Comparison of Serum and Urine as Sources of miRNA Markers for the Detection of Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. RNA Isolation
2.3. MiRNA Expression Profiling with Microarrays
2.4. Quantitative PCR Analysis of Samples
2.5. qPCR Statistics
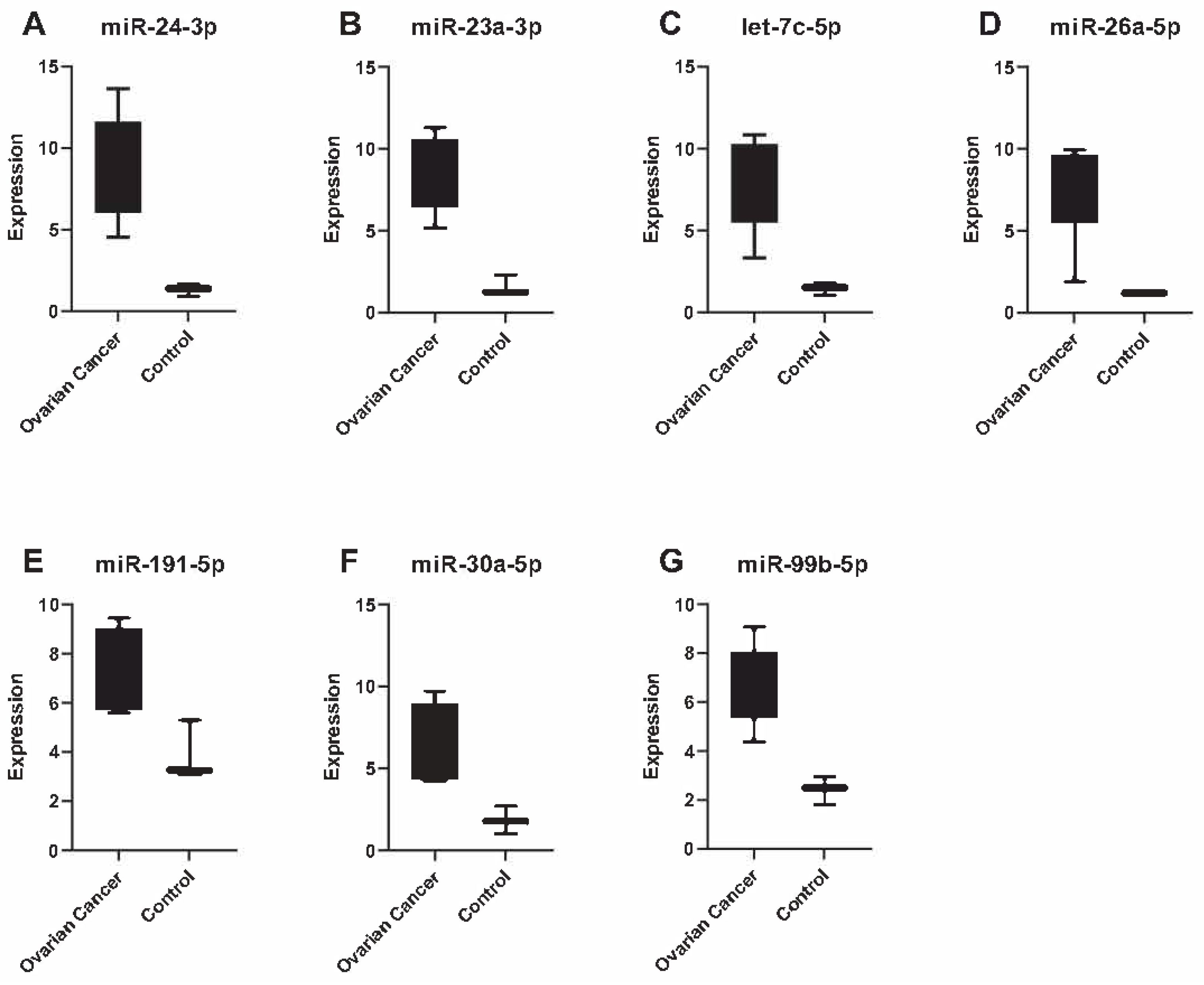
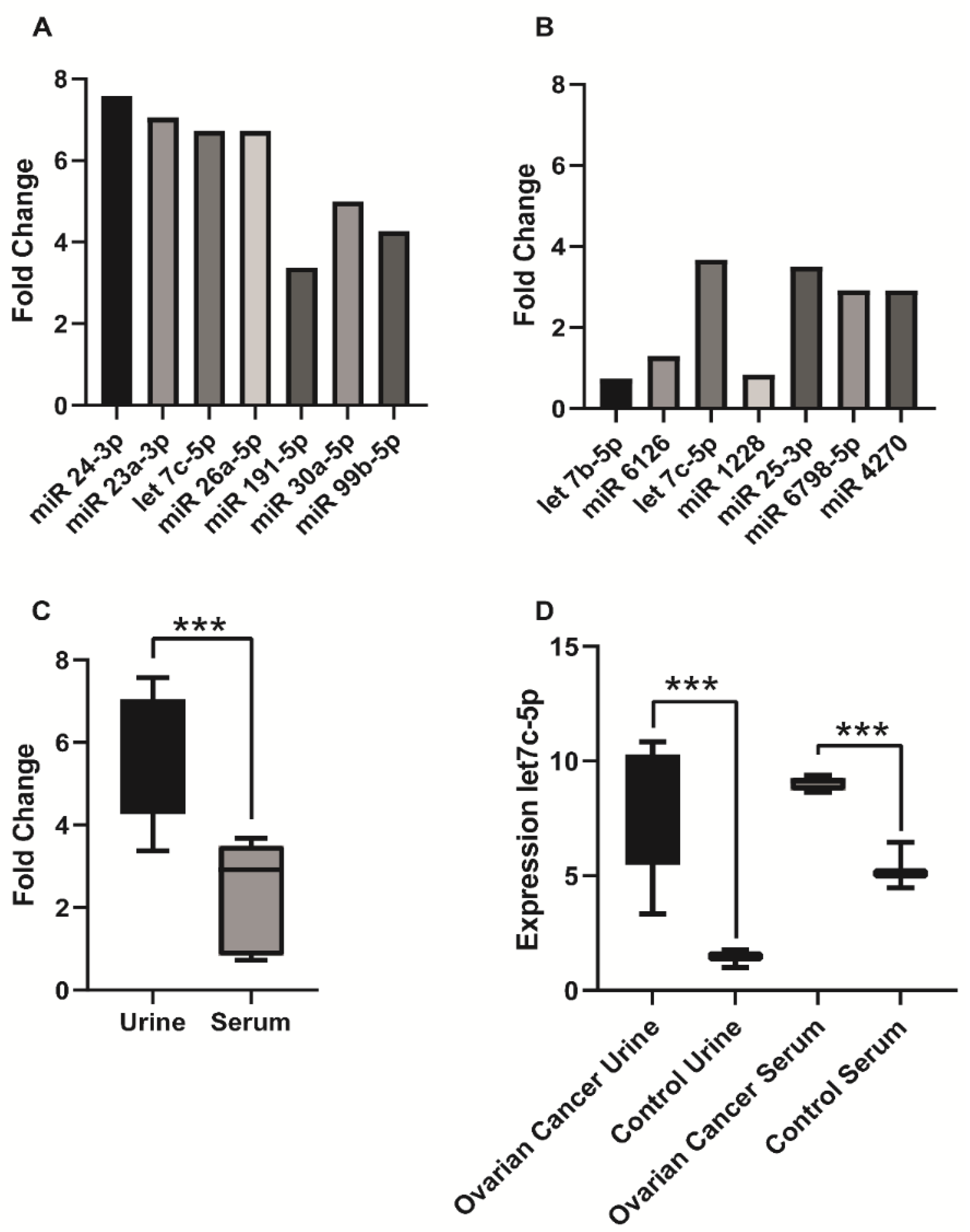
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Robert Koch-Institut und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. Krebs in Deutschland 2011/2012, 10. Ausgabe. 2015. Available online: http://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_node.html (accessed on 18 August 2016).
