Systematic Review of Platelet-Rich Plasma for Low Back Pain
Abstract
:1. Introduction
1.1. Manifestations of Degenerative Disease
- Internal disc disruption: One of the most common causes of low back pain, a painful disk, without herniation, is a consequence of internal ruptures leading to vascularized granulation tissue invasion with extensive innervation, in an attempt to heal [26,27,28]. Magnetic resonance imaging can show, in some patients, loss of T2 signal (dehydration) and/or hyperintense signal in the posterior disc from fissures called HIZ, or high-intensity zone [29]. In the vast majority of discogenic pain cases, the degenerative alterations are Pfirrmann grade II or III [30]. Modic Type I changes have also been correlated with pain and positive discography alterations [31]. The use of provocative discography can be useful, although its use should be weighed against the possible risks of this more involved procedure [32,33,34,35].
- Disc herniation is a consequence of hydration losses and capacity to absorb and distribute compressive loads, making it susceptible to internal fissures that can progress to complete ruptures and leak of disc tissues into the spinal canal [36]. This herniation can lead to radiculopathy, either through direct mechanical compression of the nerve tissue or through an inflammatory reaction triggered by the release of various cytokines, particularly TNF alpha and IL-6 [37].
- Facet joint pain/syndrome is a prevalent complaint among individuals experiencing low back pain. Facet joints are diarthrodial joints encompassing a joint capsule and synovial membrane, with surfaces covered by cartilage. The oblique orientation of these joints contributes to their resistance against shear forces and limitation of rotational movement [38]. It was demonstrated that in a degenerated disc, axial compression is not adequately absorbed, resulting in the transmission of this load to the facet joints. Consequently, the facets experience an increase in mechanical demand by four to eight times their original capacity. This heightened load, coupled with increased instability, leads to joint injuries, and initiates the degenerative process within the facets [39,40].
- The atrophy of the paravertebral musculature has been associated with low back pain [41]. Animal studies and magnetic resonance images in humans with discogenic low back pain found a causal relationship between fatty infiltration of the musculature and discogenic pain [42]. Muscle atrophy and fatty degeneration are commonly observed in individuals with chronic low back pain, indicating the vital role played by the paraspinal muscles in maintaining lumbar spine stability [43].
1.2. Treatment Options
1.2.1. Medication and Physical Activity
1.2.2. Interventional Measures
1.2.3. Surgery
1.2.4. Regenerative Medicine
1.2.5. PRP in Degenerative Spine Disease
1.2.6. Study Aims
2. Materials and Methods
2.1. Literature Search and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment and Risk of Bias
3. Results
3.1. Outcome Measure Tools—Pain and Disability
3.2. PRP Technique
Risk of Bias
4. Discussion
4.1. Intradiscal Injections
4.2. Epidural Injections
4.3. Facet Injections
4.4. Other Spinal Target Sites
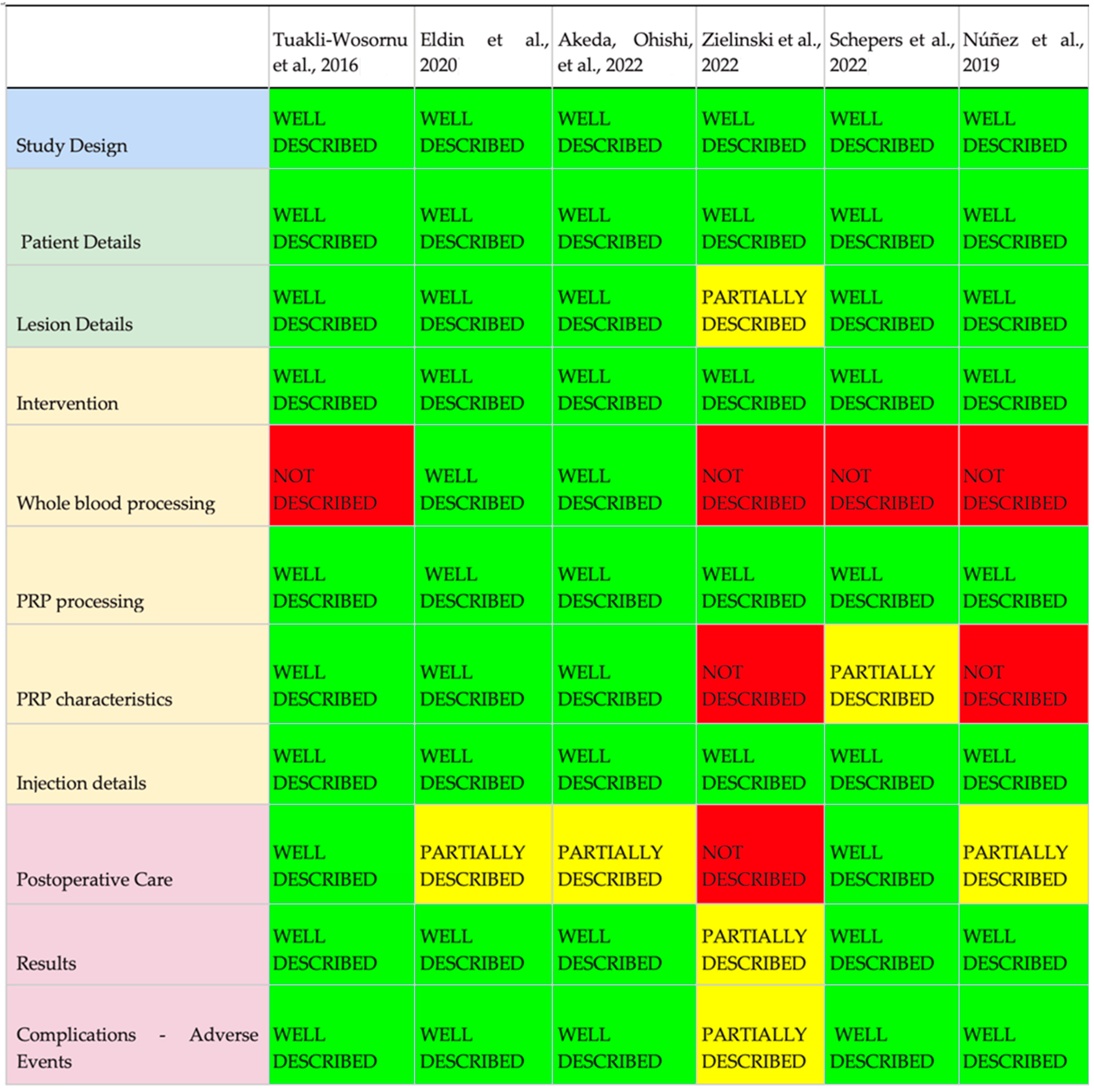
4.5. Compliance According to the MIBO Assessment Checklist
4.6. Multitarget Therapy
Evidence Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. PRISMA Checklist
| Section/Topic | # | Checklist Item | Information Reported | Line Number(s) | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Administrative Information | |||||
| Title | |||||
| Identification | 1a | Identify the report as a protocol of a systematic review | X | ☐ | |
| Update | 1b | If the protocol is for an update of a previous systematic review, identify as such | ☐ | X | |
| Registration | 2 | If registered, provide the name of the registry (e.g., PROSPERO) and registration number in the Abstract | X | ☐ | |
| Authors | |||||
| Contact | 3a | Provide name, institutional affiliation, and e-mail address of all protocol authors; provide physical mailing address of corresponding author | X | ☐ | |
| Contributions | 3b | Describe contributions of protocol authors and identify the guarantor of the review | X | ☐ | |
| Amendments | 4 | If the protocol represents an amendment of a previously completed or published protocol, identify as such and list changes; otherwise, state plan for documenting important protocol amendments | ☐ | X | |
| Support | |||||
| Sources | 5a | Indicate sources of financial or other support for the review | X | ||
| Sponsor | 5b | Provide name for the review funder and/or sponsor | X | ☐ | |
| Role of sponsor/funder | 5c | Describe roles of funder(s), sponsor(s), and/or institution(s), if any, in developing the protocol | ☐ | X | |
| Introduction | |||||
| Rationale | 6 | Describe the rationale for the review in the context of what is already known | X | ☐ | |
| Objectives | 7 | Provide an explicit statement of the question(s) the review will address with reference to participants, interventions, comparators, and outcomes (PICO) | X | ☐ | |
| Methods | |||||
| Eligibility criteria | 8 | Specify the study characteristics (e.g., PICO, study design, setting, time frame) and report characteristics (e.g., years considered, language, publication status) to be used as criteria for eligibility for the review | X | ☐ | |
| Information sources | 9 | Describe all intended information sources (e.g., electronic databases, contact with study authors, trial registers, or other grey literature sources) with planned dates of coverage | X | ☐ | |
| Search strategy | 10 | Present draft of search strategy to be used for at least one electronic database, including planned limits, such that it could be repeated | X | ☐ | |
| Study Records | |||||
| Data management | 11a | Describe the mechanism(s) that will be used to manage records and data throughout the review | X | ☐ | |
| Selection process | 11b | State the process that will be used for selecting studies (e.g., two independent reviewers) through each phase of the review (i.e., screening, eligibility, and inclusion in meta-analysis) | X | ☐ | |
| Data collection process | 11c | Describe planned method of extracting data from reports (e.g., piloting forms, done independently, in duplicate), any processes for obtaining and confirming data from investigators | X | ☐ | |
| Data items | 12 | List and define all variables for which data will be sought (e.g., PICO items, funding sources), any pre-planned data assumptions and simplifications | X | ☐ | |
| Outcomes and prioritization | 13 | List and define all outcomes for which data will be sought, including prioritization of main and additional outcomes, with rationale | X | ☐ | |
| Risk of bias in individual studies | 14 | Describe anticipated methods for assessing risk of bias of individual studies, including whether this will be done at the outcome or study level, or both; state how this information will be used in data synthesis | X | ☐ | |
