Purified Serum IgG from a Patient with Anti-IgLON5 Antibody Cause Long-Term Movement Disorders with Impaired Dopaminergic Pathways in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Human IgG Purification
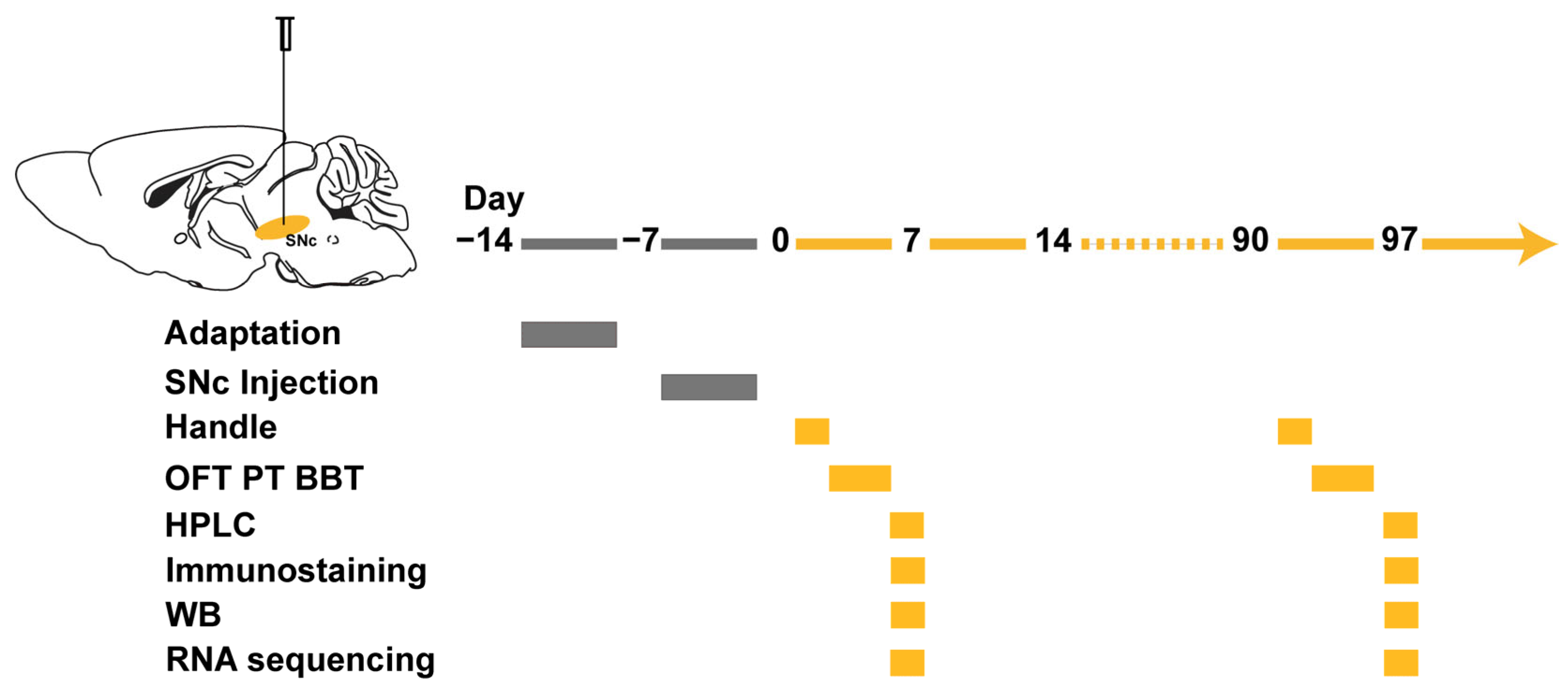
2.3. Stereotactic IgG Injection
2.4. Behavioral Tests
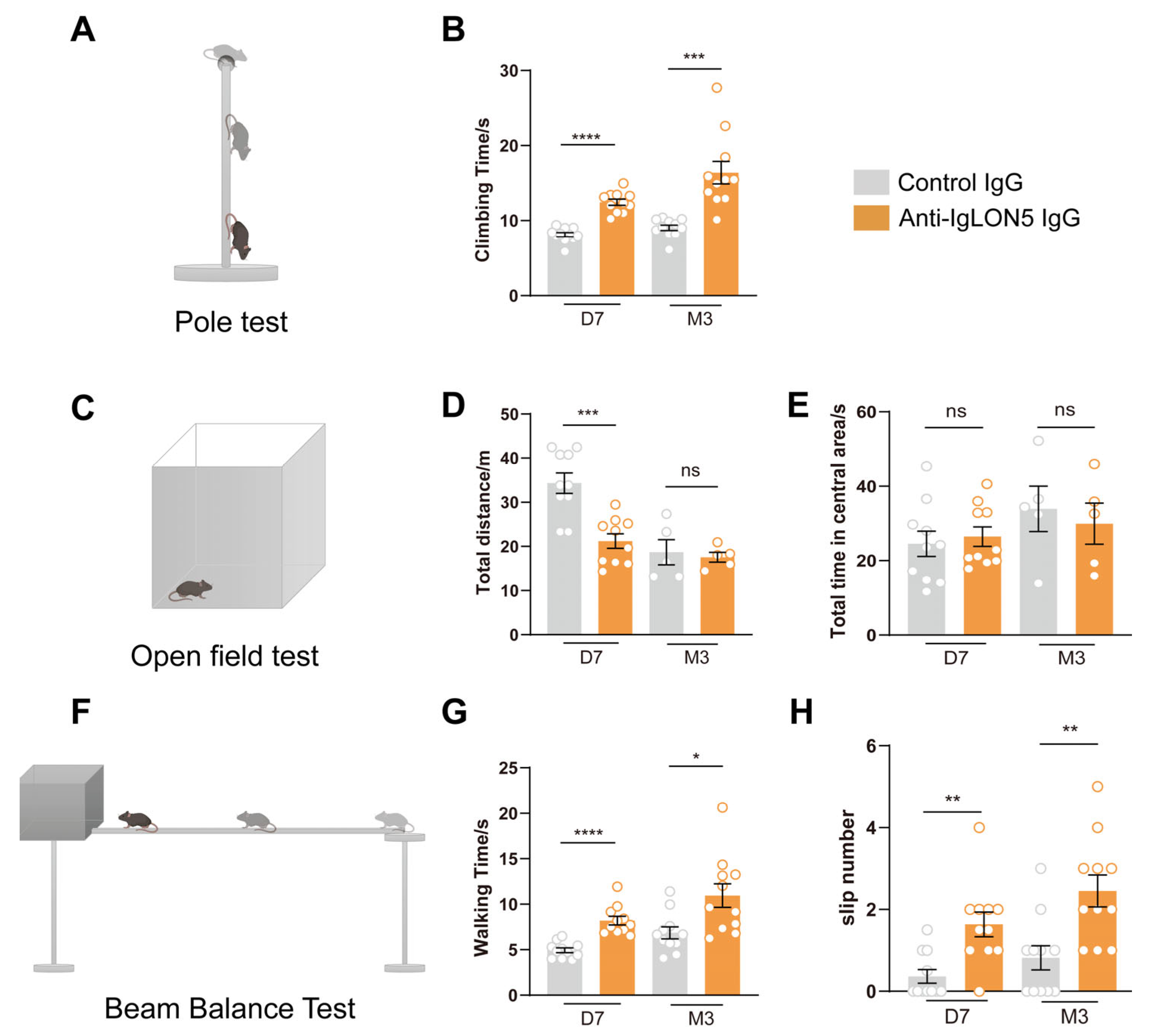
2.5. Open Field Test (OFT)
2.6. Pole Test (PT)
2.7. Beam Balance Test (BBT)
2.8. Histology and Imaging
2.9. Western Blot Analysis
2.10. RNA Extraction and RNA Sequencing
2.11. High-Performance Liquid Chromatography
2.12. Statistical Analyses
3. Results
3.1. The Patients’ Anti-IgLON5 Antibodies Induce Motor Disorders in Mice
3.2. The Anti-IgLON5 Antibodies in Patients Lead to a Reduction in SNc TH Neurons and Decreased Projections onto Basal Ganglia
3.3. The Long-Term Effects of Anti-IgLON5 Antibodies from Patients Leads to an Increase in p-Tau Protein
3.4. The Presence of Anti-IgLON5 Antibodies from Patients Leads to Sustained Activation of Microglial Cells
3.5. Whole-Genome Transcriptomic Analyses in SNc
4. Discussion
4.1. The Movement Impairment Mediated by Anti-IgLON5 Antibodies Persists in the Long Term
4.2. Motor Impairment Is Associated with the Nigrostriatal Dopaminergic Pathway
4.3. Long-Term Effects of IgLON5 Antibodies Lead to Neurodegenerative Changes
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabater, L.; Gaig, C.; Gelpi, E.; Bataller, L.; Lewerenz, J.; Torres-Vega, E.; Contreras, A.; Giometto, B.; Compta, Y.; Embid, C.; et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014, 13, 575–586. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ni, Y.; Gao, Y.N.; Shen, D.D.; He, L.; Yin, D.; Meng, H.Y.; Zhou, Q.M.; Hu, J.; Chen, S. Anti-IgLON5 disease: A novel topic beyond neuroimmunology. Neural Regen. Res. 2023, 18, 1017–1022. [Google Scholar]
- Ni, Y.; Shen, D.; Zhang, Y.; Song, Y.; Gao, Y.; Zhou, Q.; He, L.; Yin, D.; Wang, Y.; Song, F.; et al. Expanding the clinical spectrum of anti-IgLON5 disease: A multicenter retrospective study. Eur. J. Neurol. 2022, 29, 267–276. [Google Scholar] [PubMed]
