Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cultures
2.2. Chemicals and Drugs
2.3. Cell Treatment and Cell Proliferation Assay
3. Results
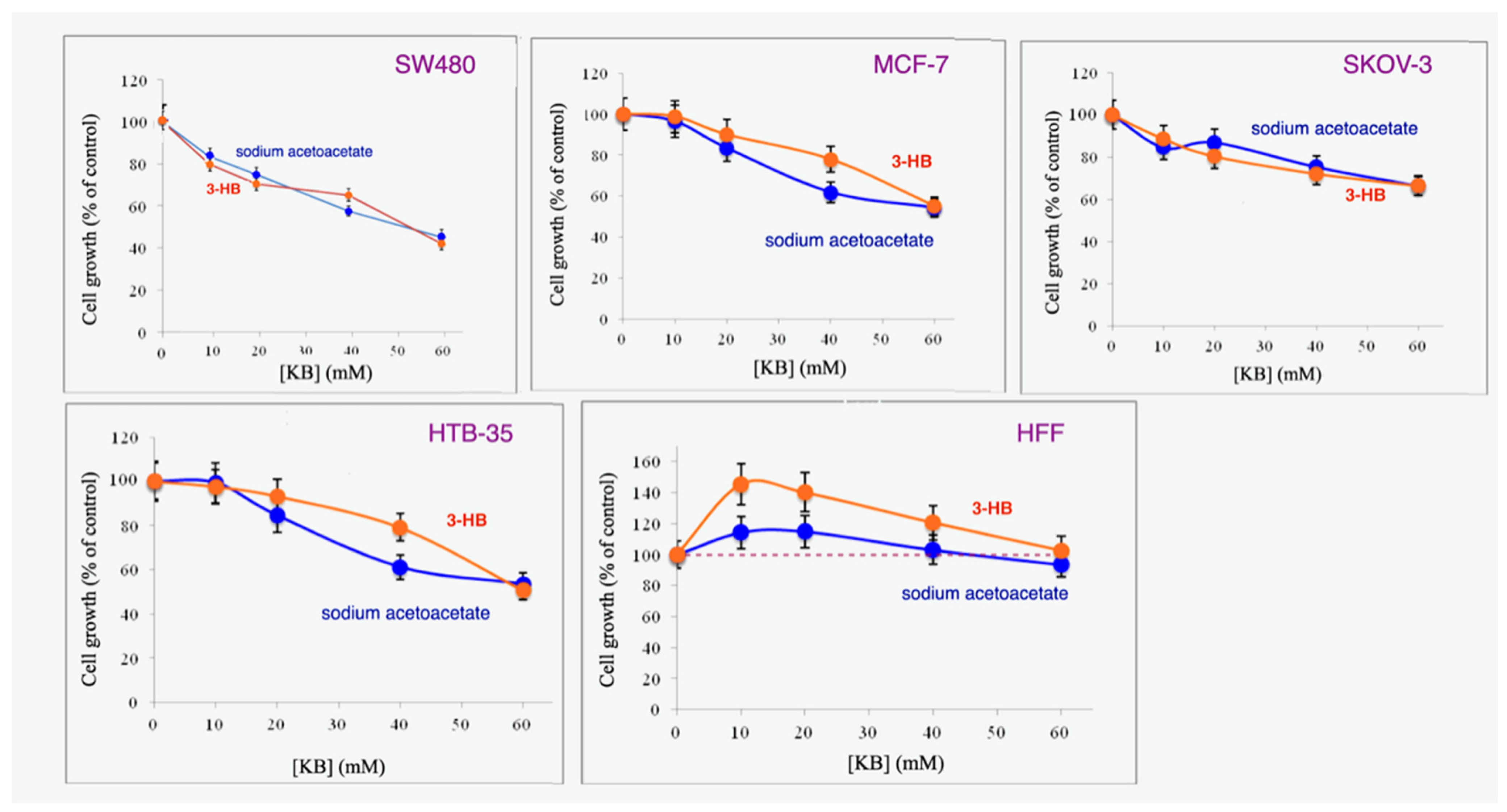
3.1. Sodium Acetoacetate and 3-Hydroxybutyrate Inhibit Cancer but Not Normal Cell Proliferation
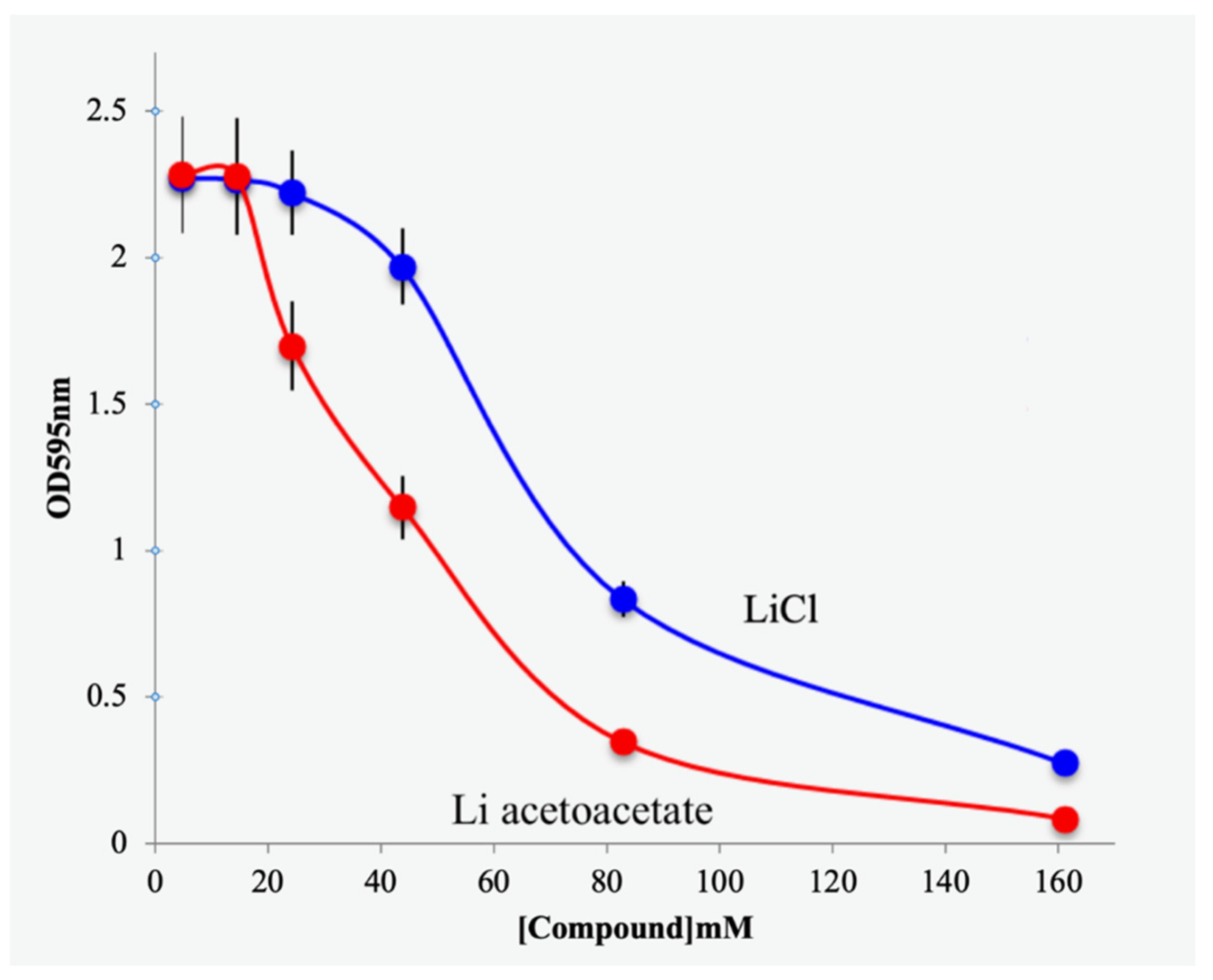
3.2. Effects of Lithium Acetoacetate on Cancer and Untransformed Cells
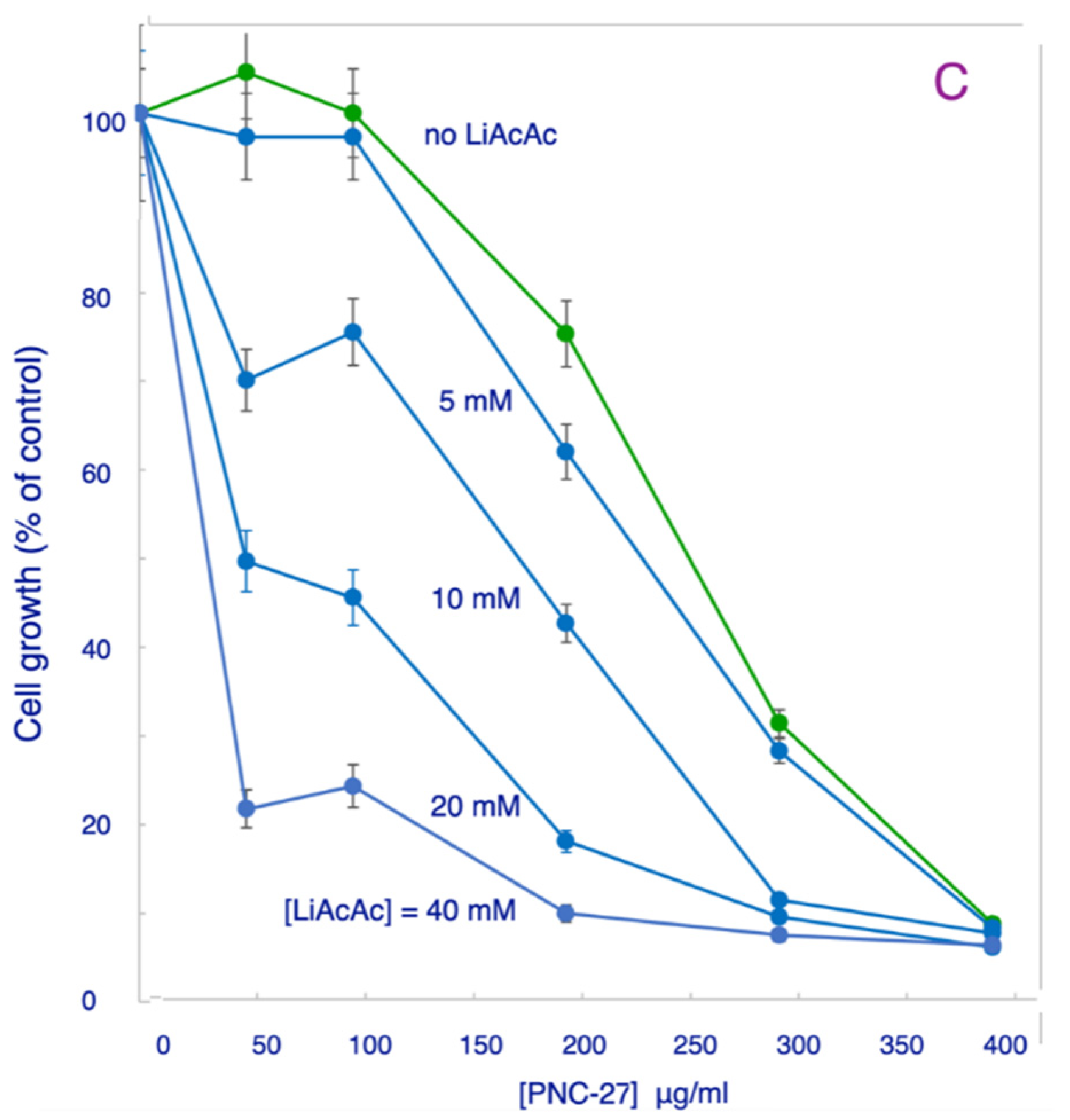
3.3. Effects of LiAcAc on Chemotherapeutic Agent-Induced Killing of Cancer Cells
4. Discussion
4.1. Ketone Bodies Enhance Chemotherapeutic Molecules in Killing Cancer Cells
4.2. Ketone Bodies Are Selectively Active in Blocking Cancer Cell Growth
4.3. Effects of Lithium and Sodium Counterions
4.4. Possible Effects of Lithium Ion on Cancer Cell Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Champ, C.E.; Palmer, J.D.; Volek, J.S.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Glass, J.; Kim, L.; Shi, W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J. Neurooncol. 2014, 117, 125–131. [Google Scholar] [CrossRef]
- Tan-Shalaby, J. Ketogenic Diets and Cancer, Emerging Evidence. Fed. Pract. 2017, 34, 37S. [Google Scholar] [PubMed]
