Blood-Biomarkers for Glucose Metabolism in Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Recruitment of the Infants
2.2. Sample Procedures
2.3. Target Analytes and Measurements
2.4. Patient Data Retrieval and Definitions
2.5. Definition of “Healthy” and “Ill” Preterm (at Least One Clinical Criteria Fulfilled) Subgroups
- “Healthy” infants, born GA < 32 weeks, AGA.
- “Ill” very preterm infants, born GA ≥ 28 weeks, A-/LGA.
- “Ill” very preterm infants, born GA ≥ 28 weeks, SGA.
- “Ill” extremely preterm infants, born GA < 28 weeks, A-/LGA.
- “Ill” extremely preterm infants, born GA < 28 weeks, SGA.
2.6. Ethics Statement
2.7. Statistical Analysis
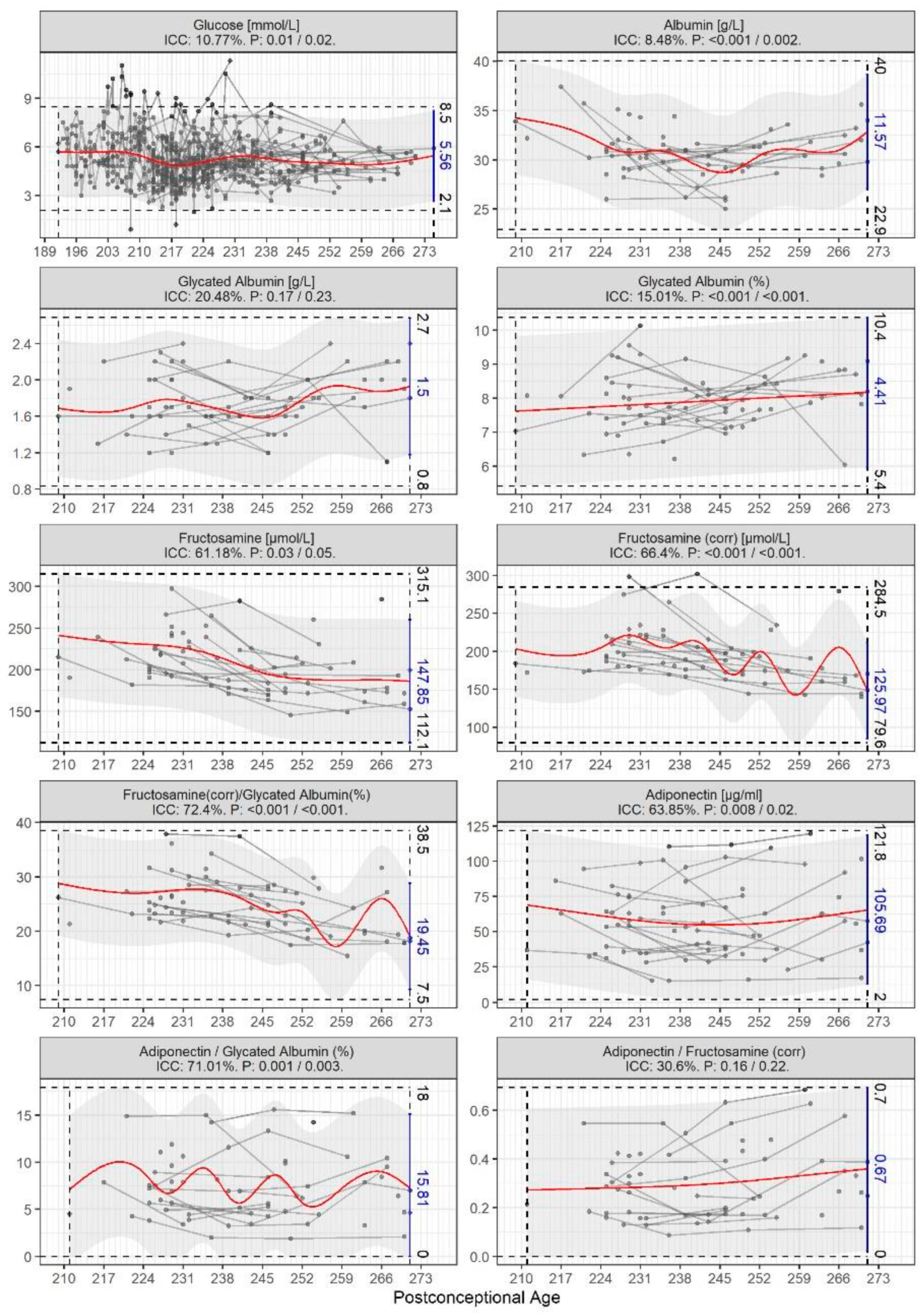
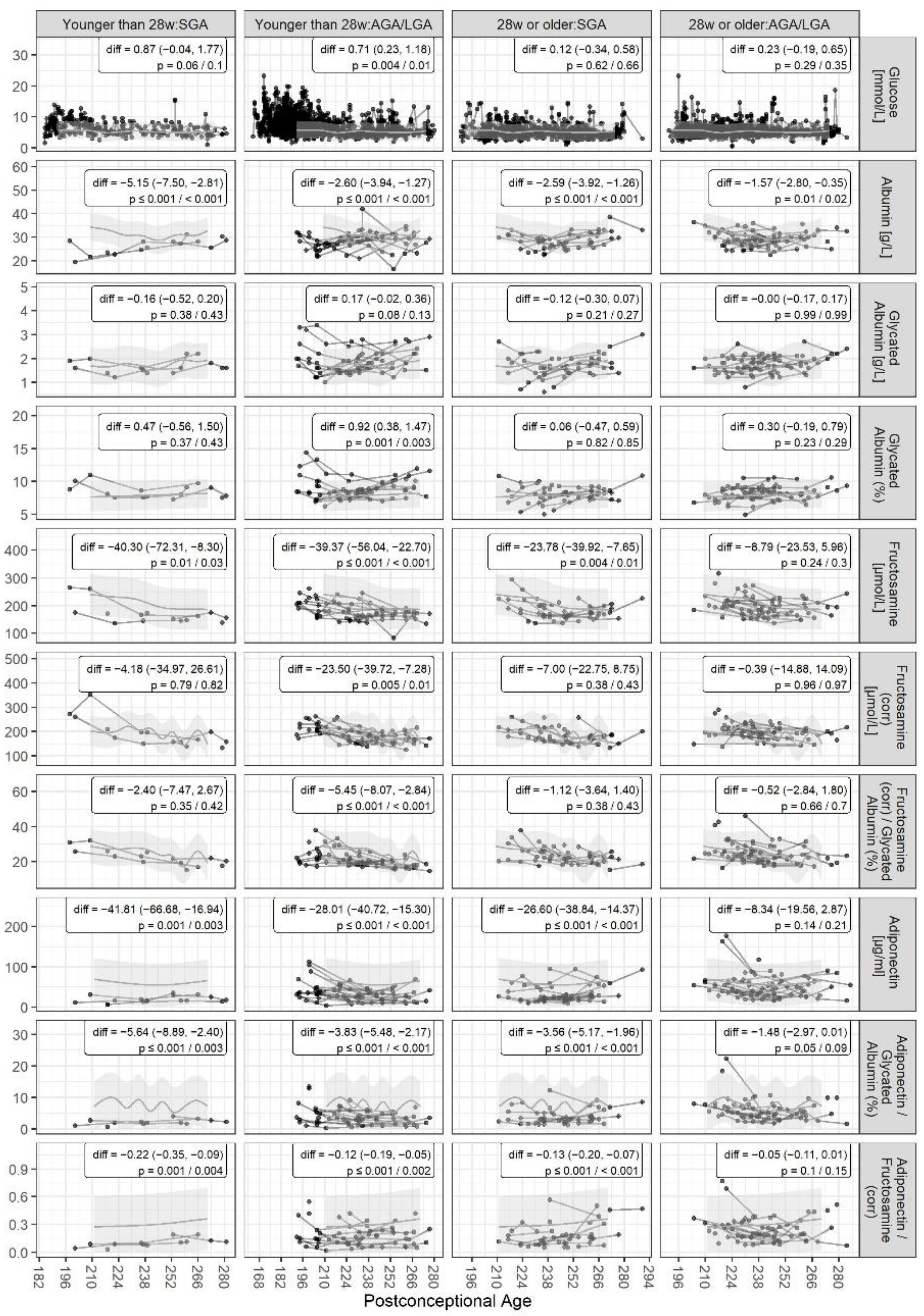
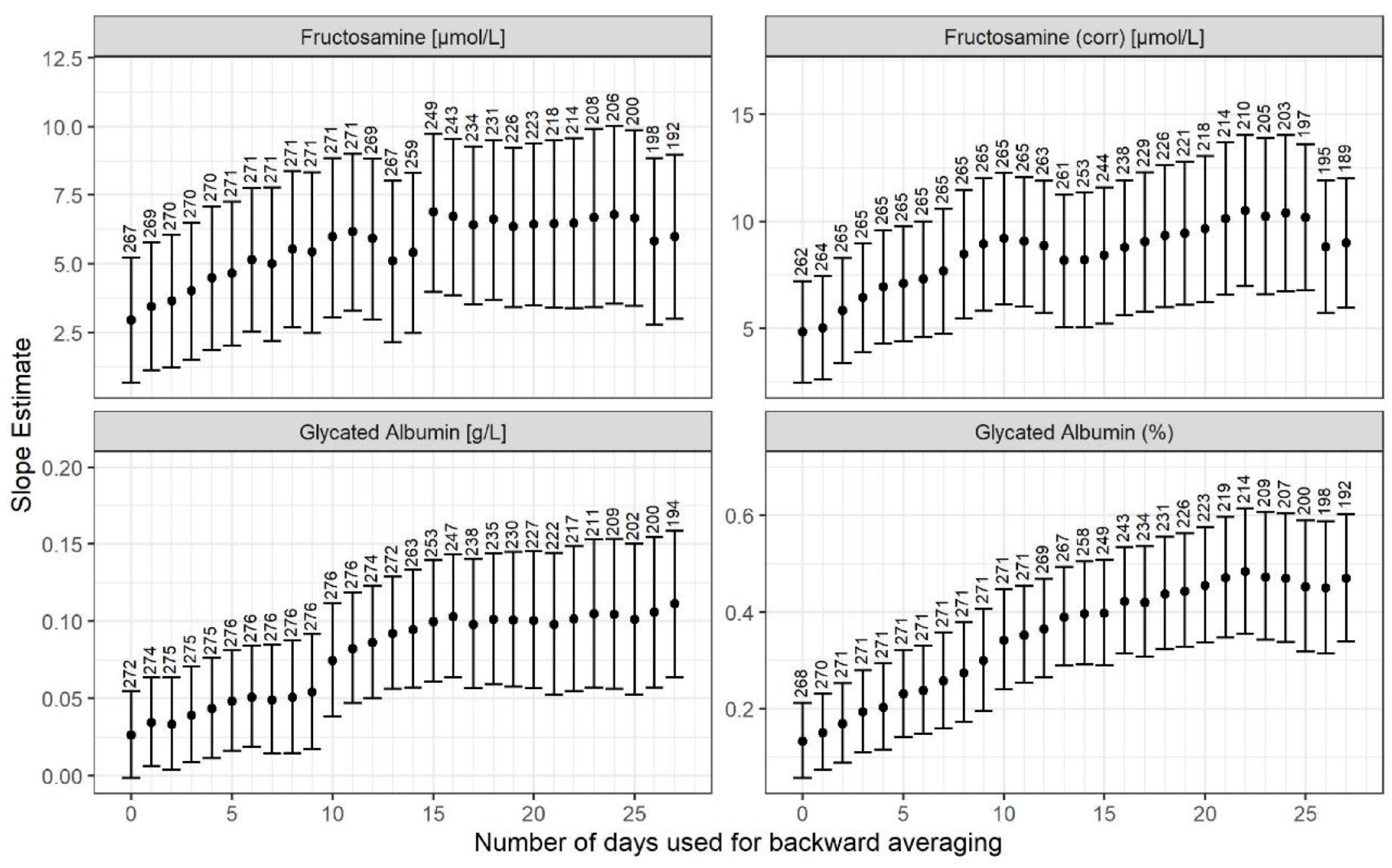
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ong, K.K.; Kennedy, K.; Castañeda-Gutiérrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr. Int. J. Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D. Glucose regulation in preterm newborn infants. Horm. Res. 2007, 68, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Louik, C.; Mitchell, A.A.; Epstein, M.F.; Shapiro, S. Risk factors for neonatal hyperglycemia associated with 10% dextrose infusion. Am. J. Dis. Child. 1985, 139, 783–786. [Google Scholar] [CrossRef]
- De Boo, H.A.; Harding, J.E. Protein metabolism in preterm infants with particular reference to intrauterine growth restriction. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Cowett, R.M.; Oh, W.; Schwartz, R. Persistent glucose production during glucose infusion in the neonate. J. Clin. Investig. 1983, 71, 467–475. [Google Scholar] [CrossRef]
- Harding, J.E.; Cormack, B.E.; Alexander, T.; Alsweiler, J.M.; Bloomfield, F.H. Advances in nutrition of the newborn infant. Lancet 2017, 389, 1660–1668. [Google Scholar] [CrossRef]