| Data | |||||
| Synthesis | 15a | Describe criteria under which study data will be quantitatively synthesized | X | ☐ | |
| 15b | If data are appropriate for quantitative synthesis, describe planned summary measures, methods of handling data, and methods of combining data from studies, including any planned exploration of consistency (e.g., I2, Kendall’s tau) | ☐ | X | ||
| 15c | Describe any proposed additional analyses (e.g., sensitivity or subgroup analyses, meta-regression) | X | |||
| 15d | If quantitative synthesis is not appropriate, describe the type of summary planned | X | |||
| Meta-bias(es) | 16 | Specify any planned assessment of meta-bias(es) (e.g., publication bias across studies, selective reporting within studies) | X | ☐ | |
| Confidence in cumulative evidence | 17 | Describe how the strength of the body of evidence will be assessed (e.g., GRADE) | X | ☐ | |
Appendix B
| PUBMED | |
| GROUP 1 Search: ((((((((((“back pain”[MeSH Terms]) OR “low back pain”[MeSH Terms]) OR “chronic pain”[MeSH Terms]) OR “chronic low back pain”) OR “facet pain”) OR “acute radicular pain”) OR sciatica[MeSH Terms]) OR “sciatic neuropathy”[MeSH Terms]) OR “facet joint pain”) OR neuralgia[MeSH Terms]) OR “facet syndrome” | GROUP 2 Search: (((((((((((((((((((((((((((((((“intervertebral disc displacement”[MeSH Terms]) OR (“intervertebral disc degeneration”[MeSH Terms])) OR (“intervertebral disc chemolysis”[MeSH Terms])) OR (joints[MeSH Terms])) OR (“joint diseases”[MeSH Terms])) OR (“spinal stenosis”[MeSH Terms])) OR (“spinal diseases”[MeSH Terms])) OR (“intervertebral disc”[MeSH Terms])) OR (“intervertebral disk”[MeSH Terms])) OR (Arthropathy, Neurogenic[MeSH Terms])) OR (“lumbar Degenerative Disc Disease”)) OR (“central canal stenosis”)) OR (“disc protrusion”)) OR (“foraminal stenosis”)) OR (“lateral recess stenosis”)) OR (radiculopathy[MeSH Terms])) OR (“facet arthropathy”)) OR (“facet arthrosis”)) OR (“facet syndrome”)) OR (sacroiliitis[MeSH Terms])) OR (“sacroiliac joint”[MeSH Terms])) OR (“disc herniation”)) OR (“disc regeneration”)) OR (“lumbar stenosis”)) OR (epidural)) OR (peridural)) OR (“paravertebral muscle atrophy”)) OR (“atrophied lumbar multifidus”)) OR (disk)) OR (slipped)) OR (disks)) OR (disc) |
| GROUP 3 Search: ((((((((((((((“Platelet-rich plasma”[MeSH Terms]) OR PRP) OR “plasma rich in growth factors”) OR PRGF) OR “conditioned serum plasma”) OR ACSP) OR CSP) OR “platelet lysate”) OR “platelet concentrate”) OR “autologous platelet”) OR “leukocyte rich plasma”) OR PLRP) OR “platelet rich fibrin”[MeSH Terms]) OR “leukocyte poor platelet rich plasma”) OR “leukocyte rich platelet rich plasma” | FINAL SEARCH Search: ((((((((((((“back pain”[MeSH Terms]) OR “low back pain”[MeSH Terms]) OR “chronic pain”[MeSH Terms]) OR “chronic low back pain”) OR “facet pain”) OR “acute radicular pain”) OR sciatica[MeSH Terms]) OR “sciatic neuropathy”[MeSH Terms]) OR “facet joint pain”) OR neuralgia[MeSH Terms]) OR “facet syndrome”) AND ((((((((((((((((((((((((((((((((“intervertebral disc displacement”[MeSH Terms]) OR (“intervertebral disc degeneration”[MeSH Terms])) OR (“intervertebral disc chemolysis”[MeSH Terms])) OR (joints[MeSH Terms])) OR (“joint diseases”[MeSH Terms])) OR (“spinal stenosis”[MeSH Terms])) OR (“spinal diseases”[MeSH Terms])) OR (“intervertebral disc”[MeSH Terms])) OR (“intervertebral disk”[MeSH Terms])) OR (Arthropathy, Neurogenic[MeSH Terms])) OR (“lumbar Degenerative Disc Disease”)) OR (“central canal stenosis”)) OR (“disc protrusion”)) OR (“foraminal stenosis”)) OR (“lateral recess stenosis”)) OR (radiculopathy[MeSH Terms])) OR (“facet arthropathy”)) OR (“facet arthrosis”)) OR (“facet syndrome”)) OR (sacroiliitis[MeSH Terms])) OR (“sacroiliac joint”[MeSH Terms])) OR (“disc herniation”)) OR (“disc regeneration”)) OR (“lumbar stenosis”)) OR (epidural)) OR (peridural)) OR (“paravertebral muscle atrophy”)) OR (“atrophied lumbar multifidus”)) OR (disk)) OR (slipped)) OR (disks)) OR (disc))) AND (((((((((((((((“Platelet-rich plasma”[MeSH Terms]) OR PRP) OR “plasma rich in growth factors”) OR PRGF) OR “conditioned serum plasma”) OR ACSP) OR CSP) OR “platelet lysate”) OR “platelet concentrate”) OR “autologous platelet”) OR “leukocyte rich plasma”) OR PLRP) OR “platelet rich fibrin”[MeSH Terms]) OR “leukocyte poor platelet rich plasma”) OR “leukocyte rich platelet rich plasma”) |
| SCOPUS | |
| GROUP 1 ALL (“back pain”) OR ALL (“low back pain”) OR ALL (“chronic pain”) OR (“chronic low back pain”) OR ALL (“facet pain”) OR ALL (“acute radicular pain”) OR ALL (sciatica) OR ALL (“sciatic neuropathy”) OR ALL (“facet joint pain”) OR ALL (neuralgia) OR ALL (“facet syndrome”) OR ALL (“vertebrogenic pain syndrome”) OR ALL (“discogenic pain”) OR ALL (“discogenic back pain”) | GROUP 2 ALL (“intervertebral disc displacement”) OR ALL (“intervertebral disc degeneration”) OR ALL (“intervertebral disc chemolysis”) OR ALL (joints) OR ALL (“joint diseases”) OR ALL (“spinal stenosis”) OR ALL (“spinal diseases”) OR ALL (“intervertebral disc”) OR ALL (“intervertebral disk”) OR ALL (arthropathy, AND neurogenic) OR ALL (“lumbar Degenerative Disc Disease”) OR ALL (“central canal stenosis”) OR ALL (“disc protrusion”) OR ALL (“foraminal stenosis”) OR ALL (“lateral recess stenosis”) OR ALL (radiculopathy) OR ALL (“facet arthropathy”) OR ALL (“facet arthrosis”) OR ALL (“facet syndrome”) OR ALL (sacroiliitis) OR ALL (“sacroiliac joint”) OR ALL (“disc herniation”) OR ALL (“disc regeneration”) OR ALL (“lumbar stenosis”) OR ALL (epidural) OR ALL (peridural) OR ALL (“paravertebral muscle atrophy”) OR ALL (“atrophied lumbar multifidus”) OR ALL (disk) OR ALL (slipped) OR ALL (disks) OR ALL (disc) OR ALL (“herniated disc”) OR ALL (“herniated disk”) OR ALL (“prolapsed disk”) OR ALL (“nerve root compressions”) OR ALL (“nerve root disorder”) OR ALL (“nerve root compression”) OR ALL (“nerve root disorders”) OR ALL (“degenerative disc disease”) OR ALL (“degeneration of the intervertebral disc joint”) OR ALL (radiculitis) OR ALL (“disc disease”) OR ALL (“intervertebral disc disease”) OR ALL (displacements) OR ALL (“intervertebral disk displacement”) OR ALL (“disk displacement”) OR ALL (“disk displacements”) OR ALL (“intervertebral displacements”) OR ALL (“intervertebral displacement”) OR ALL (“herniated disks”) OR ALL (“herniated discs”) OR ALL (“slipped disk”) OR ALL (“prolapsed disc”) OR ALL (“prolapsed discs”) OR ALL (“prolapsed disks”) OR ALL (“disc prolapse”) OR ALL (“disk prolapse”) OR ALL (“disk prolapses”) |