- Klement, R.J. Beneficial Effects of Ketogenic Diets for Cancer Patients: A Realist Review with Focus on Evidence and Confirmation. Med. Oncol. 2017, 34, 132. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Kiebish, M.; Mukherjee, P.; Marsh, J. Targeting energy metabolism in brain cancer with calorically restricted ketogenic diets. Epilepsia 2008, 49 (Suppl. S8), 114–116. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’Agostino, D.P. The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS ONE 2013, 8, e65522. [Google Scholar] [CrossRef] [PubMed]
- Fine, E.J.; Segal-Isaacson, C.J.; Feinman, R.D.; Herszkopf, S.; Romano, M.C.; Tomuta, N.; Bontempo, A.F.; Negassa, A.; Sparano, J.A. Targeting Insulin Inhibition as a Metabolic Therapy in Advanced Cancer: A Pilot Safety and Feasibility Dietary Trial in 10 Patients. Nutrition 2012, 28, 1028–1035. [Google Scholar] [CrossRef]
- Fine, E.J.; Miller, A.; Quadros, E.V.; Sequeira, J.M.; Feinman, R.D. Acetoacetate Reduces Growth and ATP Concentration in Cancer Cell Lines Which Over-Express Uncoupling Protein 2. Cancer Cell Int. 2009, 9, 14. [Google Scholar] [CrossRef]
- Zou, Y.; Fineberg, S.; Pearlman, A.; Feinman, R.D.; Fine, E.J. The Effect of a Ketogenic Diet and Synergy with Rapamycin in a Mouse Model of Breast Cancer. PLoS ONE 2020, 15, e0233662. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Villegas-Vázquez, E.Y.; Quintas-Granados, L.I.; Cortes, H.; González-Del Carmen, M.; Leyva-Gómez, G.; Rodríguez-Morales, M.; Bustamante-Montes, L.P.; Silva-Adaya, D.; Pérez-Plasencia, C.; Jacobo-Herrera, N.; et al. Lithium: A Promising Anti-Cancer Agent. Life 2023, 13, 537. [Google Scholar] [CrossRef]
- Cohen-Harazi, R.; Hofmann, S.; Kogan, V.; Fulman-Levy, H.; Abaev, K.; Shovman, O.; Brider, T.; Koman, I. Cytotoxicity of Exogenous Acetoacetate in Lithium Salt Form Is Mediated by Lithium and Not Acetoacetate. Anticancer. Res. 2020, 40, 3831–3837. [Google Scholar] [CrossRef]
- Vidali, S.; Aminzadeh-Gohari, S.; Vatrinet, R.; Iommarini, L.; Porcelli, A.M.; Kofler, B.; Feichtinger, R.G. Lithium And Not Acetoacetate Influences the Growth of Cells Treated with Lithium Acetoacetate. Int. J. Mol. Sci. 2019, 20, 3104. [Google Scholar] [CrossRef]
- Miller, A.; Lin, B.; Pincus, M.R.; Fine, E.; Feinman, R.D. Selective Enhancement of Chemotherapeutic Agent-Induced Tumor Cell Killing by Acetoacetate and 3-Hydroxybutyrate. EC Pharmacol. Toxicol. 2021, 9, 29–34. [Google Scholar]
- López-Soriano, F.J.; Argiles, J.M. A Simple Method for the Preparation of Acetoacetate. Anal. Lett. 1985, 18, 589–592. [Google Scholar] [CrossRef]