- Patel, P.; Bhatia, J. Total parenteral nutrition for the very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 2–7. [Google Scholar] [CrossRef]
- Beardsall, K.; Vanhaesebrouck, S.; Ogilvy-Stuart, A.L.; Vanhole, C.; Palmer, C.R.; Ong, K.; van Weissenbruch, M.; Midgley, P.; Thompson, M.; Thio, M.; et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: Cohort analyses of the NIRTURE study. J. Pediatr. 2010, 157, 715–719.e3. [Google Scholar] [CrossRef]
- Mitanchez-Mokhtari, D.; Lahlou, N.; Kieffer, F.; Magny, J.F.; Roger, M.; Voyer, M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics 2004, 113 Pt 1, 537–541. [Google Scholar] [CrossRef]
- Ertl, T.; Gyarmati, J.; Gaál, V.; Szabó, I. Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biol. Neonate 2006, 89, 56–59. [Google Scholar] [CrossRef]
- Slidsborg, C.; Jensen, L.B.; Rasmussen, S.C.; Fledelius, H.C.; Greisen, G.; Cour, M. Early postnatal hyperglycaemia is a risk factor for treatment-demanding retinopathy of prematurity. Br. J. Ophthalmol. 2018, 102, 14–18. [Google Scholar] [CrossRef]
- Decaro, M.H.; Vain, N.E. Hyperglycaemia in preterm neonates: What to know, what to do. Early Hum. Dev. 2011, 87, S19–S22. [Google Scholar] [CrossRef]
- Alsweiler, J.M.; Harding, J.E.; Bloomfield, F.H. Tight glycemic control with insulin in hyperglycemic preterm babies: A randomized controlled trial. Pediatrics 2012, 129, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Aynsley-Green, A.; Hawdon, J.M.; Deshpande, S.; Platt, M.W.; Lindley, K.; Lucas, A. Neonatal insulin secretion: Implications for the programming of metabolic homeostasis. Acta Paediatr. Jpn. 1997, 39 (Suppl. S1), S21–S25. [Google Scholar] [PubMed]