- Ni, Y.; Feng, Y.; Shen, D.; Chen, M.; Zhu, X.; Zhou, Q.; Gao, Y.; Liu, J.; Zhang, Q.; Shen, Y.; et al. Anti-IgLON5 antibodies cause progressive behavioral and neuropathological changes in mice. J. Neuroinflamm. 2022, 19, 140. [Google Scholar]
- Gelpi, E.; Hoftberger, R.; Graus, F.; Ling, H.; Holton, J.L.; Dawson, T.; Popovic, M.; Pretnar-Oblak, J.; Hogl, B.; Schmutzhard, E.; et al. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol. 2016, 132, 531–543. [Google Scholar] [CrossRef]
- Montojo, T.; Piren, V.; Benkhadra, F.; Codreanu, A.; Diederich, N.J. Gaze Palsy, Sleep and Gait Disorder, as Well as Tako-Tsubo Syndrome in a Patient with IgLON5 Antibodies. Mov. Disord. Clin. Pract. 2017, 4, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Fuseya, K.; Kimura, A.; Yoshikura, N.; Yamada, M.; Hayashi, Y.; Shimohata, T. Corticobasal Syndrome in a Patient with Anti-IgLON5 Antibodies. Mov. Disord. Clin. Pract. 2020, 7, 557–559. [Google Scholar] [CrossRef]
- Gonzalez-Avila, C.; Casado, L.; Muro Garcia, I.; Villacieros-Alvarez, J.; Vivancos, J.; Quintas, S. Altered ioflupane single-photon emission computed tomography in anti-IgLON5 disease: A new case mimicking probable progressive supranuclear palsy and review of the literature. Eur. J. Neurol. 2021, 28, 1392–1395. [Google Scholar] [CrossRef]
- Haitao, R.; Yingmai, Y.; Yan, H.; Fei, H.; Xia, L.; Honglin, H.; Chaiyan, L.; Stocker, W.; Liying, C.; Hongzhi, G. Chorea and parkinsonism associated with autoantibodies to IgLON5 and responsive to immunotherapy. J. Neuroimmunol. 2016, 300, 9–10. [Google Scholar]
- Ryding, M.; Gamre, M.; Nissen, M.S.; Nilsson, A.C.; Okarmus, J.; Poulsen, A.A.E.; Meyer, M.; Blaabjerg, M. Neurodegeneration Induced by Anti-IgLON5 Antibodies Studied in Induced Pluripotent Stem Cell-Derived Human Neurons. Cells 2021, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Haitao, R.; Huiqin, L.; Tao, Q.; Xunzhe, Y.; Xiaoqiu, S.; Wei, L.; Jiewen, Z.; Liying, C.; Hongzhi, G. Autoimmune encephalitis associated with vitiligo? J. Neuroimmunol. 2017, 310, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, N.; Cui, R. Serial 18F-FDG PET/CT Findings in a Patient with IgLON5 Encephalopathy. Clin. Nucl. Med. 2016, 41, 787–788. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.J.; Zwiers, M.P.; Naaijen, J.; Akkermans, S.E.A.; Openneer, T.J.C.; Visscher, F.; Dietrich, A.; Buitelaar, J.K.; Hoekstra, P.J. Basal ganglia structure in Tourette’s disorder and/or attention-deficit/hyperactivity disorder. Mov. Disord. 2017, 32, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Ding, J.; Day, M.; Wang, Z.; Shen, W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007, 30, 228–235. [Google Scholar] [CrossRef]
- Nambu, A. A new dynamic model of the cortico-basal ganglia loop. Prog. Brain Res. 2004, 143, 461–466. [Google Scholar] [PubMed]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Menassa, D.A.; Jacobson, L.; Coutinho, E.; Prota, G.; Lang, B.; Leite, M.I.; Cerundolo, V.; Liguori, R.; Vincent, A. Behaviour and neuropathology in mice injected with human contactin-associated protein 2 antibodies. Brain 2019, 142, 2000–2012. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Gao, Z.; Feng, Y.; Luo, H.; Lu, T.; Sun, X.; Hu, J.; Luo, Y. SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology 2020, 177, 108237. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, H.; Gao, Z.; Zhu, X.; Shen, Y.; Li, Y.; Hu, J.; Yang, J. Reduction of prefrontal purinergic signaling is necessary for the analgesic effect of morphine. iScience 2021, 24, 102213. [Google Scholar] [CrossRef]
- Rojas-Carvajal, M.; Brenes, J.C. Acute stress differentially affects grooming subtypes and ultrasonic vocalisations in the open-field and home-cage test in rats. Behav. Process. 2020, 176, 104140. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, N.; Ayari, R.; Malikov, S.; Mollo, M.; Branchereau, P.; Hut, F.; Branchereau, A. ‘Pole test’ measurements in critical leg ischaemia. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 253–257. [Google Scholar] [CrossRef]
- Orenduff, M.C.; Rezeli, E.T.; Hursting, S.D.; Pieper, C.F. Psychometrics of the Balance Beam Functional Test in C57BL/6 Mice. Comp. Med. 2021, 71, 302–308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.F.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflamm. 2018, 15, 112. [Google Scholar] [CrossRef]
