Piperlongumine Induces Apoptosis and Cytoprotective Autophagy via the MAPK Signaling Pathway in Human Oral Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. DAPI Staining
2.5. Annexin V/PI Staining
2.6. Acridine Orange Staining
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
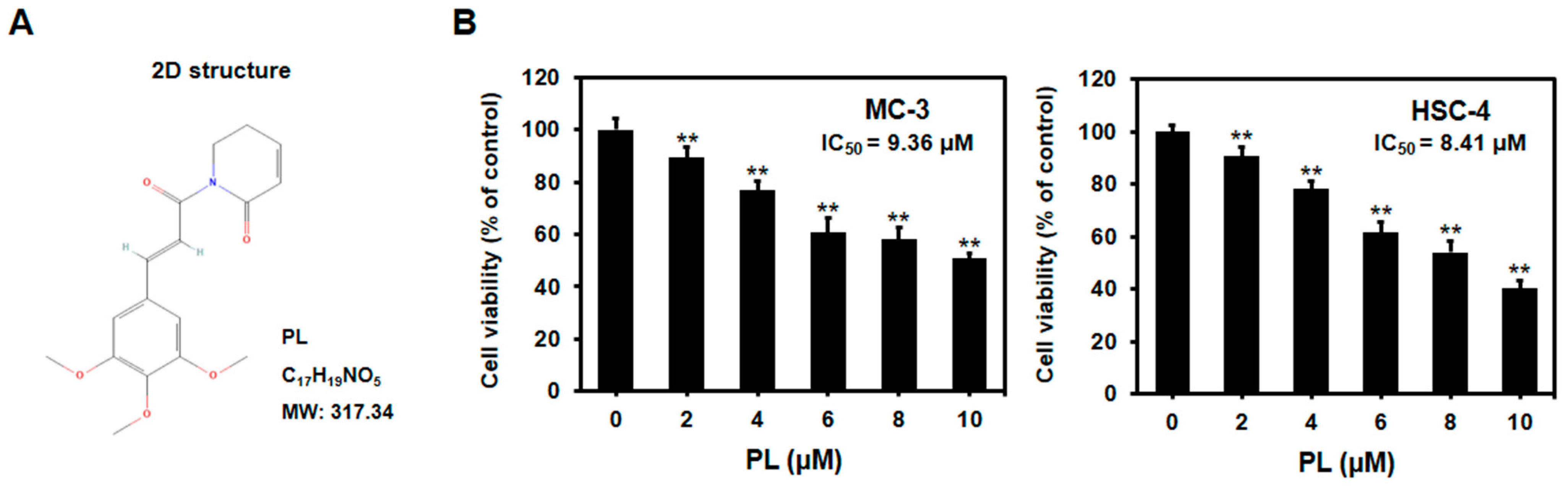
3.1. PL Reduced Cell Viability of Human Oral Cancer Cells
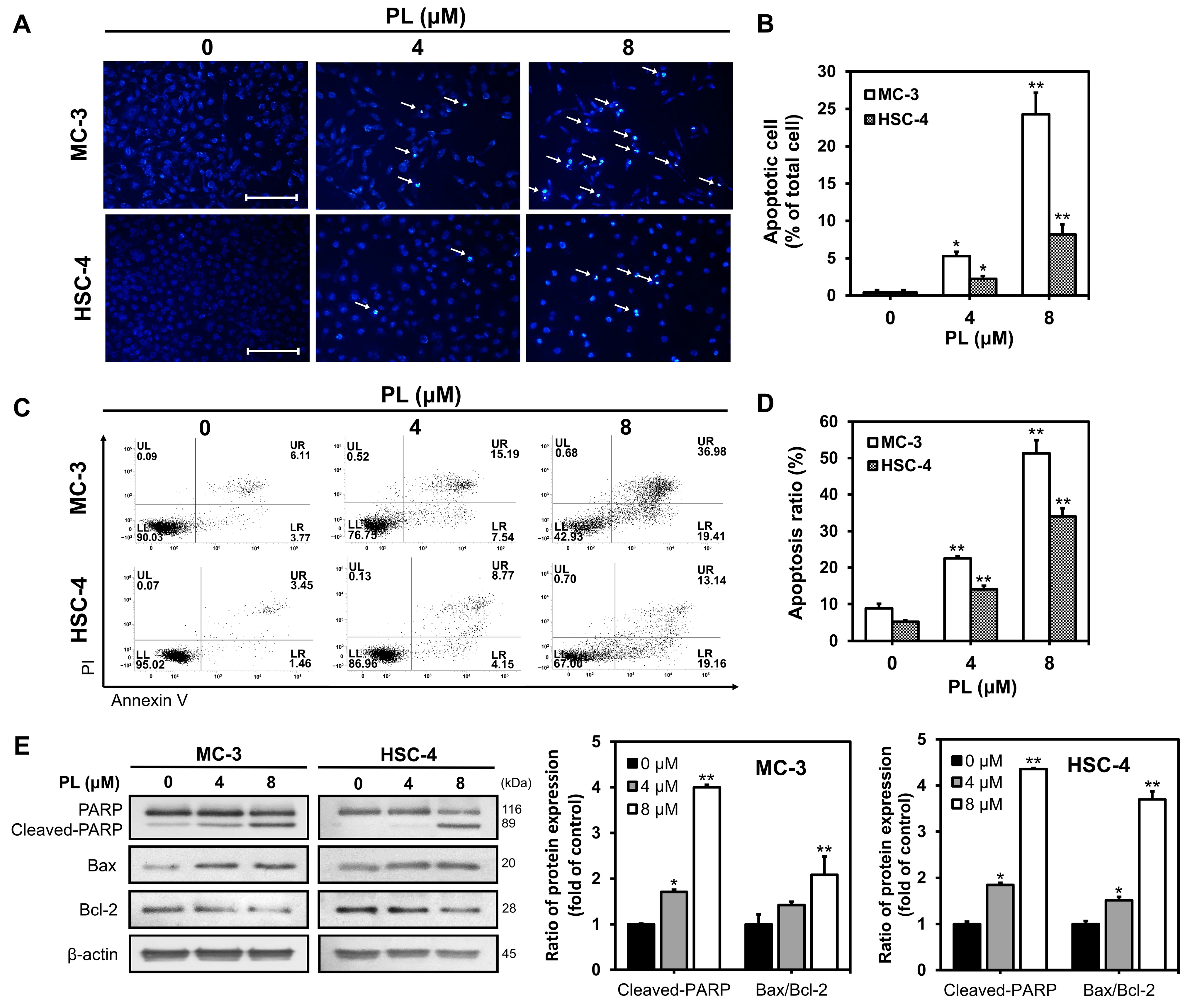
3.2. PL Induced Apoptosis in Human Oral Cancer Cells
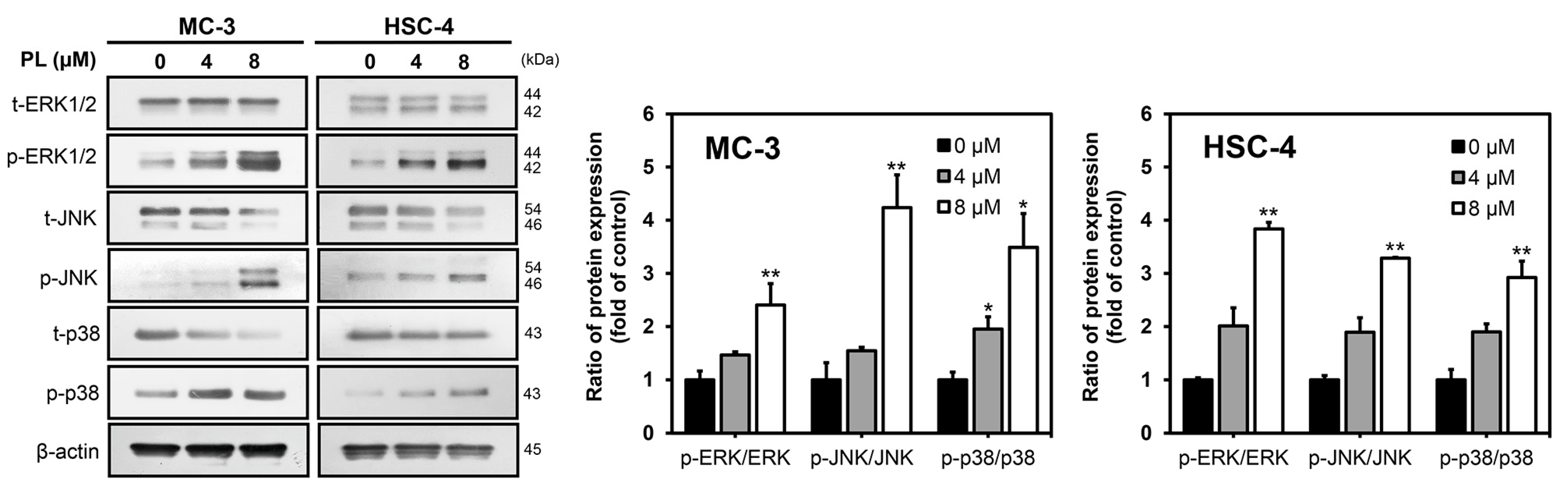
3.3. PL Induced Apoptosis via MAPK Pathway in Human Oral Cancer Cells
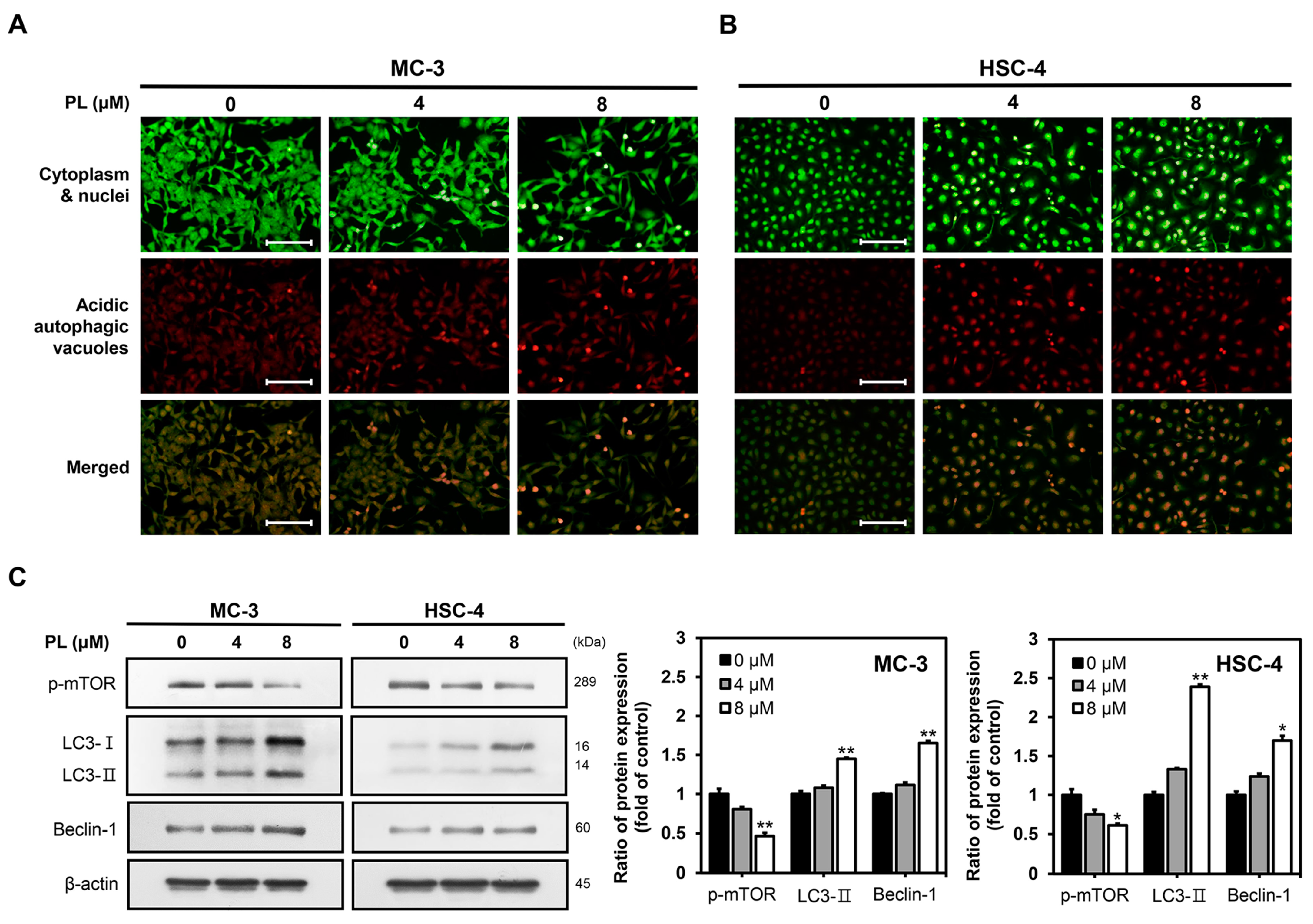
3.4. PL Induced Autophagy in Human Oral Cancer Cells
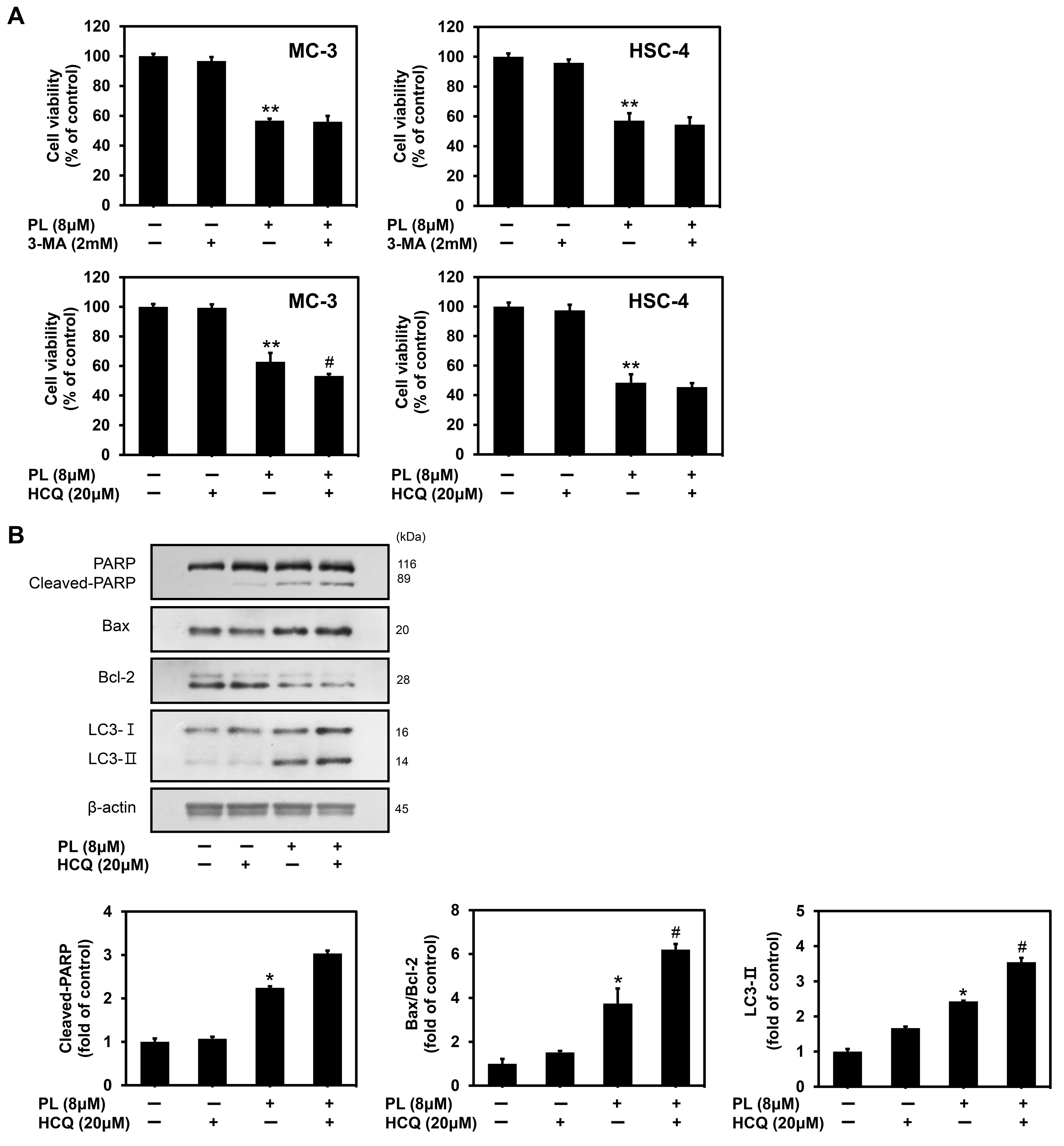
3.5. PL Induced Cytoprotective Autophagy in Human Oral Cancer Cells
3.6. PL Regulated Apoptosis and Autophagy through the Activation of JNK
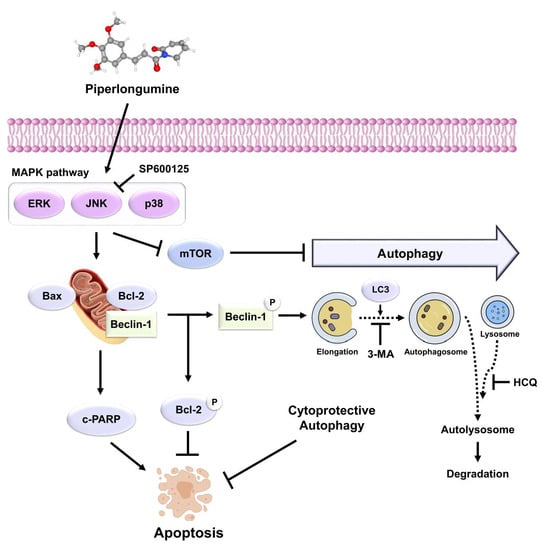
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, S.S.; Alkahtani, S.; Bharadwaj, P.; Ansari, M.T.; Alkahtani, M.D.; Pang, Z.; Hasnain, M.S.; Nayak, A.K.; Aminabhavi, T.M. Molecular insights and novel approaches for targeting tumor metastasis. Int. J. Pharm. 2020, 585, 119556. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Yadalam, P.K.; Hosmani, J.; Khan, Z.A.; Shankar, V.G.; Shaukat, L.; Khan, S.S.; Awan, K.H. Modulation of oral cancer and periodontitis using chemotherapeutic agents—A narrative review. Dis. Mon. 2023, 69, 101348. [Google Scholar] [CrossRef]
