Breaking the Fear Barrier: Aberrant Activity of Fear Networks as a Prognostic Biomarker in Patients with Panic Disorder Normalized by Pharmacotherapy
Abstract
:1. Introduction
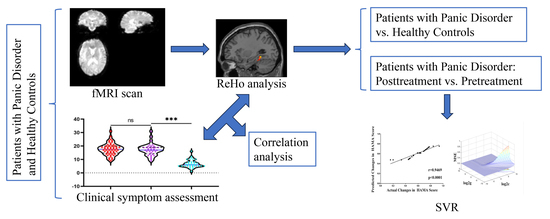
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.4. Imaging Data Acquisition and Preprocessing and ReHo Calculation
2.5. Statistical Analysis
2.6. SVR Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Paroxetine Treatment Outcome
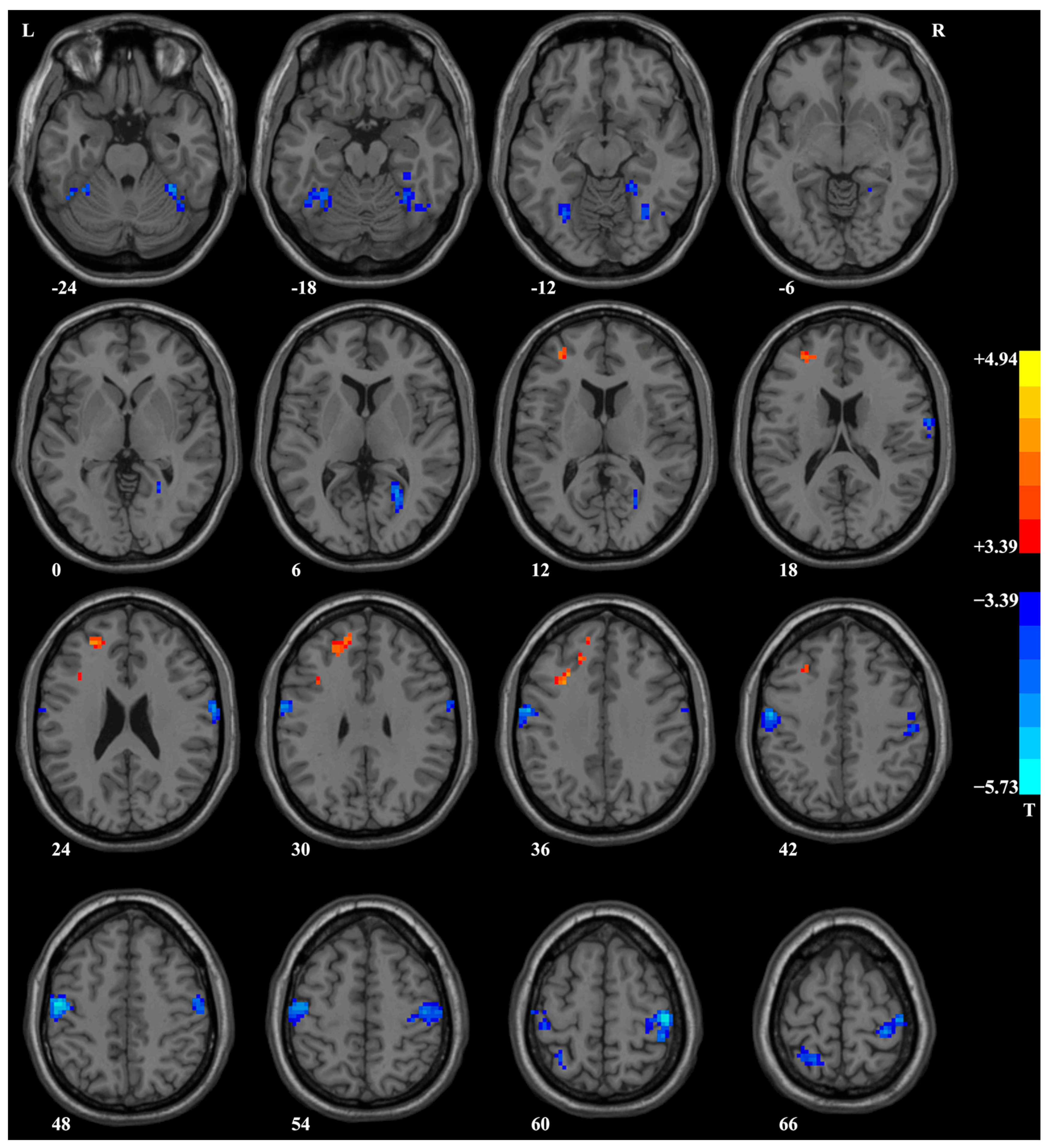
3.3. ReHo Analysis Results
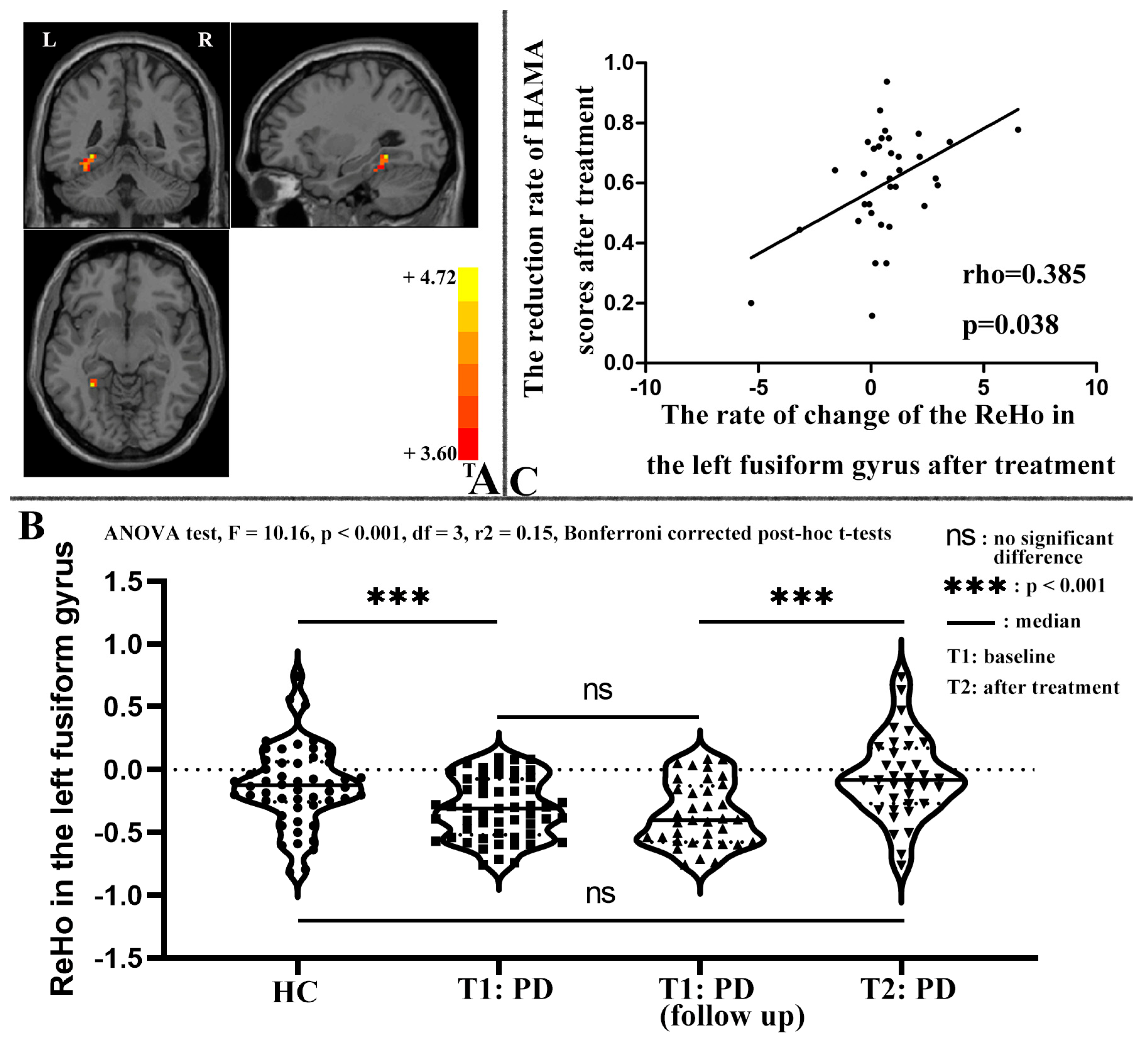
3.4. Correlation between ReHo and Clinical Symptoms of Patients with PD
3.5. SVR Analysis Results
4. Discussion
4.1. Abnormal ReHo at Baseline
4.2. Changes in ReHo after Treatment
4.3. Applying SVR to Predict the Treatment Response from Abnormal ReHo
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; Volume 21, pp. 591–643. [Google Scholar] [CrossRef]
- De Jonge, P.; Roest, A.M.; Lim, C.C.; Florescu, S.E.; Bromet, E.J.; Stein, D.J.; Harris, M.; Nakov, V.; Caldas-de-Almeida, J.M.; Levinson, D. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress. Anxiety 2016, 33, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Batelaan, N.; Smit, F.; de Graaf, R.; van Balkom, A.; Vollebergh, W.; Beekman, A. Economic costs of full-blown and subthreshold panic disorder. J. Affect. Disord. 2007, 104, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Mansueto, G. Biological and Clinical Markers in Panic Disorder. Psychiatry Investig. 2019, 16, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, G. Panic disorder: Definitions, contexts, neural correlates and clinical strategies. Curr. Trends Clin. Med. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, S.-H.; Han, C.; Jeong, H.-G.; Lee, M.-S.; Kim, J. Antidepressant-induced mania in panic disorder: A single-case study of clinical and functional connectivity characteristics. Front. Psychiatry 2023, 14, 1205126. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: Synaptic transmission and plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, C. Towards a mechanistic understanding of depression, anxiety, and their comorbidity: Perspectives from cognitive neuroscience. Front. Behav. Neurosci. 2023, 17, 1268156. [Google Scholar] [CrossRef]
- Campbell, T.L.; Kochli, D.E.; McDaniel, M.A.; Myers, M.K.; Dunn, M.E.; Diana, V.A.; Quinn, J.J. Using extinction-renewal to circumvent the memory strength boundary condition in fear memory reconsolidation. Brain Sci. 2021, 11, 1023. [Google Scholar] [CrossRef]
- Lai, C.-H.; Wu, Y.-T. The gray matter alterations in major depressive disorder and panic disorder: Putative differences in the pathogenesis. J. Affect. Disord. 2015, 186, 1–6. [Google Scholar] [CrossRef]
- Maron, E.; Hettema, J.; Shlik, J. Advances in molecular genetics of panic disorder. Mol. Psychiatry 2010, 15, 681–701. [Google Scholar] [CrossRef]