| GROUP 3 ALL (“Platelet-rich plasma”) OR ALL (prp) OR ALL (“plasma rich in growth factors”) OR ALL (prgf) OR ALL (“conditioned serum plasma”) OR ALL (acsp) OR ALL (csp) OR ALL (“platelet lysate”) OR ALL (“platelet concentrate”) OR ALL (“autologous platelet”) OR ALL (“leukocyte rich plasma”) OR ALL (plrp) OR ALL (“platelet rich fibrin”) OR ALL (“leukocyte poor platelet rich plasma”) OR ALL (“leukocyte rich platelet rich plasma”) OR ALL (“platelet rich plasma”) | (ALL (“back pain”) OR ALL (“low back pain”) OR ALL (“chronic pain”) OR (“chronic low back pain”) OR ALL (“facet pain”) OR ALL (“acute radicular pain”) OR ALL (sciatica) OR ALL (“sciatic neuropathy”) OR ALL (“facet joint pain”) OR ALL (neuralgia) OR ALL (“facet syndrome”) OR ALL (“vertebrogenic pain syndrome”) OR ALL (“discogenic pain”) OR ALL (“discogenic back pain”)) AND (ALL (“intervertebral disc displacement”) OR ALL (“intervertebral disc degeneration”) OR ALL (“intervertebral disc chemolysis”) OR ALL (joints) OR ALL (“joint diseases”) OR ALL (“spinal stenosis”) OR ALL (“spinal diseases”) OR ALL (“intervertebral disc”) OR ALL (“intervertebral disk”) OR ALL (arthropathy, AND neurogenic) OR ALL (“lumbar Degenerative Disc Disease”) OR ALL (“central canal stenosis”) OR ALL (“disc protrusion”) OR ALL (“foraminal stenosis”) OR ALL (“lateral recess stenosis”) OR ALL (radiculopathy) OR ALL (“facet arthropathy”) OR ALL (“facet arthrosis”) OR ALL (“facet syndrome”) OR ALL (sacroiliitis) OR ALL (“sacroiliac joint”) OR ALL (“disc herniation”) OR ALL (“disc regeneration”) OR ALL (“lumbar stenosis”) OR ALL (epidural) OR ALL (peridural) OR ALL (“paravertebral muscle atrophy”) OR ALL (“atrophied lumbar multifidus”) OR ALL (disk) OR ALL (slipped) OR ALL (disks) OR ALL (disc) OR ALL (“herniated disc”) OR ALL (“herniated disk”) OR ALL (“prolapsed disk”) OR ALL (“nerve root compressions”) OR ALL (“nerve root disorder”) OR ALL (“nerve root compression”) OR ALL (“nerve root disorders”) OR ALL (“degenerative disc disease”) OR ALL (“degeneration of the intervertebral disc joint”) OR ALL (radiculitis) OR ALL (“disc disease”) OR ALL (“intervertebral disc disease”) OR ALL (displacements) OR ALL (“intervertebral disk displacement”) OR ALL (“disk displacement”) OR ALL (“disk displacements”) OR ALL (“intervertebral displacements”) OR ALL (“intervertebral displacement”) OR ALL (“herniated disks”) OR ALL (“herniated discs”) OR ALL (“slipped disk”) OR ALL (“prolapsed disc”) OR ALL (“prolapsed discs”) OR ALL (“prolapsed disks”) OR ALL (“disc prolapse”) OR ALL (“disk prolapse”) OR ALL (“disk prolapses”)) AND (ALL (“Platelet-rich plasma”) OR ALL (prp) OR ALL (“plasma rich in growth factors”) OR ALL (prgf) OR ALL (“conditioned serum plasma”) OR ALL (acsp) OR ALL (csp) OR ALL (“platelet lysate”) OR ALL (“platelet concentrate”) OR ALL (“autologous platelet”) OR ALL (“leukocyte rich plasma”) OR ALL (plrp) OR ALL (“platelet rich fibrin”) OR ALL (“leukocyte poor platelet rich plasma”) OR ALL (“leukocyte rich platelet rich plasma”) OR ALL (“platelet rich plasma”)) |
| WEB OF SCIENCE | |
| GROUP 1 TS = (“back pain”) OR TS = (“low back pain”) OR TS = (“chronic pain”) OR TS = (“chronic low back pain”) OR TS = (“facet pain”) OR TS = (“acute radicular pain”) OR TS = (sciatica) OR TS = (“sciatic neuropathy”) OR TS = (“facet joint pain”) OR TS = (neuralgia) OR TS = (“facet syndrome”) OR TS = (“vertebrogenic pain syndrome”) OR TS = (“discogenic pain”) OR TS = (“discogenic back pain”) | GROUP 2 TS = (“intervertebral disc displacement”) OR TS = (“intervertebral disc degeneration”) OR TS = (“intervertebral disc chemolysis”) OR TS = (joints) OR TS = (“joint diseases”) OR TS = (“spinal stenosis”) OR TS = (“spinal diseases”) OR TS = (“intervertebral disc”) OR TS = (“intervertebral disk”) OR TS = (arthropathy, AND neurogenic) OR TS = (“lumbar Degenerative Disc Disease”) OR TS = (“central canal stenosis”) OR TS = (“disc protrusion”) OR TS = (“foraminal stenosis”) OR TS = (“lateral recess stenosis”) OR TS = (radiculopathy) OR TS = (“facet arthropathy”) OR TS = (“facet arthrosis”) OR TS = (“facet syndrome”) OR TS = (sacroiliitis) OR TS = (“sacroiliac joint”) OR TS = (“disc herniation”) OR TS = (“disc regeneration”) OR TS = (“lumbar stenosis”) OR TS = (epidural) OR TS = (peridural) OR TS = (“paravertebral muscle atrophy”) OR TS = (“atrophied lumbar multifidus”) OR TS = (disk) OR TS = (slipped) OR TS = (disks) OR TS = (disc) OR TS = (“herniated disc”) OR TS = (“herniated disk”) OR TS = (“prolapsed disk”) OR TS = (“nerve root compressions”) OR TS = (“nerve root disorder”) OR TS = (“nerve root compression”) OR TS = (“nerve root disorders”) OR TS = (“degenerative disc disease”) OR TS = (“degeneration of the intervertebral disc joint”) OR TS = (radiculitis) OR TS = (“disc disease”) OR TS = (“intervertebral disc disease”) OR TS = (displacements) OR TS = (“intervertebral disk displacement”) OR TS = (“disk displacement”) OR TS = (“disk displacements”) OR TS = (“intervertebral displacements”) OR TS = (“intervertebral displacement”) OR TS = (“herniated disks”) OR TS = (“herniated discs”) OR TS = (“slipped disk”) OR TS = (“prolapsed disc”) OR TS = (“prolapsed discs”) OR TS = (“prolapsed disks”) OR TS = (“disc prolapse”) OR TS = (“disk prolapse”) OR TS = (“disk prolapses”) |
| GROUP 3 TS = (“Platelet-rich plasma”) OR TS = (prp) OR TS = (“plasma rich in growth factors”) OR TS = (prgf) OR TS = (“conditioned serum plasma”) OR TS = (acsp) OR TS = (csp) OR TS = (“platelet lysate”) OR TS = (“platelet concentrate”) OR TS = (“autologous platelet”) OR TS = (“leukocyte rich plasma”) OR TS = (plrp) OR TS = (“platelet rich fibrin”) OR TS = (“leukocyte poor platelet rich plasma”) OR TS = (“leukocyte rich platelet rich plasma”) OR TS = (“platelet rich plasma”) |
References
- Dieleman, J.L.; Squires, E.; Bui, A.L.; Campbell, M.; Chapin, A.; Hamavid, H.; Horst, C.; Li, Z.; Matyasz, T.; Reynolds, A.; et al. Factors Associated with Increases in US Health Care Spending, 1996–2013. JAMA 2017, 318, 1668–1678. [Google Scholar] [CrossRef]
- Schofield, D.J.; Shrestha, R.N.; Passey, M.E.; Earnest, A.; Fletcher, S.L. Chronic disease and labour force participation among older Australians. Med. J. Aust. 2008, 189, 447–450. [Google Scholar] [CrossRef]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.R.C.d.; Costa, L.O.P. Prevalência da dor lombar no Brasil: Uma revisão sistemática. Cad. Saúde Pública 2015, 31, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Foizer, G.A.; de Paiva, V.C.; do Nascimento, R.D.; Gorios, C.; Cliquet Júnior, A.; de Miranda, J.B. Is There Any Association between the Severity of Disc Degeneration and Low Back Pain? Rev. Bras. Ortop. 2022, 57, 334–340. [Google Scholar] [CrossRef]
- Pang, W.W.; Mok, M.S.; Lin, M.L.; Chang, D.P.; Hwang, M.H. Application of spinal pain mapping in the diagnosis of low back pain—Analysis of 104 cases. Acta Anaesthesiol. Sin. 1998, 36, 71–74. [Google Scholar]
- Manchikanti, L.; Singh, V.; Pampati, V.; Damron, K.S.; Barnhill, R.C.; Beyer, C.; Cash, K.A. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician 2001, 4, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hicks, G.E.; Morone, N.; Weiner, D.K. Degenerative Lumbar Disc and Facet Disease in Older Adults. Spine 2009, 34, 1301–1306. [Google Scholar] [CrossRef]