- Du, J.; Zhang, L. Analysis of salivary microRNA expression profiles and identification of novel biomarkers in esophageal cancer. Oncol. Lett. 2017, 14, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Erbes, T.; Hirschfeld, M.; Rücker, G.; Jaeger, M.; Boas, J.; Iborra, S.; Mayer, S.; Gitsch, G.; Stickeler, E. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer 2015, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Erdem, A.; Ozsoz, M.; Carrara, S. microRNA biosensors: Opportunities and challenges among conventional and commercially available techniques. Biosens. Bioelectron. 2018, 99, 525–546. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Kupec, T.; Bleilevens, A.; Iborra, S.; Najjari, L.; Wittenborn, J.; Maurer, J.; Stickeler, E. Stability of circulating microRNAs in serum. PLoS ONE 2022, 17, e0268958. [Google Scholar] [CrossRef]
- Amuran, G.G.; Tinay, I.; Filinte, D.; Ilgin, C.; Eyüboğlu, I.P.; Akkiprik, M. Urinary micro-RNA expressions and protein concentrations may differentiate bladder cancer patients from healthy controls. Int. Urol. Nephrol. 2020, 52, 461–468. [Google Scholar] [CrossRef]
- Kao, H.-W.; Pan, C.-Y.; Lai, C.-H.; Wu, C.-W.; Fang, W.-L.; Huang, K.-H.; Lin, W.-C. Urine miR-21-5p as a potential non-invasive biomarker for gastric cancer. Oncotarget 2017, 8, 56389–56397. [Google Scholar] [CrossRef]
- Mosaad, H.; Ahmed, M.M.; Elaidy, M.M.; Elfarargy, O.M.; Abdelwahab, M.M.; Abdelnour, H.M. Down-regulated MiRNA 29-b as a diagonistic marker in colorectal cancer and its correlation with ETV4 and Cyclin D1 immunohistochemical expression. Cancer Biomark. 2023, 37, 179–189. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of miRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, H.; Fu, Y.; Zhang, J. Long noncoding RNA DARS-AS1 regulates TP53 ubiquitination and affects ovarian cancer progression by modulation miR-194-5p/RBX1 axis. J. Biochem. Mol. Toxicol. 2021, 35, e22865. [Google Scholar] [CrossRef]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.-G.; Alder, H.; et al. MicroRNA Signatures in Human Ovarian Cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Fountain, M.D.; Yu, Z.; Nagaraja, A.K.; Zhu, H.; Khan, M.; Olokpa, E.; Zariff, A.; Gunaratne, P.H.; Matzuk, M.M.; et al. Molecular Profiling Uncovers a p53-Associated Role for MicroRNA-31 in Inhibiting the Proliferation of Serous Ovarian Carcinomas and Other Cancers. Cancer Res. 2010, 70, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Ghafour, A.A.; Odemis, D.A.; Tuncer, S.B.; Kurt, B.; Saral, M.A.; Erciyas, S.K.; Erdogan, O.S.; Celik, B.; Saip, P.; Yazici, H. High expression level of miR-1260 family in the peripheral blood of patients with ovarian carcinoma. J. Ovarian Res. 2021, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gong, G.; Tan, H.; Dai, F.; Zhu, X.; Chen, Y.; Wang, J.; Liu, Y.; Chen, P.; Wu, X.; et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol. Rep. 2015, 33, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Záveský, L.; Jandáková, E.; Turyna, R.; Langmeierová, L.; Weinberger, V.; Zaveska Drábková, L.; Hůlková, M.; Hořínek, A.; Dušková, D.; Feyereisl, J.; et al. Evaluation of Cell-Free Urine microRNAs Expression for the Use in Diagnosis of Ovarian and Endometrial Cancers. A Pilot Study. Pathol. Oncol. Res. 2015, 21, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.-S.; Chang, J.-M.; Lertkhachonsuk, A.-A.; Rermluk, N.; Jinawath, N. Circulating Exosomal miRNAs as Biomarkers in Epithelial Ovarian Cancer. Biomedicines 2021, 9, 1433. [Google Scholar] [CrossRef]