- Otte, C. Cognitive behavioral therapy in anxiety disorders: Current state of the evidence. Dialogues Clin. Neurosci. 2011, 13, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Kent, J.M.; Sullivan, G.M.; Coplan, J.D. Neuroanatomical hypothesis of panic disorder, revised. Am. J. Psychiatry 2000, 157, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Liebowitz, M.R.; Fyer, A.J.; Stein, J. A neuroanatomical hypothesis for panic disorder. Am. J. Psychiatry 1989, 146, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.R.; Chen, Z.F.; Kang, C.Y.; Liu, R.X.; Bai, J.Y.; Cao, Y.P.; Cheng, Y.Q.; Xu, X.F.; Zhang, Y.L. Mindfulness-based cognitive therapy is associated with distinct resting-state neural patterns in patients with generalized anxiety disorder. Asia-Pac. Psychiatry 2019, 11, e12368. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The influence of vicarious fear-learning in “infecting” reactive action inhibition. Front. Behav. Neurosci. 2022, 16, 946263. [Google Scholar] [CrossRef] [PubMed]
- Dresler, T.; Guhn, A.; Tupak, S.V.; Ehlis, A.-C.; Herrmann, M.J.; Fallgatter, A.J.; Deckert, J.; Domschke, K. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. J. Neural Transm. 2013, 120, 3–29. [Google Scholar] [CrossRef]
- Lai, C.-H. Fear network model in panic disorder: The past and the future. Psychiatry Investig. 2019, 16, 16. [Google Scholar] [CrossRef]
- Deppermann, S.; Vennewald, N.; Diemer, J.; Sickinger, S.; Haeussinger, F.B.; Dresler, T.; Notzon, S.; Laeger, I.; Arolt, V.; Ehlis, A.-C.; et al. Neurobiological and clinical effects of fNIRS-controlled rTMS in patients with panic disorder/agoraphobia during cognitive-behavioural therapy. NeuroImage Clin. 2017, 16, 668–677. [Google Scholar] [CrossRef]
- Kikuchi, M.; Koenig, T.; Munesue, T.; Hanaoka, A.; Strik, W.; Dierks, T.; Koshino, Y.; Minabe, Y. EEG microstate analysis in drug-naive patients with panic disorder. PLoS ONE 2011, 6, e22912. [Google Scholar] [CrossRef]
- Sim, H.B.; Kang, E.H.; Yu, B.H. Changes in Cerebral Cortex and Limbic Brain Functions after Short-Term Paroxetine Treatment in Panic Disorder: An [F]FDG-PET Pilot Study. Psychiatry Investig. 2010, 7, 215–219. [Google Scholar] [CrossRef]
- Sakai, Y.; Kumano, H.; Nishikawa, M.; Sakano, Y.; Kaiya, H.; Imabayashi, E.; Ohnishi, T.; Matsuda, H.; Yasuda, A.; Sato, A.; et al. Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport 2005, 16, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Jun, C.S.; Jeong, H.S.; Lee, S.; Lim, S.M.; Ma, J.; Ko, E.; Cho, H.B.; Yeum, T.S.; Lyoo, I.K. Altered cortical gyrification patterns in panic disorder: Deficits and potential compensation. J. Psychiatr. Res. 2013, 47, 1446–1454. [Google Scholar] [CrossRef]
- Beutel, M.E.; Stark, R.; Pan, H.; Silbersweig, D.; Dietrich, S. Changes of brain activation pre-post short-term psychodynamic inpatient psychotherapy: An fMRI study of panic disorder patients. Psychiatry Res. Neuroimaging 2010, 184, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Wu, Y.-T. Changes in regional homogeneity of parieto-temporal regions in panic disorder patients who achieved remission with antidepressant treatment. J. Affect. Disord. 2013, 151, 709–714. [Google Scholar] [CrossRef]