- Machado, E.S.; Ambach, M.A.; Caldas, J.M.; Wei, J.J.; Bredemeier, M. Personalized multitarget biologic injection in the spine: Prospective case series of multitarget platelet-rich plasma for low back pain. Regen. Med. 2022, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, M.S.; Behera, P.; Patel, S.; Shetty, V. Orthobiologics and platelet rich plasma. Indian J. Orthop. 2014, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.S.; Soares, F.P.; Yamaguchi, R.S.; Felipone, W.K.; Meves, R.; Souza, T.A.C.; Topolniak, R.; Caldas, J.P.; Abreu, E.V.; Neto, L.S.R.; et al. A Simple Double-Spin Closed Method for Preparing Platelet-Rich Plasma. Cureus 2022, 14, e20899. [Google Scholar] [CrossRef]
- Tramś, E.; Malesa, K.; Pomianowski, S.; Kamiński, R. Role of Platelets in Osteoarthritis—Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis. Cells 2022, 11, 1080. [Google Scholar] [CrossRef]
- Thu, A.C. The use of platelet-rich plasma in management of musculoskeletal pain: A narrative review. Yeungnam Univ. J. Med. 2022, 39, 206–215. [Google Scholar] [CrossRef]
- Anitua, E.; Padilla, S. Biologic therapies to enhance intervertebral disc repair. Regen. Med. 2018, 13, 55–72. [Google Scholar] [CrossRef]
- Kim, S.H.; Kuh, S.U.; Kim, K.N.; Park, J.Y.; Cho, K.H.; Chin, D.K.; Kim, K.S.; Cho, Y.E. Biologic Response of Degenerative Living Human Nucleus Pulposus Cells to Treatment with Cytokines. Yonsei Med. J. 2015, 56, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, R.; Gan, Y.; Wang, L.; Zhao, C.; Luo, L.; Zhang, C.; Zhou, Q. Effects of osteogenic protein-1 on intervertebral disc regeneration: A systematic review of animal studies. Biomed. Pharmacother. 2017, 88, 260–266. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.S.; Pichika, R.; Attawia, M.; Thonar, E.J.-M.A.; Lenz, M.E.; Uchida, A.; Masuda, K. Platelet-Rich Plasma (PRP) Stimulates the Extracellular Matrix Metabolism of Porcine Nucleus Pulposus and Anulus Fibrosus Cells Cultured in Alginate Beads. Spine 2006, 31, 959–966. [Google Scholar] [CrossRef]
- Obata, S.; Akeda, K.; Imanishi, T.; Masuda, K.; Bae, W.; Morimoto, R.; Asanuma, Y.; Kasai, Y.; Uchida, A.; Sudo, A. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: A preclinical study. Arthritis Res. Ther. 2012, 14, R241. [Google Scholar] [CrossRef]
- Li, P.; Zhang, R.; Zhou, Q. Efficacy of Platelet-Rich Plasma in Retarding Intervertebral Disc Degeneration: A Meta-Analysis of Animal Studies. BioMed Res. Int. 2017, 2017, 7919201. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Yu, W.; Dou, Y.; Wang, T. Efficacy of Platelet-rich Plasma for Low Back Pain: A Systematic Review and Meta-analysis. J. Neurol. Surg. Part A Central Eur. Neurosurg. 2020, 81, 529–534. [Google Scholar] [CrossRef]
- Muthu, S.; Jeyaraman, M.; Chellamuthu, G.; Jeyaraman, N.; Jain, R.; Khanna, M. Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? Glob. Spine J. 2021, 12, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Navani, A.; Manchikanti, L.; Albers, S.L.; Latchaw, R.E.; Sanapati, J.; Kaye, A.D.; Atluri, S.; Jordan, S.; Gupta, A.; Cedeno, D.; et al. Responsible, Safe, and Effective Use of Biologics in the Management of Low Back Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2019, 22, S1–S74. [Google Scholar]
- Daste, C.; Laclau, S.; Boisson, M.; Segretin, F.; Feydy, A.; Lefèvre-Colau, M.-M.; Rannou, F.; Nguyen, C. Intervertebral disc therapies for non-specific chronic low back pain: A systematic review and meta-analysis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720x211028001. [Google Scholar] [CrossRef]
- Peng, B.-G. Pathophysiology, diagnosis, and treatment of discogenic low back pain. World J. Orthop. 2013, 4, 42–52. [Google Scholar] [CrossRef]
- Peng, B.; Hao, J.; Hou, S.; Wu, W.; Jiang, D.; Fu, X.; Yang, Y. Possible Pathogenesis of Painful Intervertebral Disc Degeneration. Spine 2006, 31, 560–566. [Google Scholar] [CrossRef]
- Erwin, W.M.; Hood, K.E. The cellular and molecular biology of the intervertebral disc: A clinician’s primer. J. Can. Chiropr. Assoc. 2014, 58, 246–257. [Google Scholar]
- Peng, B.; Hou, S.; Wu, W.; Zhang, C.; Yang, Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur. Spine J. 2005, 15, 583–587. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.A.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Thompson, K.J.; Dagher, A.P.; Eckel, T.S.; Clark, M.; Reinig, J.W. Modic Changes on MR Images as Studied with Provocative Diskography: Clinical Relevance—A Retrospective Study of 2457 Disks. Radiology 2009, 250, 849–855. [Google Scholar] [CrossRef]
- Cuellar, J.M.; Stauff, M.P.; Herzog, R.J.; Carrino, J.A.; Baker, G.A.; Carragee, E.J. Does provocative discography cause clinically important injury to the lumbar intervertebral disc? A 10-year matched cohort study. Spine J. 2016, 16, 273–280. [Google Scholar] [CrossRef]
- Gelalis, I.; Gkiatas, I.; Spiliotis, A.; Papadopoulos, D.; Pakos, E.; Vekris, M.; Korompilias, A. Current concepts in intradiscal percutaneous minimally invasive procedures for chronic low back pain. Asian J. Neurosurg. 2019, 14, 657–669. [Google Scholar] [CrossRef]
- Navani, A.; Ambach, M.A.; Navani, R.; Wei, J. Biologics for Lumbar Discogenic Pain: 18 Month Follow-up for Safety and Efficacy. Int. Pain Manag. Rep. 2018, 2, 8. [Google Scholar]
- Fujii, K.; Yamazaki, M.; Kang, J.D.; Risbud, M.V.; Cho, S.K.; Qureshi, S.A.; Hecht, A.C.; Iatridis, J.C. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus 2019, 3, e10180. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; McNally, D.S.; Dolan, P. “Stress” Distributions inside Intervertebral Discs. The Effects of Age and Degeneration. J. Bone Jt. Surg. 1996, 78, 965–972. [Google Scholar] [CrossRef]
- Igarashi, T.; Kikuchi, S.; Shubayev, V.; Myers, R.R. 2000 Volvo Award Winner in Basic Science Studies: Exogenous Tumor Necrosis Factor-Alpha Mimics Nucleus Pulposus-Induced Neuropathology. Molecular, Histologic, and Behavioral Comparisons in Rats. Spine 2000, 25, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Sapkas, G.; Papadopoulos, E.C.; Katonis, P. Pathophysiology and Biomechanics of the Aging Spine. Open Orthop. J. 2011, 5, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Pollintine, P.; Przybyla, A.; Dolan, P.; Adams, M. Neural arch load-bearing in old and degenerated spines. J. Biomech. 2004, 37, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Kaye, A.D.; Soin, A.; Albers, S.L.; Beall, D.; Latchaw, R.; Sanapati, M.R.; Shah, S.; Atluri, S.; Abd-Elsayed, A.; et al. Comprehensive Evidence-Based Guidelines for Facet Joint Interventions in the Management of Chronic Spinal Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines Facet Joint Interventions 2020 Guidelines. Pain Physician 2020, 23, S1–S127. [Google Scholar] [CrossRef] [PubMed]
- Seyedhoseinpoor, T.; Taghipour, M.; Dadgoo, M.; Sanjari, M.A.; Takamjani, I.E.; Kazemnejad, A.; Khoshamooz, Y.; Hides, J. Alteration of lumbar muscle morphology and composition in relation to low back pain: A systematic review and meta-analysis. Spine J. 2021, 22, 660–676. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Li, M.; Huang, J.; Huang, J.; Liang, Y.; Lu, S.; Liang, C.; Xing, T.; Su, K.; et al. Correlation between posterior paraspinal muscle atrophy and lumbar intervertebral disc degeneration in patients with chronic low back pain. Int. Orthop. 2022, 47, 793–801. [Google Scholar] [CrossRef]