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Yun, S.J.; Jeong, P.; Kim, W.-T.; Kim, T.H.; Lee, Y.-S.; Song, P.H.; Choi, Y.-H.; Kim, I.Y.; Moon, S.-K.; Kim, W.-J. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int. J. Oncol. 2012, 41, 1871–1878. [Google Scholar] [CrossRef]
- Montazerian, M.; Yasari, F.; Aghaalikhani, N. Ovarian extracellular MicroRNAs as the potential non-invasive biomarkers: An update. BioMedicine Pharmacother. 2018, 106, 1633–1640. [Google Scholar] [CrossRef]
- Hulstaert, E.; Morlion, A.; Levanon, K.; Vandesompele, J.; Mestdagh, P. Candidate RNA biomarkers in biofluids for early diagnosis of ovarian cancer: A systematic review. Gynecol. Oncol. 2021, 160, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum microRNAs. PLoS ONE 2016, 11, e0153200. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sawada, K.; Yoshimura, A.; Kinose, Y.; Nakatsuka, E.; Kimura, T. Clinical relevance of circulating cell-free microRNAs in ovarian cancer. Mol. Cancer 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, M.C.; Jeong, J.-Y.; Hwang, S.; Jung, S.G.; Joo, W.D.; Park, H.; Song, S.H.; Lee, C.; Kim, T.H.; et al. Serum exosomal miRNA-145 and miRNA-200c as promising biomarkers for preoperative diagnosis of ovarian carcinomas. J. Cancer 2019, 10, 1958–1967. [Google Scholar] [CrossRef]
- Elias, K.M.; Fendler, W.; Stawiski, K.; Fiascone, S.J.; Vitonis, A.F.; Berkowitz, R.S.; Frendl, G.; Konstantinopoulos, P.; Crum, C.P.; Kedzierska, M. Diagnostic potential for a serum miRNA neural network for detection of ovarian cancer. eLife 2017, 6, e28932. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, S.; Chaurasia, A.; Sachan, M. Evaluation of Diagnostic Potential of Epigenetically Deregulated MiRNAs in Epithelial Ovarian Cancer. Front. Oncol. 2021, 11, 681872. [Google Scholar] [CrossRef]
- Záveský, L.; Jandáková, E.; Weinberger, V.; Minář, L.; Hanzíková, V.; Dušková, D.; Drábková, L.Z.; Hořínek, A. Ovarian Cancer: Differentially Expressed microRNAs in Tumor Tissue and Cell-Free Ascitic Fluid as Potential Novel Biomarkers. Cancer Investig. 2019, 37, 440–452. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar]
- Wong, N.W.; Chen, Y.; Chen, S.; Wang, X. OncomiR: An online resource for exploring pan-cancer microRNA dysregulation. Bioinformatics 2018, 34, 713–715. [Google Scholar] [CrossRef]



| Sample No. | Age (Years) | Menopausal Status | Histological Subtype | Initial Tumor Stage | FIGO Staging | Nodal Status | Tumor Residues After Surgery |
|---|---|---|---|---|---|---|---|
| 1 | 57 | Postmenopausal | Serous adenocarcinoma | pT3 | IIIc | pN1 | 0 cm |
| 2 | 67 | Postmenopausal | Serous adenocarcinoma | pT3 | IIIc | n.a. | >1 cm |
| 3 | 66 | Postmenopausal | Serous adenocarcinoma | pT3 | IIIc | n.a. | >1 cm |
| 4 | 56 | Postmenopausal | Serous adenocarcinoma | >pT3 | IVb | n.a. | >1 cm |
| 5 | 55 | Postmenopausal | Serous adenocarcinoma | pT3 | IIIc | n.a. | >1 cm |