- Tanaka, M.; Diano, M.; Battaglia, S. Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Schmidt, A.; Hassel, S.; Tanaka, M. Case reports in neuroimaging and stimulation. Front. Psychiatry 2023, 14, 1264669. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Wu, Y.-T. Frontal regional homogeneity increased and temporal regional homogeneity decreased after remission of first-episode drug-naive major depressive disorder with panic disorder patients under duloxetine therapy for 6 weeks. J. Affect. Disord. 2012, 136, 453–458. [Google Scholar] [CrossRef]
- Neufang, S.; Geiger, M.J.; Homola, G.A.; Mahr, M.; Schiele, M.A.; Gehrmann, A.; Schmidt, B.; Gajewska, A.; Nowak, J.; Meisenzahl-Lechner, E.; et al. Cognitive-behavioral therapy effects on alerting network activity and effective connectivity in panic disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 587–598. [Google Scholar] [CrossRef]
- Yang, Y.; Lueken, U.; Richter, J.; Hamm, A.; Wittmann, A.; Konrad, C.; Ströhle, A.; Pfleiderer, B.; Herrmann, M.J.; Lang, T.; et al. Effect of CBT on biased semantic network in panic disorder: A multicenter fMRI study using semantic priming. Am. J. Psychiatry 2020, 177, 254–264. [Google Scholar] [CrossRef]
- Hahn, T.; Kircher, T.; Straube, B.; Wittchen, H.-U.; Konrad, C.; Ströhle, A.; Wittmann, A.; Pfleiderer, B.; Reif, A.; Arolt, V.; et al. Predicting treatment response to cognitive behavioral therapy in panic disorder with agoraphobia by integrating local neural information. JAMA Psychiatry 2015, 72, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, A.; Thilo, K.; Filippini, N.; Croft, A.; Harmer, C.J. Predicting rapid response to cognitive-behavioural treatment for panic disorder: The role of hippocampus, insula, and dorsolateral prefrontal cortex. Behav. Res. Ther. 2014, 62, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Pérez Benítez, C.I.; Shea, M.T.; Raffa, S.; Rende, R.; Dyck, I.R.; Ramsawh, H.J.; Edelen, M.O.; Keller, M.B. Anxiety sensitivity as a predictor of the clinical course of panic disorder: A 1-year follow-up study. Depress. Anxiety 2009, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Teachman, B.A.; Marker, C.D.; Clerkin, E.M. Catastrophic misinterpretations as a predictor of symptom change during treatment for panic disorder. J. Consult. Clin. Psychol. 2010, 78, 964. [Google Scholar] [CrossRef] [PubMed]
- Albus, M.; Lecrubier, Y.; Maier, W.; Buller, R.; Rosenberg, R.; Hippius, H. Drug treatment of panic disorder: Early response to treatment as a predictor of final outcome. Acta Psychiatr. Scand. 1990, 82, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Santacana, M.; Arias, B.; Mitjans, M.; Bonillo, A.; Montoro, M.; Rosado, S.; Guillamat, R.; Vallès, V.; Pérez, V.; Forero, C.G.; et al. Predicting response trajectories during cognitive-behavioural therapy for panic disorder: No association with the BDNF gene or childhood maltreatment. PLoS ONE 2016, 11, e0158224. [Google Scholar] [CrossRef]