- Hodges, P.W.; Danneels, L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2016, 389, 736–747. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.-W.C.; Chenot, J.-F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Malmivaara, A.; van Tulder, M.W. Exercise therapy for chronic low back pain. Cochrane Database Syst. Rev. 2021, 9, CD009790. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Torres-Costoso, A.; Pozuelo-Carrascosa, D.P.; Reina-Gutiérrez, S.; Pascual-Morena, C.; Martínez-Vizcaíno, V. Best Exercise Options for Reducing Pain and Disability in Adults With Chronic Low Back Pain: Pilates, Strength, Core-Based, and Mind-Body. A Network Meta-analysis. J. Orthop. Sports Phys. Ther. 2022, 52, 505–521. [Google Scholar] [CrossRef]
- Owen, P.J.; Miller, C.T.; Mundell, N.L.; Verswijveren, S.J.J.M.; Tagliaferri, S.D.; Brisby, H.; Bowe, S.J.; Belavy, D.L. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sports Med. 2019, 54, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chung, C.K.; Park, C.S.; Choi, B.; Kim, M.J.; Park, B.J. Reoperation Rate After Surgery for Lumbar Herniated Intervertebral Disc Disease: Nationwide Cohort Study. Spine 2013, 38, 581–590. [Google Scholar] [CrossRef]
- Lang, S.A.J.; Bohn, T.; Barleben, L.; Pumberger, M.; Roll, S.; Büttner-Janz, K. Advanced meta-analyses comparing the three surgical techniques total disc replacement, anterior stand-alone fusion and circumferential fusion regarding pain, function and complications up to 3 years to treat lumbar degenerative disc disease. Eur. Spine J. 2021, 30, 3688–3701. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Schneider, M.D. Regenerative Medicine: Prometheus unbound. Nature 2004, 432, 451–453. [Google Scholar] [CrossRef]
- Collins, T.; Alexander, D.; Barkatali, B. Platelet-rich plasma: A narrative review. EFORT Open Rev. 2021, 6, 225–235. [Google Scholar] [CrossRef]
- Nazaroff, J.; Oyadomari, S.; Brown, N.; Wang, D. Reporting in clinical studies on platelet-rich plasma therapy among all medical specialties: A systematic review of Level I and II studies. PLoS ONE 2021, 16, e0250007. [Google Scholar] [CrossRef]
- Bugarin, A.; Schroeder, G.; Shi, B.Y.; Jones, K.J.; Kremen, T.J. Assessment of Characteristics and Methodological Quality of the Top 50 Most Cited Articles on Platelet-Rich Plasma in Musculoskeletal Medicine. Orthop. J. Sports Med. 2022, 10, 23259671221093070. [Google Scholar] [CrossRef]
- Akeda, K.; Ohishi, K.; Masuda, K.; Bae, W.C.; Takegami, N.; Yamada, J.; Nakamura, T.; Sakakibara, T.; Kasai, Y.; Sudo, A. Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain: A Preliminary Clinical Trial. Asian Spine J. 2017, 11, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; Takegami, N.; Yamada, J.; Fujiwara, T.; Ohishi, K.; Tamaru, S.; Sudo, A. Platelet-Rich Plasma-Releasate (PRPr) for the Treatment of Discogenic Low Back Pain Patients: Long-Term Follow-Up Survey. Medicina 2022, 58, 428. [Google Scholar] [CrossRef]
- Akeda, K.; Ohishi, K.; Takegami, N.; Sudo, T.; Yamada, J.; Fujiwara, T.; Niimi, R.; Matsumoto, T.; Nishimura, Y.; Ogura, T.; et al. Platelet-Rich Plasma Releasate versus Corticosteroid for the Treatment of Discogenic Low Back Pain: A Double-Blind Randomized Controlled Trial. J. Clin. Med. 2022, 11, 304. [Google Scholar] [CrossRef]
- Gui, K.; Ren, W.; Yu, Y.; Li, X.; Dong, J.; Yin, W. Inhibitory Effects of Platelet-Rich Plasma on Intervertebral Disc Degeneration: A Preclinical Study in a Rabbit Model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1368–1375. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Jin, J.-Y.; Guo, Y.-D.; Ma, L.-Y.; Chang, Q.; Peng, X.-G.; Guo, F.-F.; Zhang, H.-X.; Hu, X.-F.; Wang, C. Intervertebral disc regeneration using platelet-rich plasma-containing bone marrow-derived mesenchymal stem cells: A preliminary investigation. Mol. Med. Rep. 2016, 13, 3475–3481. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Hussein, T. Effect of autologous platelet leukocyte rich plasma injections on atrophied lumbar multifidus muscle in low back pain patients with monosegmental degenerative disc disease. SICOT-J 2016, 2, 12. [Google Scholar] [CrossRef]
- Sanapati, J.; Manchikanti, L.; Atluri, S.; Jordan, S.; Albers, S.L.; Pappolla, M.A.; Kaye, A.D.; Candido, K.D.; Pampati, V.; Hirsch, J.A. Do Regenerative Medicine Therapies Provide Long-Term Relief in Chronic Low Back Pain: A Systematic Review and Metaanalysis. Pain Physician 2018, 21, 515–540. [Google Scholar]
- Wu, J.; Zhou, J.; Liu, C.; Zhang, J.; Xiong, W.; Lv, Y.; Liu, R.; Wang, R.; Du, Z.; Zhang, G.; et al. A Prospective Study Comparing Platelet-Rich Plasma and Local Anesthetic (LA)/Corticosteroid in Intra-Articular Injection for the Treatment of Lumbar Facet Joint Syndrome. Pain Pract. 2017, 17, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Hyzy, M.; Williams, C.; Freeman, M. The use of lumbar epidural injection of platelet lysate for treatment of radicular pain. J. Exp. Orthop. 2017, 4, 914–924. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Williams, C.A.; Wadey, C.; Pieles, G.; Stuart, G.; Taylor, R.S.; Long, L. Physical activity interventions for people with congenital heart disease. Cochrane Database Syst. Rev. 2020, 2021, CD013400. [Google Scholar] [CrossRef]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J. Bone Jt. Surg. 2017, 99, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM&R 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Eldin, M.M.; Hassan, A.S.A.; Baraka, M.A.A.; Khorshied, M. Intradiscal Injection of Autologous Platelet-Rich Fibrin versus Platelet-Rich Plasma in Discogenic Lumbar Pain: An Applied Comparative Study. J. Orthop. Trauma Surg. Relat. Res. 2020, 15, 1. [Google Scholar]
- Zielinski, M.A.; Evans, N.E.; Bae, H.; Kamrava, E.; Calodney, A.; Remley, K.; Benyamin, R.; Franc, D.; Peterson, M.R.; Lovine, J.; et al. Safety and Efficacy of Platelet Rich Plasma for Treatment of Lumbar Discogenic Pain: A Prospective, Multicenter, Randomized, Double-blind Study. Pain Physician 2022, 25, 29–34. [Google Scholar]
- Schepers, M.; Groot, D.; Kleinjan, E.; Pol, M.; Mylenbusch, H.; Klopper-Kes, A. Effectiveness of intradiscal platelet rich plasma for discogenic low back pain without Modic changes: A randomized controlled trial. Interv. Pain Med. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Núñez, P.P.B.; Pérez, M.G.; Farril, D.T.O.; González, M.M. Efficacy of intradiscal application of platelet-rich plasma as a treatment for lumbar discogenic pain. Investig. Medicoquirúrgicas 2019, 11. [Google Scholar]
- Navani, A.; Ambach, M.; Calodney, A.; Rosenthal, R.; Li, G.; Mahoney, C.B.; Everts, P.A. The Safety and Effectiveness of Orthobiologic Injections for Discogenic Chronic Low Back Pain: A Multicenter Prospective, Crossover, Randomized Controlled Study. Pain Physician Journal. in peer review process.