- Schilke, R.E.; Johnson, R.E. A Colorimetric Method for Estimating Acetoacetate. Am. J. Clin. Pathol. 1965, 43, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, D.; Nguyen, L.X.; Wu, H.; Li, L.; Dong, D.; Troadec, E.; Zhu, Y.; Hoang, D.H.; Stein, A.S.; et al. Targeting cell membrane HDM2: A novel therapeutic approach for acute myeloid leukemia. Leukemia 2020, 34, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2016, 111, A3. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, E.; Mumin, S.; Cheung, D.; Fridman, D.; Lin, B.; Wong, L.; Rosal, R.; Rudolph, R.; Frenkel, M.; Thadi, A.; et al. PNC-27, a Chimeric p53-Penetratin Peptide Binds to HDM-2 in a p53 Peptide-like Structure, Induces Selective Membrane-Pore Formation and Leads to Cancer Cell Lysis. Biomedicines 2022, 10, 945. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Penso, J.; Beitner, R. Lithium Detaches Hexokinase from Mitochondria and Inhibits Proliferation of B16 Melanoma Cells. Mol. Genet. Metab. 2003, 78, 74–78. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | Ketone Body | Chemotherapeutic Agent | IC50 | IC50 Ratio 1 |
|---|---|---|---|---|
| SW480 | None | Rapamycin | 1.655 nM | |
| SW480 | 10 mM LiAcAc | Rapamycin | 0.394 nM | 4.2 |
| SW480 | 20 mM LiAcAc | Rapamycin | 0.0692 nM | 23.94 |
| SW480 | None | Methotrexate | 0.1965 nM | |
| SW480 | 15 mM LiAcAc | Methotrexate | 0.0863 nM | 2.28 |
| MCF-7 | None | Rapamycin | 1.822 nM | |