- Sharp, D.; Power, K. Predicting treatment outcome for panic disorder and agoraphobia in primary care. Clin. Psychol. Psychother. Int. J. Theory Pract. 1999, 6, 336–348. [Google Scholar] [CrossRef]
- Chambless, D.L.; Milrod, B.; Porter, E.; Gallop, R.; McCarthy, K.S.; Graf, E.; Rudden, M.; Sharpless, B.A.; Barber, J.P. Prediction and moderation of improvement in cognitive-behavioral and psychodynamic psychotherapy for panic disorder. J. Consult. Clin. Psychol. 2017, 85, 803. [Google Scholar] [CrossRef]
- Ramnerö, J.; Öst, L.-G. Prediction of outcome in the behavioural treatment of panic disorder with agoraphobia. Cogn. Behav. Ther. 2004, 33, 176–180. [Google Scholar] [CrossRef]
- Yan, H.; Shan, X.; Li, H.; Liu, F.; Guo, W. Abnormal spontaneous neural activity as a potential predictor of early treatment response in patients with obsessive–compulsive disorder. J. Affect. Disord. 2022, 309, 27–36. [Google Scholar] [CrossRef]
- Mariette, A.; Khanna, R. Support Vector Regression. Effic. Learn. Mach. 2015, 11, 203–224. [Google Scholar] [CrossRef]
- Murphy, K.; Garavan, H. An empirical investigation into the number of subjects required for an event-related fMRI study. Neuroimage 2004, 22, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Pajula, J.; Tohka, J. How many is enough? Effect of sample size in inter-subject correlation analysis of fMRI. Comput. Intell. Neurosci. 2016, 2016, 2094601. [Google Scholar] [CrossRef] [PubMed]
- Thirion, B.; Pinel, P.; Mériaux, S.; Roche, A.; Dehaene, S.; Poline, J.-B. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage 2007, 35, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Churchill, N.W.; Yourganov, G.; Strother, S.C. Comparing within-subject classification and regularization methods in fMRI for large and small sample sizes. Hum. Brain Mapp. 2014, 35, 4499–4517. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.R.P.; Pereira, V.M.; Machado, S.; Nardi, A.E. Cognitive functions in patients with panic disorder: A literature review. Braz. J. Psychiatry 2013, 35, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Takaishi, M.; Nakamura, R.; Yoshida, H.; Yoshimi, A.; Whitford, T.J.; Inoue, T.; Hirayasu, Y. Cortical thickness reductions in the middle frontal cortex in patients with panic disorder. J. Affect. Disord. 2018, 240, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Wu, Y.T. Decreased regional homogeneity in lingual gyrus, increased regional homogeneity in cuneus and correlations with panic symptom severity of first-episode, medication-naïve and late-onset panic disorder patients. Psychiatry Res. 2013, 211, 127–131. [Google Scholar] [CrossRef]
- Lopes, F.L.; Faria, C.G.F.; Dias, G.P.; Mallmann, M.B.; Mendes, V.; Horato, N.; de-Melo-Neto, V.L.; Veras, A.B.; Magalhães, F.V.; Malaspina, D.; et al. Neural correlates of negative and disease-specific emotional stimuli in panic disorder: A functional magnetic resonance imaging study. Braz. J. Psychiatry 2021, 43, 605–612. [Google Scholar] [CrossRef]
- Shao, R.; He, P.; Ling, B.; Tan, L.; Xu, L.; Hou, Y.; Kong, L.; Yang, Y. Prevalence of depression and anxiety and correlations between depression, anxiety, family functioning, social support and coping styles among Chinese medical students. BMC Psychol. 2020, 8, 38. [Google Scholar] [CrossRef]