- Ruiz-Lopez, R.; Tsai, Y. A Randomized Double-Blind Controlled Pilot Study Comparing Leucocyte-Rich Platelet-Rich Plasma and Corticosteroid in Caudal Epidural Injection for Complex Chronic Degenerative Spinal Pain. Pain Pract. 2020, 20, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, S.; Li, X.; Liu, C.; Fan, S.; Ma, C. Ultrasound-Guided Transforaminal Injections of Platelet-Rich Plasma Compared with Steroid in Lumbar Disc Herniation: A Prospective, Randomized, Controlled Study. Neural Plast. 2021, 2021, 5558138. [Google Scholar] [CrossRef] [PubMed]
- Núñez, P.P.B.; Cedeño, J.L.H.; Oliver, T.G.; Gómez, M.P.; Peguera, I.F.; Piedra, M.G.; Santiesteban, Y.Z.; Gómez, M.P.; Bosque, Y.C. Efficacy of parasargital translaminar epidural application of growth factors derived from Platelet Rich Plasma as a treatment for unilateral root pain caused by multisegmental disc disease. Investig. Medicoquirúrgicas 2021, 13. [Google Scholar]
- Byvaltsev, V.A.; Kalinin, A.A.; Okoneshnikova, A.K. Comparative analysis of the effectiveness of PRP therapy and facetoplasty in older patients with isolated lumbar facet syndrome: Long-term results of a randomized controlled trial. Adv. Gerontol. Uspekhi Gerontol. 2019, 32, 804–811. [Google Scholar]
- Won, S.J.; Kim, D.-Y.; Kim, J.M. Effect of platelet-rich plasma injections for chronic nonspecific low back pain: A Randomized Controlled Study. Medicine 2022, 101, e28935. [Google Scholar] [CrossRef]
- Levi, D.; Horn, S.; Tyszko, S.; Levin, J.; Hecht-Leavitt, C.; Walko, E. Intradiscal Platelet-Rich Plasma Injection for Chronic Discogenic Low Back Pain: Preliminary Results from a Prospective Trial. Pain Med. 2015, 17, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Sevgili, U.; Sari, A.S. May Single One Level Intradiscal Autologous Platelet Rich Plasma Injection Play a Role in the Treatment of Discogenic Pain? Results at Six Month Follow-Up. Kırıkkale Üniversitesi Tıp Fakültesi Derg. 2020, 22, 39–49. [Google Scholar] [CrossRef]
- Jain, D.; Goyal, T.; Verma, N.; Paswan, A.K.; Dubey, R.K. Intradiscal Platelet-Rich Plasma Injection for Discogenic Low Back Pain and Correlation with Platelet Concentration: A Prospective Clinical Trial. Pain Med. 2020, 21, 2719–2725. [Google Scholar] [CrossRef]
- Lutz, C.; Cheng, J.; Prysak, M.; Zukofsky, T.; Rothman, R.; Lutz, G. Clinical outcomes following intradiscal injections of higher-concentration platelet-rich plasma in patients with chronic lumbar discogenic pain. Int. Orthop. 2022, 46, 1381–1385. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Gong, Q.; Chen, J.; Wan, L. Intradiscal Autologous Platelet-Rich Plasma Injection for Discogenic Low Back Pain: A Clinical Trial. BioMed Res. Int. 2022, 2022, 9563693. [Google Scholar] [CrossRef]
- Bhatia, R.; Chopra, G. Efficacy of Platelet Rich Plasma via Lumbar Epidural Route in Chronic Prolapsed Intervertebral Disc Patients-A Pilot Study. J. Clin. Diagn. Res. 2016, 10, UC05–UC07. [Google Scholar] [CrossRef]
- Correa, J.; Cortés, H.; Coral, O.; García, E. PRP epidural en el manejo de la enfermedad discal degenerativa y dolor axial. Estudio preliminar. Rev. Soc. Esp. Dolor 2017, 24, 85–95. [Google Scholar]
- Bise, S.; Dallaudiere, B.; Pesquer, L.; Pedram, M.; Meyer, P.; Antoun, M.B.; Hocquelet, A.; Silvestre, A. Comparison of interlaminar CT-guided epidural platelet-rich plasma versus steroid injection in patients with lumbar radicular pain. Eur. Radiol. 2020, 30, 3152–3160. [Google Scholar] [CrossRef]
- Jose, C.; Henry, C.; Patricia, A.; Edwin, G. Epidural Plasma Rich in Growth Factors for Degenerative Disc Disease: A Valuable Alternative to Conventional “Palliative Medicine”. Int. J. Anesth. Clin. Med. 2019, 7, 1. [Google Scholar] [CrossRef]
- Le, V.-T.; Dao, L.T.N.; Nguyen, A.M. Transforaminal Injection with Autologous Platelet-Rich Plasma in Lumbar Disc Herniation: A Single-Center Prospective Study in Vietnam. Asian J. Surg. 2023, 46, 438–443. [Google Scholar] [CrossRef]
- Wu, J.; Du, Z.; Lv, Y.; Zhang, J.; Xiong, W.; Wang, R.; Liu, R.; Zhang, G.; Liu, Q. A New Technique for the Treatment of Lumbar Facet Joint Syndrome Using Intra-articular Injection with Autologous Platelet Rich Plasma. Pain Physician 2016, 19, 617–625. [Google Scholar]
- Byvaltsev, V.A.; Kalinin, A.A.; Okoneshnikova, A.K.; Satardinova, E.E. Analysis of the clinical efficacy of platelet-rich plasma therapy in the treatment of patients with isolated facet-syndrome of the lumbar spine. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 2019, 119, 27–31. [Google Scholar] [CrossRef]
- Byvaltsev, V.; Kalinin, A.; Okoneshnikova, A.; Biryuchkov, M. PRP Therapy for the Treatment of Lumbar Facet Syndrome in Professional Team Sports Athletes. Hum. Sport Med. 2022, 22, 169–178. [Google Scholar]
- Darrow, M.; Shaw, B.; Nicholas, S.; Li, X.; Boeger, G. Treatment of unresolved lower back pain with platelet-rich plasma injections. Cogent Med. 2019, 6, 1581449. [Google Scholar] [CrossRef]
- Schwartz, A.; Meléndez, C.; Martínez, M. Ozone and Ozonated Growth Factors in the Treatment of Disc Herniation and Discartrosis Lumbar Spine. Ozone Ther. Glob. J. 2013, 3, 21–33. [Google Scholar]
- Kirchner, F. Tratamiento de las patologías discales y degenerativas de la columna vertebral con Plasma Rico en Factores de Crecimiento Plaquetario Ozonizados. Ozone Ther. Glob. J. 2012, 2, 91–106. [Google Scholar]
- Anitua, E.; Kirchner, F. Intradiscal and intra articular facet infiltrations with plasma rich in growth factors reduce pain in patients with chronic low back pain. J. Craniovertebr. Junction Spine 2016, 7, 251–256. [Google Scholar] [CrossRef]
- Cameron, J.A.; Thielen, K.M. Autologous Platelet Rich Plasma for Neck and Lower Back Pain Secondary to Spinal Disc Herniation: Midterm Results. Spine Res. 2017, 3, 10. [Google Scholar] [CrossRef]
- Morera, L.M.T.; García-Palacios, M.V.; Pareja, D.B.; Rebollar, R.E.; Lopez, J.A.L.; Matas, R.S.d.L.; Seoane, E.C. Tratamiento con la administración intradiscal de plasma rico en plaquetas para dolor crónico discogénico cervical y lumbar. Multidiscip. Pain J. 2021, 1, 123–130. [Google Scholar] [CrossRef]
- Kirchner, F.; Milani, I.; Martinez, A.; Kirchner-Bossi, N.; Prado, R.; Padilla, S.; Anitua, E. Plasma Rich in Growth Factors (PRGF) in the Treatment of Cervical and Lumbar Back Pain: A Retrospective Observational Clinical Study. Pain Physician 2021, 24, E649–E660. [Google Scholar]
- Godek, P. High Volume PRP Therapy. Ortop. Traumatol. Rehabil. 2022, 24, 43–60. [Google Scholar] [CrossRef]
- Machado, E.S. A Regenerative Interventional Approach to the Management of Degenerative Low Back Pain. J. Regen. Med. Biol. Res. 2022, 3, 1–16. [Google Scholar] [CrossRef]
- Cheng, J.; Santiago, K.A.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Treatment of symptomatic degenerative intervertebral discs with autologous platelet-rich plasma: Follow-up at 5–9 years. Regen. Med. 2019, 14, 831–840. [Google Scholar] [CrossRef]
- Manchikanti, L.; Abdi, S.; Atluri, S.; Benyamin, R.M.; Boswell, M.V.; Buenaventura, R.M.; Bryce, D.A.; Burks, P.A.; Caraway, D.L.; Calodney, A.K.; et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: Guidance and recommendations. Pain Physician 2013, 16 (Suppl. S2), S49–S283. [Google Scholar] [PubMed]
- Manchikanti, L. Lack of Superiority of Epidural Injections with Lidocaine with Steroids Compared to Without Steroids in Spinal Pain: A Systematic Review and Meta-Analysis. Pain Physician 2020, 23, S239–S270. [Google Scholar] [CrossRef]