- Shanmugam, K.; Sellappan, S.; Alahmadi, T.A.; Almoallim, H.S.; Natarajan, N.; Veeraraghavan, V.P. Green synthesized zinc oxide nanoparticles from Cinnamomum verum bark extract inhibited cell growth and induced caspase-mediated apoptosis in oral cancer KB cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103577. [Google Scholar] [CrossRef]
- Kademani, D. Oral cancer. Mayo Clin. Proc. 2007, 82, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Logan, R.M.; Luke, C.; Rees, G.L. Retrospective study of survival and treatment pattern in a cohort of patients with oral and oropharyngeal tongue cancers from 1987 to 2004. Oral Oncol. 2007, 43, 150–158. [Google Scholar] [CrossRef]
- Mascitti, M.; Orsini, G.; Tosco, V.; Monterubbianesi, R.; Balercia, A.; Putignano, A.; Procaccini, M.; Santarelli, A. An overview on current non-invasive diagnostic devices in oral oncology. Front. Physiol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Biswal, B.K. Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol. Res. 2020, 156, 104772. [Google Scholar] [CrossRef]
- Park, B.S.; Son, D.J.; Park, Y.H.; Kim, T.W.; Lee, S.E. Antiplatelet effects of acidamides isolated from the fruits of Piper longum L. Phytomedicine 2007, 14, 853–855. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Son, D.J.; Kim, S.Y.; Han, S.S.; Kim, C.W.; Kumar, S.; Park, B.S.; Lee, S.E.; Yun, Y.P.; Jo, H.; Park, Y.H. Piperlongumine inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochem. Biophys. Res. Commun. 2012, 427, 349–354. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.G.; Lee, M.Y.; Bae, J.S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; DeMoraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ma, Y.; Guo, Z.; Liu, L.; Yang, Y.; Wang, Y.; Pang, B.; Wu, L.; Hui, Y.; Yang, W. Two natural alkaloids synergistically induce apoptosis in breast cancer cells by inhibiting STAT3 activation. Molecules 2020, 25, 216. [Google Scholar] [CrossRef]