- Zugliani, M.M.; Martin-Santos, R.; Nardi, A.E.; Freire, R.C. Personality traits in panic disorder patients with and without comorbidities. J. Nerv. Ment. Dis. 2017, 205, 855–858. [Google Scholar] [CrossRef]
- Clark, D.B.; Donovan, J.E. Reliability and validity of the Hamilton Anxiety Rating Scale in an adolescent sample. J. Am. Acad. Child Adolesc. Psychiatry 1994, 33, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Phillips, M.; Liu, J.; Cai, M.; Sun, S.; Huang, M. Validity and reliability of the Chinese Hamilton depression rating scale. Br. J. Psychiatry 1988, 152, 660–664. [Google Scholar] [CrossRef]
- Hurford, I.M.; Ventura, J.; Marder, S.R.; Reise, S.P.; Bilder, R.M. A 10-minute measure of global cognition: Validation of the Brief Cognitive Assessment Tool for Schizophrenia (B-CATS). Schizophr. Res. 2018, 195, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Weng, Y.; Li, W.; Gao, L.; Chen, G.; Xie, L.; Chang, Y.; Tang, W.-K.; Ungvari, G.S. Training patients with schizophrenia with the community re-entry module: A controlled study. Soc. Psychiatry Psychiatr. Epidemiol. 2006, 41, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Hurford, I.M.; Marder, S.R.; Keefe, R.S.; Reise, S.P.; Bilder, R.M. A brief cognitive assessment tool for schizophrenia: Construction of a tool for clinicians. Schizophr. Bull. 2011, 37, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y. Reliability and validity of the simplified coping style questionnaire. Chin. J. Clin. Psychol. 1998, 6, 114–115. [Google Scholar]
- Gong, Y. Eysenck personality questionnaire revised in china. Inf. Psychol. Sci. 1984, 4, 11–18. [Google Scholar]
- Wu, D.; Yin, H.; Xu, S.; Zhao, Y. Risk factors for posttraumatic stress reactions among Chinese students following exposure to a snowstorm disaster. BMC Public Health 2011, 11, 96. [Google Scholar] [CrossRef]
- Yan, C.; Chao-Gan, Y.; Yu-Feng, Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef]
- Huang, C.-M.; Lee, S.-H.; Hsiao, T.; Kuan, W.-C.; Wai, Y.-Y.; Ko, H.-J.; Wan, Y.-L.; Hsu, Y.-Y.; Liu, H.-L. Study-specific EPI template improves group analysis in functional MRI of young and older adults. J. Neurosci. Methods 2010, 189, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sala-Llonch, R.; Smith, S.M.; Woolrich, M.; Duff, E.P. Spatial parcellations, spectral filtering, and connectivity measures in fMRI: Optimizing for discrimination. Hum. Brain Mapp. 2019, 40, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004, 22, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Mikl, M.; Mareček, R.; Hluštík, P.; Pavlicová, M.; Drastich, A.; Chlebus, P.; Brázdil, M.; Krupa, P. Effects of spatial smoothing on fMRI group inferences. Magn. Reson. Imaging 2008, 26, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C. A Library for Support Vector Machines. 2001. Available online: http://www.csie.ntu.edu.tw/~cjlin/libsvm (accessed on 20 August 2023).