- DeClercq, M.G.; Fiorentino, A.M.; Lengel, H.A.; Ruzbarsky, J.J.; Robinson, S.K.; Oberlohr, V.T.; Whitney, K.E.; Millett, P.J.; Huard, J. Systematic Review of Platelet-Rich Plasma for Rotator Cuff Repair: Are We Adhering to the Minimum Information for Studies Evaluating Biologics in Orthopaedics? Orthop. J. Sports Med. 2021, 9, 23259671211041971. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, A.; Fitzpatrick, J.; O’donnell, J.M. Treatment of Gluteal Tendinopathy: A Systematic Review and Stage-Adjusted Treatment Recommendation. Orthop. J. Sports Med. 2021, 9, 23259671211016850. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Braun, H.J.; Durham, J.L.; Ridley, B.A.; Odegaard, J.I.; Luong, R.; Arnoczky, S.P. Comparison of the Acute Inflammatory Response of Two Commercial Platelet-Rich Plasma Systems in Healthy Rabbit Tendons. Am. J. Sports Med. 2012, 40, 1274–1281. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, Y.-B.; Ha, C.-W.; Roh, Y.J.; Park, J.-G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211011948. [Google Scholar] [CrossRef]
- Abbas, A.; Du, J.T.; Dhotar, H.S. The Effect of Leukocyte Concentration on Platelet-Rich Plasma Injections for Knee Osteoarthritis: A Network Meta-Analysis. J. Bone Jt. Surg. 2021, 104, 559–570. [Google Scholar] [CrossRef]
- Niemiec, P.; Szyluk, K.; Jarosz, A.; Iwanicki, T.; Balcerzyk, A. Effectiveness of Platelet-Rich Plasma for Lateral Epicondylitis: A Systematic Review and Meta-analysis Based on Achievement of Minimal Clinically Important Difference. Orthop. J. Sports Med. 2022, 10, 23259671221086920. [Google Scholar] [CrossRef]
- Shim, J.W.; Lee, J.-S.; Park, Y.-B.; Cho, H.-C.; Jung, H.-S. The effect of leucocyte concentration of platelet-rich plasma on outcomes in patients with lateral epicondylitis: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2021, 31, 634–645. [Google Scholar] [CrossRef]
- Muthu, S.; Patel, S.; Selvaraj, P.; Jeyaraman, M. Comparative analysis of leucocyte poor vs leucocyte rich platelet-rich plasma in the management of lateral epicondylitis: Systematic review & meta-analysis of randomised controlled trials. J. Clin. Orthop. Trauma 2021, 19, 96–107. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci.-Landmark 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, C.-W.; Xu, H.; Wang, Y.-F.; Yung, P.S.H.; Jiang, Y.; Lee, O.K. Phenotypic alteration of macrophages during osteoarthritis: A systematic review. Arthritis Res. Ther. 2021, 23, 110. [Google Scholar] [CrossRef]
- Nishio, H.; Saita, Y.; Kobayashi, Y.; Takaku, T.; Fukusato, S.; Uchino, S.; Wakayama, T.; Ikeda, H.; Kaneko, K. Platelet-rich plasma promotes recruitment of macrophages in the process of tendon healing. Regen. Ther. 2020, 14, 262–270. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Yin, J. Platelet-Rich Plasma: A Promising Product for Treatment of Peripheral Nerve Regeneration After Nerve Injury. Int. J. Neurosci. 2011, 121, 176–180. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-Rich Plasma: Growth Factors and Pro- and Anti-Inflammatory Properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef]
- Yadav, A.; Ramasamy, T.S.; Lin, S.-C.; Chen, S.-H.; Lu, J.; Liu, Y.-H.; Lu, F.-I.; Hsueh, Y.-Y.; Lin, S.-P.; Wu, C.-C. Autologous Platelet-Rich Growth Factor Reduces M1 Macrophages and Modulates Inflammatory Microenvironments to Promote Sciatic Nerve Regeneration. Biomedicines 2022, 10, 1991. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef]
- Tisot, R.A.; Vieira, J.D.S.; Collares, D.D.S.; Tisatto, D.L.; Pasini, A.; Gobetti, B.; Coronel, E.; Scharnovski, E.; Agostini, M.; Borin, M.; et al. Facet joint degeneration in patients with lumbar disc herniation and probable determining factors. Coluna/Columna 2020, 19, 262–265. [Google Scholar] [CrossRef]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Krittanawong, C.; Johnson, K.W.; Tang, W.W. How artificial intelligence could redefine clinical trials in cardiovascular medicine: Lessons learned from oncology. Pers. Med. 2019, 16, 87–92. [Google Scholar] [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- Dickson, D.; Johnson, J.; Bergan, R.; Owens, R.; Subbiah, V.; Kurzrock, R. The Master Observational Trial: A New Class of Master Protocol to Advance Precision Medicine. Cell 2020, 180, 9–14. [Google Scholar] [CrossRef] [PubMed]


| Criteria | Determinants |
|---|---|
| P (Population) | People with low back pain |
| I (Intervention) | PRP injection |
| C (Comparator) | Steroids, hyaluronic acid, ozone, saline, contrast medium |
| O (Outcome) | Pain control, less disability, |
| Study Design | RCTs, NRCTs, case series with more than 10 patients. |
| Level I | Strong evidence obtained from multiple relevant high-quality randomized controlled trials for effectiveness. |
| Level II | Moderate evidence obtained from at least one relevant high quality randomized controlled trial or multiple relevant |
| moderate or low-quality randomized controlled trials. | |
| Level III | Fair evidence obtained from at least one relevant high quality nonrandomized trial or observational study with |
| multiple moderate or low-quality observational studies. | |
| Level IV | Limited Evidence obtained from multiple moderate or low quality relevant observational studies. |
| Level V | Consensus-based opinion or consensus of large group of clinicians and/or scientists for effectiveness, as well as to assess |
| preventive measures, adverse consequences, and effectiveness of other measures. |
| Author (Reference) | Comparator Control Group | PRP Processing | PRP Characteristics | Sample (n) | Adverse Event | Follow-Up | |
|---|---|---|---|---|---|---|---|
| 1 | (Tuakli-Wosornu et al., 2016) [68] | placebo (contrast agent) | PRP Harvest Technol. Corporation (Plymouth, Ma) centrifuge | LR-PRP | 47 | Not related | 12 months |
| 2 | (Eldin et al., 2020) [69] | PRF | Single spin 1000 rpm 6 min | LP-PRP | 132 | Not related | 6 months |
| 3 | (Akeda, Ohishi, et al., 2022) [58] | betamethasone | PRP Relesate | PRP Rel | 16 | Pain: 1 case | 12 months |
| 4 | (Zielinski et al., 2022) [70] | placebo (saline) | PUREPRP 1st Spin 3800 RPM 1.5 min 2nd Spin 3800 RPM 5 min | LP-PRP | 36 | Pain: 1 case | 8 weeks |
| 5 | (Schepers et al., 2022) [71] | placebo (saline + kefazol) | SmartPReP Single spin 1000 RPM 15 min | LR-PRP | 89 | Discitis: 1 case | 12 months |
| 6 | (Núñez et al., 2019) [72] | ozone | 1st Spin 1200 rpm 8 min 2nd Spin 1200 rpm 8 min | NR | 67 | Vagal crisis: 2 cases | 12 months |
| 7 | (Navani et al. 2023) [73] | placebo (saline) | Emcyte Pure PRP | LP-PRP | 40 | None | 12 months |
| Author (Reference) | Comparator Control Group | PRP Processing | PRP Processing | Sample (n) | Adverse Event | Follow-Up | |
|---|---|---|---|---|---|---|---|
| 1 | (Ruiz-Lopez & Tsai, 2020) [74] Caudal Epidural | triamcinolone | Single spin 14 min 1568 g | LR-PRP | 50 | 1 case pruritus | 6 months |
| 2 | (Xu et al., 2021) [75] Transforaminal | betamethasone | 1st Spin 1600 rpm 10 min 2nd Spin 3200 rpm 10 min | LP-PRP | 124 | Not related | 12 months |
| 3 | (Núñez et al., 2021a) [76] Interlaminar | triamcinolone | Plasmaferesis—Plasma rich in growth factors | PRGF | 93 | 1 case headache | 12 months |
| 4 | (Wu et al., 2017) [63] Facet | betamethasone | PRP 1st Spin 200 g 10 min 2nd Spin 400 g 10 min | LR-PRP | 46 | Not related | 6 months |
| 5 | (Byvaltsev et al., 2019) [77] Facet | hyaluronic acid | PRP Single spin 450 g 20 min | LP-PRP | 144 | Not related | 12 months |
| 6 | (Won, Kim & Kim, 2022) [78] Muscle | lidocaine | Prosys system (Korea) | LR-PRP | 30 | Not related | 6 months |
| Author (Reference) | PRP Processing | PRP Characteristics | Target | Sample (n) | Adverse Event | Follow-Up | |
|---|---|---|---|---|---|---|---|
| 1 | Levi et al. 2015 [79] | Smartprep (Harvest) | Disc | 22 | Not related | 6 months | |
| 2 | Akeda et al. 2017 [56] | 1st Spin 3000 g 15 min 2nd Spin 180 g 15 min | PRP | Disc | 14 | Not related | 12 months |
| 3 | Navani et al. 2019 [34] | Emcyte Pure PRP system | PRP | Disc | 14 | Not related | 18 months |
| 4 | Sevgili et al., 2020 [80] | GPS III Biomet | PRP | Disc | 22 | Not related | 6 months |