| MCF-7 | 10 mM LiAcAc | Rapamycin | 0.368 nM | 4.95 |
| MCF-7 | None | PNC-27 | 207 μg/mL | |
| MCF-7 | 20 mM LiAcAc | PNC-27 | 56.7 μg/mL | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, A.I.; Diaz, D.; Lin, B.; Krzesaj, P.K.; Ustoyev, S.; Shim, A.; Fine, E.J.; Sarafraz-Yazdi, E.; Pincus, M.R.; Feinman, R.D. Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents. Biomedicines 2023, 11, 2515. https://doi.org/10.3390/biomedicines11092515
Miller AI, Diaz D, Lin B, Krzesaj PK, Ustoyev S, Shim A, Fine EJ, Sarafraz-Yazdi E, Pincus MR, Feinman RD. Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents. Biomedicines. 2023; 11(9):2515. https://doi.org/10.3390/biomedicines11092515
Chicago/Turabian StyleMiller, Anna I., David Diaz, Bo Lin, Patryk K. Krzesaj, Sarah Ustoyev, Alfred Shim, Eugene J. Fine, Ehsan Sarafraz-Yazdi, Matthew R. Pincus, and Richard D. Feinman. 2023. "Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents" Biomedicines 11, no. 9: 2515. https://doi.org/10.3390/biomedicines11092515
APA StyleMiller, A. I., Diaz, D., Lin, B., Krzesaj, P. K., Ustoyev, S., Shim, A., Fine, E. J., Sarafraz-Yazdi, E., Pincus, M. R., & Feinman, R. D. (2023). Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents. Biomedicines, 11(9), 2515. https://doi.org/10.3390/biomedicines11092515