- Phillips, D.I.; Barker, D.J.; Hales, C.N.; Hirst, S.; Osmond, C. Thinness at birth and insulin resistance in adult life. Diabetologia 1994, 37, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.L.; Regan, F.; Harris, M.; Robinson, E.; Jackson, W.; Cutfield, W.S. The metabolic consequences of prematurity. Growth Horm. IGF Res. 2004, 14, 136–139. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Zanetti, M.B.R.; Kiwanuka, E.; Vettore, M.; Vedovato, M.; Tessari, P. Accelerated whole-body protein catabolism in subjects with type 2 Diabetes Mellitus and albuminuria. PLoS Negl. Trop. Dis. 2020, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Marsal, K.; Persson, P.H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- RCT. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Suzuki, S.; Koga, M.; Niizeki, N.; Furuya, A.; Matsuo, K.; Tanahashi, Y.; Azuma, H. Age-adjusted glycated albumin: A more robust parameter to establish glycaemic control in neonatal diabetes mellitus. Ann. Clin. Biochem. 2014, 51, 602–605. [Google Scholar] [CrossRef][Green Version]
- Viera, C.S.; Barreto Gmssrco, H.R.; Toso, B.R.; Rover, M.S.; Grassioli, S.; Guimaraes, A.T.B.; Balbo, S.L. Biochemical predictors for metabolic syndrome in preterm infants according to weight ratio. Arch. Endocrinol. Metab. 2020, 64, 7. [Google Scholar] [CrossRef] [PubMed]
- Farrag, H.M.; Cowett, R.M. Glucose homeostasis in the micropremie. Clin. Perinatol. 2000, 27, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lilien, L.D.; Rosenfield, R.L.; Baccaro, M.M.; Pildes, R.S. Hyperglycemia in stressed small premature neonates. J. Pediatr. 1979, 94, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Heimann, K.; Peschgens, T.; Kwiecien, R.; Stanzel, S.; Hoernchen, H.; Merz, U. Are recurrent hyperglycemic episodes and median blood glucose level a prognostic factor for increased morbidity and mortality in premature infants </=1500 g? J. Perinat Med. 2007, 35, 245–248. [Google Scholar]