- Randhawa, H.; Kibble, K.; Zeng, H.; Moyer, M.P.; Reindl, K.M. Activation of ERK signaling and induction of colon cancer cell death by piperlongumine. Toxicol. Vitro 2013, 27, 1626–1633. [Google Scholar] [CrossRef]
- Rawat, L.; Hegde, H.; Hoti, S.L.; Nayak, V. Piperlongumine induces ROS mediated cell death and synergizes paclitaxel in human intestinal cancer cells. Biomed. Pharmacother. 2020, 128, 110243. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Xia, Y.; Ji, J.; Chen, W.; Zhang, J.; Chen, X.; Rajamanickam, V.; Chen, G.; Wang, Z.; Chen, L.; et al. Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer. Cancer Lett. 2016, 375, 114–126. [Google Scholar] [CrossRef]
- Seok, J.S.; Jeong, C.H.; Petriello, M.C.; Seo, H.G.; Yoo, H.; Hong, K.; Han, S.G. Piperlongumine decreases cell proliferation and the expression of cell cycle-associated proteins by inhibiting Akt pathway in human lung cancer cells. Food Chem. Toxicol. 2018, 111, 9–18. [Google Scholar] [CrossRef]
- Fan, J.; Ren, D.; Wang, J.; Liu, X.; Zhang, H.; Wu, M.; Yang, G. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020, 11, 126. [Google Scholar] [CrossRef]
- Dhillon, H.; Chikara, S.; Reindl, K.M. Piperlongumine induces pancreatic cancer cell death by enhancing reactive oxygen species and DNA damage. Toxicol. Rep. 2014, 1, 309–318. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta. Biochimi. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Han, S.I.; Kim, Y.S.; Kim, T.H. Role of apoptotic and necrotic cell death under physiologic conditions. BMB Rep. 2008, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Tsuyoshi, H.; Orisaka, M.; Shieh, D.B.; Yoshida, Y.; Tsang, B.K. Mitochondrial dynamics regulating chemoresistance in gynecological cancers. Ann. N. Y. Acad. Sci. 2015, 1350, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Del Poeta, G.; Venditti, A.; Del Principe, M.I.; Maurillo, L.; Buccisano, F.; Tamburini, A.; Christina Cox, M.; Franchi, A.; Bruno, A.; Mazzone, C.; et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003, 101, 2125–2131. [Google Scholar] [CrossRef]
- Yoshino, T.; Shiina, H.; Urakami, S.; Kikuno, N.; Yoneda, T.; Shigeno, K.; Igawa, M. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin. Cancer Res. 2006, 12, 6116–6124. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, X.; Yao, Z.; Wen, F.; Fu, Z.; Zhang, J.; Zhong, Z.; Huang, Y.; Qu, S. EA ameliorated depressive behaviors in CUMS rats and was related to its suppressing autophagy in the hippocampus. Neural Plast. 2020, 2020, 8860968. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.U.; Sim, S.J. Nanoplasmonic biosensing of specific LC3 autophagy markers enabling drug discovery of autophagy modulators. Sens. Actuators B Chem. 2022, 363, 131744. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Jung, H.J.; Kang, J.H.; Choi, S.; Son, Y.K.; Lee, K.R.; Seong, J.K.; Kim, S.Y.; Oh, S.H. Pharbitis Nil (PN) induces apoptosis and autophagy in lung cancer cells and autophagy inhibition enhances PN-induced apoptosis. J. Ethnopharmacol. 2017, 208, 253–263. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Bora, G.; Yaba, A. The role of mitogen-activated protein kinase signalling pathway in endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 1610–1623. [Google Scholar] [CrossRef]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Zhang, K. Mitogen-activated protein kinase pathway in amyotrophic lateral sclerosis. Biomedicines 2021, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Movva, S.; Rodriguez, L.; Arias-Pulido, H.; Verschraegen, C. Novel chemotherapy approaches for cervical cancer. Cancer 2009, 115, 3166–3180. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.M.; Han, C.; Chen, C.; Li, L.N.; Wang, X.B.; Yang, M.H.; Gu, Y.C.; Luo, J.G.; Kong, L.Y. Artemisians A-D, diseco-guaianolide involved heterodimeric [4+2] adducts from Artemisia argyi. Org. Lett. 2017, 19, 5410–5413. [Google Scholar] [CrossRef]
- Gong, L.H.; Chen, X.X.; Wang, H.; Jiang, Q.W.; Pan, S.S.; Qiu, J.G.; Mei, X.L.; Xue, Y.Q.; Qin, W.M.; Zheng, F.Y.; et al. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid. Med. Cell. Longev. 2014, 2014, 906804. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Park, J.Y.; Kim, J.W.; Kwon, M.; Lee, B.H. Piperlongumine selectively kills cancer cells and increases cispla tin antitumor activity in head and neck cancer. Oncotarget 2014, 5, 9227. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol simultaneously induces apoptosis and protective autophagy by inhibiting Akt/mTOR pathway in gastric cancer cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef]