| 5 | Jain et al., 2020 [81] | Double spin DrPRP kit (Dr PRP USA LLC) | LR-PRP | Disc | 20 | Not related | 6 months |
| 6 | Lutz et al., 2022 [82] | Emcyte PurePRP II kit. | LR-PRP | Disc | 37 | 1 discitis case | 18 months |
| 7 | Zhang et al., 2022 [83] | Regen Laboratories SA Harvest Techonol. Corp | LP-PRP | Disc | 31 | 1 case of discitis | 48 weeks |
| 8 | Jose Correa et al., 2017 [85] | Not described | Not described | Epidural | 70 | Not related | 3 months |
| 9 | Bhatia, 2016 [84] | Not described | Not described | Epidural | 10 | Not related | 3 months |
| 10 | Centeno, 2017 [64] | Platelet lysate | Platelet lysate | Epidural | 470 | Headache, Dural lesion | 24 months |
| 11 | Bise et al., 2020 [86] | Single spin 620 g 15 min | PRP | Epidural | 60 | Not related | 6 weeks |
| 12 | Jose Correa et al., 2019 [87] | Not described | PRGF | Epidural | 175 | Not related | 24 months |
| 13 | Viet-Thang Le 2022 [88] | 1st Spin 1600 rpm 10 min 2nd Spin 3200 rpm 10 min | PRP | Epidural TFI | 25 | Not related | 12 months |
| 14 | Wu et al., 2016 [89] | 1st Spin 200 g 10 min 2nd Spin 400 g 10 min | PRP | Facet | 19 | Not related | 3 months |
| 15 | Byvaltsev, 2019 [90] | Single spin 450 g 20 min | PRP | Facet | 49 | Not related | 18 months |
| 16 | Byvaltsev, 2022 [91] | Single spin 450 g 20 min | PRP | Facet | 41 | Hematoma | 18 months |
| 17 | Hussein and Hussein 2016 [61] | 1st Spin 1500 rpm 15 min 2nd Spin 3000 rpm 20 min PLRP. | LR-PRP | Lumbar Muscle | 104 | Not related | 24 months |
| 18 | Darrow et al., 2019 [92] | Not described | PRP | Lumbar Muscles | 67 | Not related | 4 months |
| Author (Reference) | PRP Processing | PRP Characteristics | Sample (n) | Adverse Event | Follow-Up | |
|---|---|---|---|---|---|---|
| 1 | Schwartz et al. 2013 [93] | Kit Closed System (PROTEAL ®) | LP-PRP | 60 | Headache | 6 months |
| 2 | Kirchner, 2012 [94] | PRGF—Endoret ® | PRGF + O3 | 82 | Not related | 12 months |
| 3 | Kirchner, Anitua, 2016 [95] | PRGF—Endoret ® | PRGF | 86 | Headache | 18 months |
| 4 | Cameron 2017 [96] | Not Reported | Not Reported | 50 | Not related | 6 months |
| 5 | Machado et al., 2021 [10] | 1st Spin 200 g 15 min 2nd Spin 1600 g 10 min | LR-PRP | 46 | Not related | 12 months |
| 6 | Torres Morera et al., 2021 [97] | Not Reported | LR-PRP | 24 | Not Related | 18 months |
| 7 | Kirchner et al., 2021 [98] | PRGF—Endoret ® | PRGF | 47 | Not Reported | 48 weeks |
| 8 | Godek et al., 2022 [99] | Angel System ®—Arthrex | LR-PRP | 91 | Not related | 3 months |
| 9 | Machado et al., 2022 [100] | 1st Spin 200 g 12 min 2nd Spin 1600 g 8 min | LR-PRP | 23 | Not related | 12 months |
| Author Year | Evaluation Method | Sample Size | PRP Group | Control Group | Follow-Up Time | PRP Group | Control Group |
|---|---|---|---|---|---|---|---|
| Baseline Values (*) | Follow-Up Values (*) | ||||||
| Tuakli-Wosornu et al., 2016 [68] | NRS | 47 | 7.98 (1.56) | 7.72 (1.53) | 8 weeks | 5.82 (2.33) | 6.83 (2.33) |
| 12 months | 5.86 (2.20) | - | |||||
| Eldin et al., 2020 [69] | VAS | 8.45 ± 0.59 | 8.34 ± 0.77 | 6 months | 6.84 ± 1.58 | 4.95 ± 2.07 | |
| Akeda, Ohishi, et al., 2022 [58] | VAS | 16 | 6.83 (1.33) | 5.94 (1.24) | 12 months | 1.49 (2.47) | 2.3 (2.37) |
| Schepers et al., 2022 [71] | NRS | 89 | 6.29 (1.23) | 6.02 (1.48) | 12 months | 5.3 | 5.1 |
| Núñez et al., 2019 [72] | Lattinen | 67 | EVA > 5 in both groups | 12 months | 90% EVA less than 2 | 20% EVA less than 2 | |
| Ruiz-Lopez & Tsai, 2020 [74] | VAS | 50 | 7.48 (1.12) | 7.18 (0.95) | 6 months | 6.08 (0.99) | 7.53 (0.60) |
| Xu et al., 2021 [75] | VAS | 124 | 6.0 (6.0–7.25) | 6.0 (5.0–7.0) | 12 months | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| Núñez et al., 2021 [76] | Lattinen | 93 | 8.5 | 8.5 | 12 months | 1.5 | 6.5 |
| Wu et al., 2017 [63] | VAS | 46 | 7.09 (1.08) | 6.74 (1.10) | 6 months | 2.7 | 4.5 |
| Byvaltsev et al., 2019 [77] | VAS | 144 | 6.85 (5.5–7.6) | 6.6 (6.0–7.4) | 18 months | 1 (0.8–1.8) | 1.7 (0.5–2.0) |
| Author Year | Evaluation Method | Sample Size | PRP Group | Control Group | Follow-Up Time | PRP Group | Control Group |
|---|---|---|---|---|---|---|---|
| Baseline Values (*) | Follow-up Values (*) | ||||||
| Tuakli-Wosornu et al., 2016 [68] | FRI | 47 | 51.47 (15.62) | 45.37. (15.61) | 8 weeks | 37.99 (19.60) | 44.45 (19.60) |
| 12 months | 33.98 (20.35) | ||||||
| Akeda, Ohishi, et al., 2022 [58] | ODI | 16 | 36.0 ± 11.8 | 33.3 ± 11.6 | 12 months | −26.6 ± 14.8 | −13.9 ± 9.7 |
| RMQ | 8.6 ± 4.8 | 9.3 ± 4.7 | 12 months | −8.8 ± 5.0 | −4.2 ± 4.5 | ||
| Schepers et al., 2022 [71] | RMQ | 89 | 12.63 (5.35) | 13.42 (4.39) | 12 month | 9.6 (3.1) | 10.1 (3.3) |
| Ruiz-Lopez & Tsai, 2020 [74] | SF-36 | 50 | 31.30 (20.80) | 34.74 (18.42 | 6 months | 59.74 (22.57) | 35.42 (21.32) |
| Xu et al., 2021 [75] | ODI | 124 | 35.0 (26.35–44.0) | 27.0 (21.0–43.0) | 12 months | 19.0 (15.5–30.0) | 20.0 (17.3–40.0) |
| Núñez et al., 2021 [76] | LATINEN | 93 | 15.9 | 15.6 | 12 months | 1.7 | 12.3 |
| Wu et al., 2017 [63] | ODI | 46 | 60.64 (10.84) | 59.66 (10.35) | 6 months | 29.41 (7.76) | 44.11 (7.30) |
| RMQ | 17.15 (3.13) | 17.28 (2.27) | 6 months | 8.19 (3.48) | 13.60 (2.90) | ||
| Byvaltsev et al., 2019 [77] | ODI | 144 | 68 (53; 78) | 66 (58; 75) | 18 months | 6.5 (2; 10) | 14 (12; 20) |
| Won, Kim & Kim, 2022 [78] | ODI | 30 | 32.7 ± 9.8 | 32.2 ± 10.5 | 6 months | 16.1 ± 11.9 | 20.2 ± 7.9 |
| RMQ | 12.2 ± 2.9 | 11.6 ± 3.9 | 6 months | 4.0 ± 2.9 | 5.6 ± 3.2 | ||
| Author/ Year | Randomization Process | Deviations from Intended Intervention | Missing Outcome Data | Measurement of Outcome | Selection of Reported Result | Overall Bias |
|---|---|---|---|---|---|---|
| Tuakli-Wosornu et al., 2016 [68] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Akeda et al., 2022 [69] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Schepers et al., 2022 [71] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Núñez et al., 2019 [72] | Low Risk | Low Risk | High Risk | High Risk | Some Concerns | High Risk |
| Ruiz-Lopez & Tsai, 2020 [74] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Xu et al., 2021 [75] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Núñez et al., 2021 [76] | Low Risk | High Risk | High Risk | High Risk | Some Concerns | High Risk |
| Wu et al., 2017 [63] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Byvaltsev, 2019 [77] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Won, Kim & Kim, 2022 [78] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Navani, 2023 [73] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, E.S.; Soares, F.P.; Vianna de Abreu, E.; de Souza, T.A.d.C.; Meves, R.; Grohs, H.; Ambach, M.A.; Navani, A.; de Castro, R.B.; Pozza, D.H.; et al. Systematic Review of Platelet-Rich Plasma for Low Back Pain. Biomedicines 2023, 11, 2404. https://doi.org/10.3390/biomedicines11092404
Machado ES, Soares FP, Vianna de Abreu E, de Souza TAdC, Meves R, Grohs H, Ambach MA, Navani A, de Castro RB, Pozza DH, et al. Systematic Review of Platelet-Rich Plasma for Low Back Pain. Biomedicines. 2023; 11(9):2404. https://doi.org/10.3390/biomedicines11092404
Chicago/Turabian StyleMachado, Edilson Silva, Fabiano Pasqualotto Soares, Ernani Vianna de Abreu, Taís Amara da Costa de Souza, Robert Meves, Hans Grohs, Mary A. Ambach, Annu Navani, Renato Bevillaqua de Castro, Daniel Humberto Pozza, and et al. 2023. "Systematic Review of Platelet-Rich Plasma for Low Back Pain" Biomedicines 11, no. 9: 2404. https://doi.org/10.3390/biomedicines11092404
APA StyleMachado, E. S., Soares, F. P., Vianna de Abreu, E., de Souza, T. A. d. C., Meves, R., Grohs, H., Ambach, M. A., Navani, A., de Castro, R. B., Pozza, D. H., & Caldas, J. M. P. (2023). Systematic Review of Platelet-Rich Plasma for Low Back Pain. Biomedicines, 11(9), 2404. https://doi.org/10.3390/biomedicines11092404