- da Silva Santos, C.E.; Sampaio, R.C.; dos Santos Coelho, L.; Bestard, G.A.; Llanos, C.H. Multi-objective adaptive differential evolution for SVM/SVR hyperparameters selection. Pattern Recognit. 2021, 110, 107649. [Google Scholar] [CrossRef]
- Mi, X.; Zou, B.; Zou, F.; Hu, J. Permutation-based identification of important biomarkers for complex diseases via machine learning models. Nat. Commun. 2021, 12, 3008. [Google Scholar] [CrossRef]
- Ni, M.-F.; Zhang, B.-W.; Chang, Y.; Huang, X.-F.; Wang, X.-M. Altered resting-state network connectivity in panic disorder: An independent ComponentAnalysis. Brain Imaging Behav. 2021, 15, 1313–1322. [Google Scholar] [CrossRef]
- Seo, H.-J.; Choi, Y.H.; Chung, Y.-A.; Rho, W.; Chae, J.-H. Changes in cerebral blood flow after cognitive behavior therapy in patients with panic disorder: A SPECT study. Neuropsychiatr. Dis. Treat. 2014, 10, 661. [Google Scholar] [CrossRef]
- Lai, C.-H.; Wu, Y.-T. The changes in the low-frequency fluctuations of cingulate cortex and postcentral gyrus in the treatment of panic disorder: The MRI study. World J. Biol. Psychiatry 2016, 17, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, Y. Hierarchical somatosensory processing. Curr. Opin. Neurobiol. 1998, 8, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Satoh, M.; Tabei, K.I.; Ueda, Y.; Taniguchi, A.; Matsuura, K.; Asahi, M.; Ii, Y.; Niwa, A.; Tomimoto, H. Impairment of intermediate somatosensory function in corticobasal syndrome. Sci. Rep. 2020, 10, 11155. [Google Scholar] [CrossRef]
- Boshuisen, M.L.; Ter Horst, G.J.; Paans, A.M.; Reinders, A.A.; den Boer, J.A. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol. Psychiatry. 2002, 52, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Wu, Y.T. Changes in gray matter volume of remitted first-episode, drug-naïve, panic disorder patients after 6-week antidepressant therapy. J. Psychiatr. Res. 2013, 47, 122–127. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoon, H.K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Zhong, Y.; Hao, Z.; Xu, H.; Wu, Y.; Teng, C.; Li, J.; Xiao, C.; Fox, P.T.; Zhang, N.; et al. Resting-state causal connectivity of the bed nucleus of the stria terminalis in panic disorder. Brain Imaging Behav. 2021, 15, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.F.; Wang, X.M.; Wang, H.Y.; Chang, Y.; Huang, X.F.; Zhang, B.W. Regional cortical thinning and cerebral hypoperfusion in patients with panic disorder. J. Affect. Disord. 2020, 277, 138–145. [Google Scholar] [CrossRef]
- Ghuman, A.S.; Brunet, N.M.; Li, Y.; Konecky, R.O.; Pyles, J.A.; Walls, S.A.; Destefino, V.; Wang, W.; Richardson, R.M. Dynamic encoding of face information in the human fusiform gyrus. Nat. Commun. 2014, 5, 5672. [Google Scholar] [CrossRef]
- Weiner, K.S.; Natu, V.S.; Grill-Spector, K. On object selectivity and the anatomy of the human fusiform gyrus. Neuroimage 2018, 173, 604–609. [Google Scholar] [CrossRef]
- Beelen, C.; Blockmans, L.; Wouters, J.; Ghesquière, P.; Vandermosten, M. Brain-behavior dynamics between the left fusiform and reading. Brain Struct. Funct. 2022, 227, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.M.; Pizzagalli, D.A. One-year-old fear memories rapidly activate human fusiform gyrus. Soc. Cogn. Affect. Neurosci. 2016, 11, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lipka, J.; Miltner, W.H.; Straube, T. Vigilance for threat interacts with amygdala responses to subliminal threat cues in specific phobia. Biol. Psychiatry 2011, 70, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Krause, B.J.; Weiss, P.H.; Fink, G.R.; Shah, N.J.; Amorim, M.A.; Müller, H.W.; Berthoz, A. Visuospatial working memory and changes of the point of view in 3D space. Neuroimage 2007, 36, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Wu, Y.T. The Explorative Analysis to Revise Fear Network Model for Panic Disorder: Functional Connectome Statistics. Medicine 2016, 95, e3597. [Google Scholar] [CrossRef]
- Carlson, J.M.; Reinke, K.S.; Habib, R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia 2009, 47, 1386–1389. [Google Scholar] [CrossRef] [PubMed]