- Vijayarathna, S.; Gothai, S.; Jothy, S.L.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Can cancer therapy be achieved by bridging apoptosis and autophagy: A method based on microRNA-dependent gene therapy and phytochemical targets. Asian Pac. J. Cancer Prev. 2015, 16, 7435–7439. [Google Scholar] [CrossRef]
- Wang, F.; Mao, Y.; You, Q.; Hua, D.; Cai, D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. Immunopathol. Pharmacol. 2015, 28, 362–373. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Kulkarni, P.; Thummuri, D.; Jeengar, M.K.; Naidu, V.G.M.; Alvala, M.; Redddy, G.B.; Ramakrishna, S. Piperlongumine, an alkaloid causes inhibition of PI3K/Akt/mTOR signaling axis to induce caspase-dependent apoptosis in human triple-negative breast cancer cells. Apoptosis 2014, 19, 1148–1164. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, E.S.; Han, S.H.; Jung, G.H.; Han, E.J.; Jung, S.H.; Kim, B.S.; Cho, S.D.; Nam, J.S.; Choi, C.; et al. Oleanolic acid induces apoptosis and autophagy via the PI3K/AKT/mTOR pathway in AGS human gastric cancer cells. J. Funct. Foods 2021, 87, 104854. [Google Scholar] [CrossRef]
- Jung, G.H.; Lee, J.H.; Han, S.H.; Woo, J.S.; Choi, E.Y.; Jeon, S.J.; Han, E.J.; Jung, S.H.; Park, Y.S.; Park, B.K.; et al. Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells. Int. J. Mol. Sci. 2022, 23, 15747. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.D.; Hou, C.Y.; Li, Z.Y.; Li, Q.; Miao, S.Y.; Zhang, Q.; Zhang, X.Y.; Zhu, X.F.; Jiang, J.W. EM-2 inhibited autophagy and promoted G2/M phase arrest and apoptosis by activating the JNK pathway in hepatocellular carcinoma cells. Acta Pharmacol. Sin. 2021, 42, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wen, C.; Bai, H.; Wang, X.; Zhang, X.; Huang, L.; Yang, X.; Iwamoto, A.; Liu, H. JNK signaling pathway is involved in piperlongumine-mediated apoptosis in human colorectal cancer HCT116 cells. Oncol. Lett. 2015, 10, 709–715. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Cho, H.J.; Gowrisankar, Y.V.; Thiyagarajan, V.; Chen, X.Z.; Lin, K.Y.; Huang, H.C.; Yang, H.L. Kalantuboside B induced apoptosis and cytoprotective autophagy in human melanoma A2058 cells: An in vitro and in vivo study. Free Radic. Biol. Med. 2019, 143, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Jiang, X.X.; Zhu, X.; He, W.Y.; Kuang, Y.L.; Ren, K.; Lin, Y.; Gou, X. ROS activates JNK-mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol. Sin. 2015, 36, 1473–1479. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.-Y.; Han, E.-J.; Jeon, S.-J.; Lee, S.-W.; Moon, J.-M.; Jung, S.-H.; Jung, J.-Y. Piperlongumine Induces Apoptosis and Cytoprotective Autophagy via the MAPK Signaling Pathway in Human Oral Cancer Cells. Biomedicines 2023, 11, 2442. https://doi.org/10.3390/biomedicines11092442
Choi E-Y, Han E-J, Jeon S-J, Lee S-W, Moon J-M, Jung S-H, Jung J-Y. Piperlongumine Induces Apoptosis and Cytoprotective Autophagy via the MAPK Signaling Pathway in Human Oral Cancer Cells. Biomedicines. 2023; 11(9):2442. https://doi.org/10.3390/biomedicines11092442
Chicago/Turabian StyleChoi, Eun-Young, Eun-Ji Han, Su-Ji Jeon, Sang-Woo Lee, Jun-Mo Moon, Soo-Hyun Jung, and Ji-Youn Jung. 2023. "Piperlongumine Induces Apoptosis and Cytoprotective Autophagy via the MAPK Signaling Pathway in Human Oral Cancer Cells" Biomedicines 11, no. 9: 2442. https://doi.org/10.3390/biomedicines11092442