- Taleb, A.; Zhou, Y.P.; Meng, L.T.; Zhu, M.Y.; Zhang, Q.; Naveed, M.; Li, L.D.; Wang, P.; Zhou, Q.G.; Meng, F.; et al. New application of an old drug proparacaine in treating epilepsy via liposomal hydrogel formulation. Pharmacol. Res. 2021, 169, 105636. [Google Scholar] [CrossRef]
- Werner, J.; Jelcic, I.; Schwarz, E.I.; Probst-Muller, E.; Nilsson, J.; Schwizer, B.; Bloch, K.E.; Lutterotti, A.; Jung, H.H.; Schreiner, B. Anti-IgLON5 Disease: A New Bulbar-Onset Motor Neuron Mimic Syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e962. [Google Scholar] [CrossRef]
- Garcia-Serra, A.; Radosevic, M.; Pupak, A.; Brito, V.; Rios, J.; Aguilar, E.; Maudes, E.; Arino, H.; Spatola, M.; Mannara, F.; et al. Placental transfer of NMDAR antibodies causes reversible alterations in mice. Neurol. Neuroimmunol. Neuroinflamm. 2020, 8, e915. [Google Scholar] [CrossRef] [PubMed]
- Carceles-Cordon, M.; Mannara, F.; Aguilar, E.; Castellanos, A.; Planaguma, J.; Dalmau, J. NMDAR Antibodies Alter Dopamine Receptors and Cause Psychotic Behavior in Mice. Ann. Neurol. 2020, 88, 603–613. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, H.; Luo, H.; Ni, Y.; Feng, Y.; He, L.; Zhou, Q.; Hu, J.; Chen, S. Purified Serum IgG from a Patient with Anti-IgLON5 Antibody Cause Long-Term Movement Disorders with Impaired Dopaminergic Pathways in Mice. Biomedicines 2023, 11, 2483. https://doi.org/10.3390/biomedicines11092483
Gao Y, Li H, Luo H, Ni Y, Feng Y, He L, Zhou Q, Hu J, Chen S. Purified Serum IgG from a Patient with Anti-IgLON5 Antibody Cause Long-Term Movement Disorders with Impaired Dopaminergic Pathways in Mice. Biomedicines. 2023; 11(9):2483. https://doi.org/10.3390/biomedicines11092483
Chicago/Turabian StyleGao, Yining, Hongxia Li, Huoqing Luo, You Ni, Yifan Feng, Lu He, Qinming Zhou, Ji Hu, and Sheng Chen. 2023. "Purified Serum IgG from a Patient with Anti-IgLON5 Antibody Cause Long-Term Movement Disorders with Impaired Dopaminergic Pathways in Mice" Biomedicines 11, no. 9: 2483. https://doi.org/10.3390/biomedicines11092483