- Carlisi, C.O.; Norman, L.; Murphy, C.M.; Christakou, A.; Chantiluke, K.; Giampietro, V.; Simmons, A.; Brammer, M.; Murphy, D.G.; Mataix-Cols, D.; et al. Disorder-Specific and Shared Brain Abnormalities During Vigilance in Autism and Obsessive-Compulsive Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 644–654. [Google Scholar] [CrossRef]
- Nehlig, A.; Armspach, J.P.; Namer, I.J. SPECT assessment of brain activation induced by caffeine: No effect on areas involved in dependence. Dialogues Clin. Neurosci. 2010, 12, 255–263. [Google Scholar] [CrossRef]
- Moon, C.M.; Jeong, G.W. Functional neuroanatomy on the working memory under emotional distraction in patients with generalized anxiety disorder. Psychiatry Clin. Neurosci. 2015, 69, 609–619. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamada, K.; Nishimura, T.; Kinoshita, S. Tractography to depict three layers of visual field trajectories to the calcarine gyri. Am. J. Ophthalmol. 2005, 140, 781–785. [Google Scholar] [CrossRef]
- Abend, R.; Gold, A.L.; Britton, J.C.; Michalska, K.J.; Shechner, T.; Sachs, J.F.; Winkler, A.M.; Leibenluft, E.; Averbeck, B.B.; Pine, D.S. Anticipatory Threat Responding: Associations With Anxiety, Development, and Brain Structure. Biol. Psychiatry. 2020, 87, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.K.; Barton, J.L.; Gladwell, V.F. Viewing nature scenes positively affects recovery of autonomic function following acute-mental stress. Environ. Sci. Technol. 2013, 47, 5562–5569. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, J.N.; Veer, I.M.; van Tol, M.J.; van der Werff, S.J.; Demenescu, L.R.; Aleman, A.; Veltman, D.J.; Zitman, F.G.; Rombouts, S.A.; van der Wee, N.J. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J. Affect. Disord. 2013, 145, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, C.H. The alterations of degree centrality in the frontal lobe of patients with panic disorder. Int. J. Med. Sci. 2022, 19, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, Y.; Zheng, G.; Liu, Y.; Pang, M.; Xu, H.; Ding, H.; Wang, C.; Zhang, N. Disrupted fronto-temporal function in panic disorder: A resting-state connectome study. Brain Imaging Behav. 2022, 16, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, B.; Cui, H.; Li, W.; Li, H.; Hu, Q.; Wang, J.; Li, C. Altered Function of Superior Parietal Lobule Associated with Perceptive Awareness in First-Episode Drug-Naïve Panic Disorders: A Preliminary fMRI Study. Neuropsychiatr. Dis. Treat. 2020, 16, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Lueken, U.; Straube, B.; Reinhardt, I.; Maslowski, N.I.; Wittchen, H.U.; Ströhle, A.; Wittmann, A.; Pfleiderer, B.; Konrad, C.; Ewert, A.; et al. Altered top-down and bottom-up processing of fear conditioning in panic disorder with agoraphobia. Psychol. Med. 2014, 44, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Kircher, T.; Arolt, V.; Jansen, A.; Pyka, M.; Reinhardt, I.; Kellermann, T.; Konrad, C.; Lueken, U.; Gloster, A.T.; Gerlach, A.L.; et al. Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol. Psychiatry 2013, 73, 93–101. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Lisberger, S.G.; Raymond, J.L. Diversity and dynamism in the cerebellum. Nat. Neurosci. 2021, 24, 160–167. [Google Scholar] [CrossRef]
- Bodranghien, F.; Bastian, A.; Casali, C.; Hallett, M.; Louis, E.D.; Manto, M.; Mariën, P.; Nowak, D.A.; Schmahmann, J.D.; Serrao, M.; et al. Consensus Paper: Revisiting the Symptoms and Signs of Cerebellar Syndrome. Cerebellum 2016, 15, 369–391. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Liu, J.; Yao, L.; Bishop, J.R.; Lencer, R.; Gong, Q.; Yang, Z.; Lui, S. Disrupted subcortical functional connectome gradient in drug-naïve first-episode schizophrenia and the normalization effects after antipsychotic treatment. Neuropsychopharmacology 2023, 48, 789–796. [Google Scholar] [CrossRef]
