Blood-Based MicroRNAs in Psychotic Disorders—A Systematic Review
Abstract
:1. Introduction
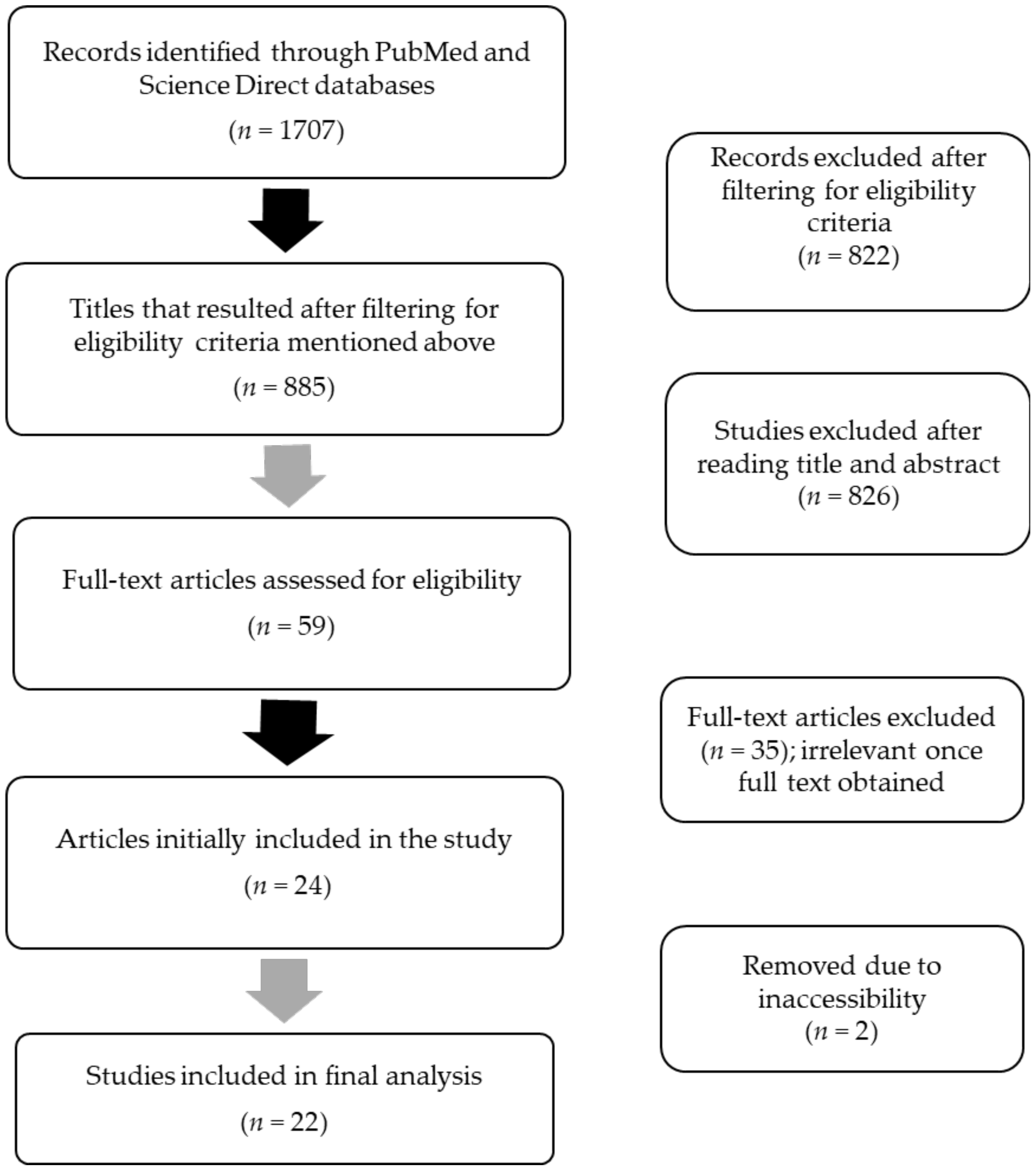
2. Methods
2.1. Data Sources, Search Strategy, and Eligibility Criteria for Article Inclusion
2.2. Data Extraction and Risk of Bias Assessment
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno-Küstner, B.; Martín, C.; Pastor, L. Prevalence of Psychotic Disorders and Its Association with Methodological Issues. A Systematic Review and Meta-Analyses. PLoS ONE 2018, 13, e0195687. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Dong, M.; Lu, L.; Zhang, L.; Zhang, Y.-S.; Ng, C.H.; Ungvari, G.S.; Li, G.; Meng, X.; Wang, G.; Xiang, Y.-T. Quality of Life in Schizophrenia: A Meta-Analysis of Comparative Studies. Psychiatr. Q. 2019, 90, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, D.B. Psychosis. Contin. Lifelong Learn. Neurol. 2015, 21, 715. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, M.H.; Avramopoulos, D. Gene-Environment Interactions in Schizophrenia: A Literature Review. Genes 2021, 12, 1850. [Google Scholar] [CrossRef]
- Popov, N.T.; Stoyanova, V.K.; Madzhirova, N.P.; Vachev, T.I. Epigenetic Aspects in Schizophrenia Etiology and Pathogenesis. Folia Med. 2012, 54, 12–16. [Google Scholar] [CrossRef]
- Hilker, R.; Helenius, D.; Fagerlund, B.; Skytthe, A.; Christensen, K.; Werge, T.M.; Nordentoft, M.; Glenthøj, B. Heritability of Schizophrenia and Schizophrenia Spectrum Based on the Nationwide Danish Twin Register. Biol. Psychiatry 2017, 83, 492–498. [Google Scholar] [CrossRef]