- Ribeiro, R.T.; Macedo, M.P.; Raposo, J.F. HbA1c, Fructosamine, and Glycated Albumin in the Detection of Dysglycaemic Conditions. Curr. Diabetes Rev. 2016, 12, 14–19. [Google Scholar] [CrossRef]
- Ochiai, M.; Matsushita, Y.; Inoue, H.; Kusuda, T.; Kang, D.; Ichihara, K.; Nakashima, N.; Ihara, K.; Ohga, S.; Hara, T.; et al. Blood Reference Intervals for Preterm Low-Birth-Weight Infants: A Multicenter Cohort Study in Japan. PLoS ONE 2016, 11, e0161439. [Google Scholar] [CrossRef]
- De Schepper, J.; Derde, M.P.; Goubert, P.; Gorus, F. Reference values for fructosamine concentrations in children’s sera: Influence of protein concentration, age, and sex. Clin. Chem. 1988, 34, 2444–2447. [Google Scholar] [CrossRef]
- Oimomi, M.; Masuta, S.; Sakai, M.; Ohara, T.; Hata, F.; Baba, S. Influence of Serum Protein Levels on Serum Fructosamine Levels. Jpn. J. Med. 1989, 28, 312–315. [Google Scholar] [CrossRef]
- Gyarmati, J.; Tokes-Fuzesi, M.; Kovacs, G.L.; Gaal, V.; Vida, G.; Ertl, T. Fructosamine levels and hyperglycemia in preterm neonates. Neonatology 2009, 95, 267–270. [Google Scholar] [CrossRef]
- Torer, B.; Hanta, D.; Yapakci, E.; Gokmen, Z.; Parlakgumus, A.; Gulcan, H.; Tarcan, A. Association of Serum Albumin Level and Mortality in Premature Infants. J. Clin. Lab. Anal. 2016, 30, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Da-Silva, L.; Virella, D.; Fusch, C. Nutritional assessment in preterm infants: A Practical Approach in the NICU. Nutrients 2019, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Oberthuer, A.; Donmez, F.; Oberhauser, F.; Hahn, M.; Hoppenz, M.; Hoehn, T.; Roth, B.; Laudes, M. Hypoadiponectinemia in extremely low gestational age newborns with severe hyperglycemia--a matched-paired analysis. PLoS ONE 2012, 7, e38481. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, S.; Martinez, C.; Maiorana, A.; Scirè, G.; Spadoni, G.L.; Boemi, S. Adiponectin levels are reduced in children born small for gestational age and are inversely related to postnatal catch-up growth. J. Clin. Endocrinol. Metab. 2004, 89, 1346–1351. [Google Scholar] [CrossRef]
- Fu, Z.; Löfqvist, C.A.; Liegl, R.; Wang, Z.; Sun, Y.; Gong, Y.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO Mol. Med. 2018, 10, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Pădureanu, V.; Moța, M.; Ștefan, A.G.; Comănescu, A.C.; Radu, L.; Mazilu, E.R.; Vladu, I.M. Analysis of maternal and neonatal complications in a group of patients with gestational diabetes mellitus. Medicina 2021, 57, 1170. [Google Scholar] [CrossRef]

| Variable | Ill Infants GA < 28 Wks SGA, | Ill Infants GA < 28 Wks AGA/LGA | Ill Infants GA ≥ 28 Wks SGA | Ill Infants GA ≥ 28 Wks AGA/LGA | Healthy Infants AGA |
|---|---|---|---|---|---|
| Group (n, %) | 3 (3%) | 18 (17%) | 22 (20%) | 31 (29%) | 34 (31%) |
| Male (n, %) | 0 (0%) | 8 (44%) | 16 (73%) | 17 (55%) | 16 (47%) |
| SGA (n, %) | 3 (100%) | 0 (0%) | 22 (100%) | 0 (0%) | 0 (0%) |
| Gestational age (Median; Q1, Q3; wks.) | 26.7 (26.5, 26.8) | 25.8 (25.1, 26.4) | 30.6 (28.9, 31.1) | 29.1 (28.1, 30.5) | 30.4 (29.0, 31.1) |
| Birth weight (Mean ± SD; gram) | 642 ± 59 | 812 ± 133 | 1026 ± 221 | 1384 ± 341 | 1513 ± 272 |
| Major neonatal morbidity: BPD/DAP/IVH/NEC/ROP [any, %] | 2/1/0/0/2 [2, 67%] | 8/12/3/4/9 [15, 83%] | 1/3/1/1/4 [6, 27%] | 4/6/4/1/7 [10, 32%] | 0/0/0/0/0 [0, 0%] |