- Pae, C.; Kim, H.-J.; Bang, M.; Lee, S.-H. Prediction of prognosis in patients with panic disorder using pre-treatment brain white matter features. J. Affect. Disord. 2022, 313, 214–221. [Google Scholar] [CrossRef]
- Ironside, M.; DeVille, D.C.; Kuplicki, R.T.; Burrows, K.P.; Smith, R.; Teed, A.R.; Paulus, M.P.; Khalsa, S.S. The unique face of comorbid anxiety and depression: Increased interoceptive fearfulness and reactivity. Front. Behav. Neurosci. 2023, 16, 1083357. [Google Scholar] [CrossRef]
- Li, Z.; Kadivar, A.; Pluta, J.; Dunlop, J.; Wang, Z. Test-retest stability analysis of resting brain activity revealed by blood oxygen level-dependent functional MRI. J. Magn. Reson. Imaging 2012, 36, 344–354. [Google Scholar] [CrossRef]


| Variables | Patients (Mean ± SD, n = 54) | Controls (Mean ± SD, n = 54) | U/χ2 | p-Value | df | Effect Size: r/Cramer’s V |
|---|---|---|---|---|---|---|
| Age (years) | 34.78 ± 9.67 | 32.28 ± 10.56 | 1151.50 | 0.06 a | 106 | −0.18 |
| Sex (male/female) | 25/29 | 21/33 | 0.61 | 0.44 b | 1 | 0.08 |
| Years of education (years) | 13.15 ± 3.45 | 13.43 ± 3.22 | 1410.00 | 0.76 a | 106 | −0.03 |
| Illness duration (months) | 14.22 ± 21.88 | |||||
| HAMD | 14.07 ± 4.01 | |||||
| HAMA | 17.59 ± 4.37 | |||||
| SDSS | 3.28 ± 3.31 | |||||
| CSQ | ||||||
| Active coping | 20.04 ± 6.24 | |||||
| Passive coping | 11.35 ± 4.38 | |||||
| B-CATS | ||||||
| Digit symbol substitution | 52.57 ± 17.05 | |||||
| Trail making test part A | 38.31 ± 15.87 | |||||
| Trail making test part B | 75.59 ± 43.38 | |||||
| Category fluency | 17.89 ± 6.73 | |||||
| EPQ | ||||||
| E | 50.74 ± 9.83 | |||||
| P | 65.28 ± 15.49 | |||||
| N | 45.09 ± 9.39 | |||||
| L | 39.91 ± 12.83 |
| Cluster Location | Peak (MNI) | Number of Voxels | T Value | p | df | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Patients with PD at baseline versus controls | ||||||||
| Right postcentral/precentral gyrus | 51 | −18 | 42 | 253 | −3.39 | <0.001 | 106 | −0.65 |
| Left postcentral/precentral gyrus | −57 | −6 | 48 | 220 | −3.39 | <0.001 | 106 | −0.65 |
| Right fusiform gyrus/cerebellum VI | 18 | −45 | −12 | 121 | −3.39 | <0.001 | 106 | −0.65 |
| Left superior/middle frontal gyrus | −27 | 45 | 21 | 88 | 4.43 | <0.001 | 106 | 0.85 |
| Left fusiform gyrus/cerebellum VI | −30 | −48 | −24 | 85 | −3.40 | <0.001 | 106 | −0.65 |
| Right calcarine/lingual gyrus | 27 | −57 | 9 | 58 | −3.42 | <0.001 | 106 | −0.66 |
| Right postcentral gyrus | 63 | −6 | 33 | 53 | −3.40 | <0.001 | 106 | −0.65 |
| Left superior parietal lobule | −15 | −57 | 66 | 44 | −3.41 | <0.001 | 106 | −0.66 |
| Left middle/inferior frontal gyrus (triangular part) | −30 | 15 | 33 | 38 | 4.94 | <0.001 | 106 | 0.95 |
| Patients with PD after 4-week treatment versus at baseline | ||||||||
| Left fusiform gyrus | −30 | −45 | −9 | 41 | 4.57 | <0.001 | 35 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Han, Y.; Shan, X.; Li, H.; Liu, F.; Li, P.; Zhao, J.; Guo, W. Breaking the Fear Barrier: Aberrant Activity of Fear Networks as a Prognostic Biomarker in Patients with Panic Disorder Normalized by Pharmacotherapy. Biomedicines 2023, 11, 2420. https://doi.org/10.3390/biomedicines11092420
Yan H, Han Y, Shan X, Li H, Liu F, Li P, Zhao J, Guo W. Breaking the Fear Barrier: Aberrant Activity of Fear Networks as a Prognostic Biomarker in Patients with Panic Disorder Normalized by Pharmacotherapy. Biomedicines. 2023; 11(9):2420. https://doi.org/10.3390/biomedicines11092420
Chicago/Turabian StyleYan, Haohao, Yiding Han, Xiaoxiao Shan, Huabing Li, Feng Liu, Ping Li, Jingping Zhao, and Wenbin Guo. 2023. "Breaking the Fear Barrier: Aberrant Activity of Fear Networks as a Prognostic Biomarker in Patients with Panic Disorder Normalized by Pharmacotherapy" Biomedicines 11, no. 9: 2420. https://doi.org/10.3390/biomedicines11092420
APA StyleYan, H., Han, Y., Shan, X., Li, H., Liu, F., Li, P., Zhao, J., & Guo, W. (2023). Breaking the Fear Barrier: Aberrant Activity of Fear Networks as a Prognostic Biomarker in Patients with Panic Disorder Normalized by Pharmacotherapy. Biomedicines, 11(9), 2420. https://doi.org/10.3390/biomedicines11092420