| Expression Levels in OvCa (log2) | Expression Compared to All Cancer Types | Ovarian Carcinoma Specific Regulated Genes | Gene ID | |
|---|---|---|---|---|
| hsa-miR-24-3p | 10.44 | median high expression | ||
| neuronal differentiation 1 | NEUROD1 | |||
| poliovirus receptor-related 1 | PVRL1 | |||
| neurofilament, medium polypeptide | NEFM | |||
| abhydrolase domain containing 2 | ABHD2 | |||
| tumor protein p53 inducible protein 11 | TP53I11 | |||
| hsa-miR-23a-3p | 11.44 | median expression | ||
| fucosyltransferase 4 | FUT4 | |||
| UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 5 | B3GNT5 | |||
| ryanodine receptor 3 | RYR3 | |||
| E74-like factor 5 | ELF5 | |||
| cellular repressor of E1A-stimulated genes 2 | ELF5 | |||
| hsa-let-7c-5p | 14.16 | highest expression | ||
| retinol dehydrogenase 10 | RDH10 | |||
| zinc finger protein 697 | ZNF697 | |||
| solute carrier organic anion transporter family, member 2A1 | SLCO2A1 | |||
| syntaxin 17 | STX17 | |||
| interleukin 13 | IL13 | |||
| hsa-miR-26a-5p | 9.65 | median expression | ||
| pleckstrin homology-like domain, family B, member 2 | PHLDB2 | |||
| UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 5 | B3GNT5 | |||
| hepatocyte growth factor | HGF | |||
| solute carrier family 24 | SLC24A4 | |||
| homeobox A9 | HOXA9 | |||
| hsa-miR-191-5p | 10.57 | median expression | no OvCa-specific genes | |
| hsa-miR-30a-5p | 12.28 | median expression | ||
| neuronal differentiation 1 | NEUROD1 | |||
| UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 5 | B3GNT5 | |||
| galanin receptor 1 | GALR1 | |||
| neurofilament, medium polypeptide | NEFM | |||
| syntaxin 17 | STX17 | |||
| hsa-miR-99b-5p | 18.06 | highest expression | ||
| frizzled class receptor 8 | FZD8 | |||
| hsa-let-7b-5p | 16.39 | highest expression | ||
| retinol dehydrogenase 10 (all-trans) | RDH10 | |||
| syntaxin 17 | STX17 | |||
| solute carrier organic anion transporter family, member 2A1 | SLCO2A1 | |||
| ADAM metallopeptidase with thrombospondin type 1 motif, 15 | ADAMTS15 | |||
| R-spondin 2 | RSPO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupec, T.; Bleilevens, A.; Klein, B.; Hansen, T.; Najjari, L.; Wittenborn, J.; Stickeler, E.; Maurer, J. Comparison of Serum and Urine as Sources of miRNA Markers for the Detection of Ovarian Cancer. Biomedicines 2023, 11, 2508. https://doi.org/10.3390/biomedicines11092508
Kupec T, Bleilevens A, Klein B, Hansen T, Najjari L, Wittenborn J, Stickeler E, Maurer J. Comparison of Serum and Urine as Sources of miRNA Markers for the Detection of Ovarian Cancer. Biomedicines. 2023; 11(9):2508. https://doi.org/10.3390/biomedicines11092508
Chicago/Turabian StyleKupec, Tomas, Andreas Bleilevens, Birgit Klein, Thomas Hansen, Laila Najjari, Julia Wittenborn, Elmar Stickeler, and Jochen Maurer. 2023. "Comparison of Serum and Urine as Sources of miRNA Markers for the Detection of Ovarian Cancer" Biomedicines 11, no. 9: 2508. https://doi.org/10.3390/biomedicines11092508
APA StyleKupec, T., Bleilevens, A., Klein, B., Hansen, T., Najjari, L., Wittenborn, J., Stickeler, E., & Maurer, J. (2023). Comparison of Serum and Urine as Sources of miRNA Markers for the Detection of Ovarian Cancer. Biomedicines, 11(9), 2508. https://doi.org/10.3390/biomedicines11092508






