± 17.4 | 18 (82) | 4 (18) |
| Patient no. | P/C Ratio | Ki-67 SI (%) | Ki-67 SI Ratio | Fluorescence Intensity (a.u.) at Final Resection (Peritumoral Area) | Type of Invasiveness | ||||
|---|---|---|---|---|---|---|---|---|---|
| (CD44) | A0 (≥5000) | A1 (3000–5000) | A2 (1000–3000) | A1/A0 | A2/A0 | on MRI | on 5-ALA | ||
| High (s) | |||||||||
| A1 | 1.3 | 73.3 | 40 | <1 | 0.55 | 0.014 | <1000 | L | L |
| A2 | 14.2 | 18.4 | 18.8 | 3.5 | 1.00 | 0.180 | 3000–5000 | H | H (m) |
| A3 | 18.3 | 44.9 | 18.6 | 2.8 | 0.41 | 0.062 | 1000–3000 | H | H (w) |
| A4 | 9.8 | 27.1 | 15.3 | 2 | 0.56 | 0.070 | 3000–5000 | H | H (m) |
| A5 | 0.7 | 26 | 20.5 | <1 | 0.79 | 0.038 | <1000 | L | L |
| A6 | 46.5 | 26.1 | 13.3 | 2.5 | 0.51 | 0.096 | 1000–3000 | H | H (w) |
| A7 | 16.3 | 23.2 | 17.2 | 2.3 | 0.74 | 0.099 | 1000–3000 | H | H(w) |
| A8 | 2 | 30 | 20 | <1 | 0.67 | 0.030 | <1000 | L | L |
| A9 | 16.8 | 22.9 | 16.3 | 3 | 0.71 | 0.130 | 3000–5000 | H | H (m) |
| A10 | 0.8 | 20 | 10 | 1 | 0.50 | 0.050 | 3000–5000 | L | * H (m) |
| A11 | 1 | 20 | 18 | 0.5 | 0.90 | 0.025 | <1000 | L | L |
| A12 | 8.1 | 30 | 13 | 2.5 | 0.43 | 0.080 | 3000–5000 | H | H (m) |
| A13 | 7.5 | 40 | 22 | 2 | 0.55 | 0.050 | 1000–3000 | H | H (w) |
| A14 | 8.1 | 44.9 | 20.2 | 2 | 0.45 | 0.045 | 1000–3000 | H | H (w) |
| A15 | 0.9 | 45 | 30 | 0.5 | 0.67 | 0.010 | ≥5000 | L | L |
| A16 | 10.3 | 40 | 15 | 2 | 0.38 | 0.050 | 1000–3000 | H | H (w) |
| A17 | 9.2 | 26 | 12 | 2 | 0.46 | 0.076 | ≥5000 | H | H (s) |
| A18 | 8.4 | 35 | 15 | 2 | 0.43 | 0.080 | ≥5000 | H | H (s) |
| A19 | 2.4 | 20 | 14 | 0.8 | 0.70 | 0.040 | <1000 | L | L |
| A20 | 0.03 | 48 | 25 | 1 | 0.69 | 0.034 | <1000 | L | L |
| A21 | 15.5 | 33 | 14 | 2.5 | 0.42 | 0.075 | ≥5000 | H | H (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, A.; Ohnishi, T.; Nishikawa, M.; Watanabe, H.; Kusakabe, K.; Taniwaki, M.; Yano, H.; Ohtsuka, Y.; Matsumoto, S.; Suehiro, S.; et al. Identification of CD44 as a Reliable Biomarker for Glioblastoma Invasion: Based on Magnetic Resonance Imaging and Spectroscopic Analysis of 5-Aminolevulinic Acid Fluorescence. Biomedicines 2023, 11, 2369. https://doi.org/10.3390/biomedicines11092369
Inoue A, Ohnishi T, Nishikawa M, Watanabe H, Kusakabe K, Taniwaki M, Yano H, Ohtsuka Y, Matsumoto S, Suehiro S, et al. Identification of CD44 as a Reliable Biomarker for Glioblastoma Invasion: Based on Magnetic Resonance Imaging and Spectroscopic Analysis of 5-Aminolevulinic Acid Fluorescence. Biomedicines. 2023; 11(9):2369. https://doi.org/10.3390/biomedicines11092369
Chicago/Turabian StyleInoue, Akihiro, Takanori Ohnishi, Masahiro Nishikawa, Hideaki Watanabe, Kosuke Kusakabe, Mashio Taniwaki, Hajime Yano, Yoshihiro Ohtsuka, Shirabe Matsumoto, Satoshi Suehiro, and et al. 2023. "Identification of CD44 as a Reliable Biomarker for Glioblastoma Invasion: Based on Magnetic Resonance Imaging and Spectroscopic Analysis of 5-Aminolevulinic Acid Fluorescence" Biomedicines 11, no. 9: 2369. https://doi.org/10.3390/biomedicines11092369
APA StyleInoue, A., Ohnishi, T., Nishikawa, M., Watanabe, H., Kusakabe, K., Taniwaki, M., Yano, H., Ohtsuka, Y., Matsumoto, S., Suehiro, S., Yamashita, D., Shigekawa, S., Takahashi, H., Kitazawa, R., Tanaka, J., & Kunieda, T. (2023). Identification of CD44 as a Reliable Biomarker for Glioblastoma Invasion: Based on Magnetic Resonance Imaging and Spectroscopic Analysis of 5-Aminolevulinic Acid Fluorescence. Biomedicines, 11(9), 2369. https://doi.org/10.3390/biomedicines11092369