| Bronchopulmonary dysplasia [n, % of group] | 2 [67%] | 8 [44%] | 1 [55%] | 4 [13%] | 0 [0, 0%] |
| Ductus arteriosus persistence [n, % of group] | 1 [33%] | 12 [67%] | 3 [14%] | 6 [19%] | 0 [0, 0%] |
| Intraventricular hemorrhage [n, % of group] | 0 [0%] | 3 [17%] | 1 [5%] | 4 [13%] | 0 [0, 0%] |
| Necrotizing enterocolitis [n, % of group] | 0 [0%] | 4 [22%] | 1 [5%] | 1 [3%] | 0 [0%] |
| Retinopathy of prematurity [n, % of group] | 2 [67%] | 9 [50%] | 4 [18%] | 7 [23%] | 0 [0, 0%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjerager, M.O.; Hansen, B.M.; Sørensen, F.; Petersen, J.R.; Jensen, K.V.; Hjelvang, B.R.; Hvelplund, A.C.; Olsen, D.A.; Nielsen, A.A.; Forman, J.L.; et al. Blood-Biomarkers for Glucose Metabolism in Preterm Infants. Biomedicines 2023, 11, 2377. https://doi.org/10.3390/biomedicines11092377
Bjerager MO, Hansen BM, Sørensen F, Petersen JR, Jensen KV, Hjelvang BR, Hvelplund AC, Olsen DA, Nielsen AA, Forman JL, et al. Blood-Biomarkers for Glucose Metabolism in Preterm Infants. Biomedicines. 2023; 11(9):2377. https://doi.org/10.3390/biomedicines11092377
Chicago/Turabian StyleBjerager, Mia O., Bo M. Hansen, Frederik Sørensen, Jes R. Petersen, Kristian V. Jensen, Brian R. Hjelvang, Anna C. Hvelplund, Dorte A. Olsen, Aneta A. Nielsen, Julie L. Forman, and et al. 2023. "Blood-Biomarkers for Glucose Metabolism in Preterm Infants" Biomedicines 11, no. 9: 2377. https://doi.org/10.3390/biomedicines11092377
APA StyleBjerager, M. O., Hansen, B. M., Sørensen, F., Petersen, J. R., Jensen, K. V., Hjelvang, B. R., Hvelplund, A. C., Olsen, D. A., Nielsen, A. A., Forman, J. L., Brandslund, I., Greisen, G., & Slidsborg, C. (2023). Blood-Biomarkers for Glucose Metabolism in Preterm Infants. Biomedicines, 11(9), 2377. https://doi.org/10.3390/biomedicines11092377