- Cardno, A.G.; Marshall, E.J.; Coid, B.; Macdonald, A.M.; Ribchester, T.R.; Davies, N.J.; Venturi, P.; Jones, L.A.; Lewis, S.W.; Sham, P.C.; et al. Heritability Estimates for Psychotic Disorders: The Maudsley Twin Psychosis Series. Arch. Gen. Psychiatry 1999, 56, 162–168. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a Complex Trait: Evidence from a Meta-Analysis of Twin Studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Robinson, N.; Bergen, S.E. Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front. Genet. 2021, 12, 686666. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Eyles, D.W. Vitamin D and Schizophrenia: 20 Years on. Mol. Psychiatry 2021, 26, 2708–2720. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Trzaskowski, M.; Vinkhuyzen, A.A.E.; Mattheisen, M.; Meier, S.; Gooch, H.; Anggono, V.; Cui, X.; Tan, M.C.; Burne, T.H.J.; et al. The Association between Neonatal Vitamin D Status and Risk of Schizophrenia. Sci. Rep. 2018, 8, 17692. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.J.; Eyles, D.W.; Pedersen, C.B.; Anderson, C.; Ko, P.; Burne, T.H.; Norgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, P.B. Neonatal Vitamin D Status and Risk of Schizophrenia: A Population-Based Case-Control Study. Arch. Gen. Psychiatry 2010, 67, 889–894. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar] [CrossRef]
- Laruelle, M.; Abi-Dargham, A.; Gil, R.; Kegeles, L.; Innis, R. Increased Dopamine Transmission in Schizophrenia: Relationship to Illness Phases. Biol. Psychiatry 1999, 46, 56–72. [Google Scholar] [CrossRef]
- Kapur, S.; Zipursky, R.; Jones, C.; Remington, G.; Houle, S. Relationship between Dopamine D(2) Occupancy, Clinical Response, and Side Effects: A Double-Blind PET Study of First-Episode Schizophrenia. Am. J. Psychiatry 2000, 157, 514–520. [Google Scholar] [CrossRef]
- Nordström, A.L.; Farde, L.; Wiesel, F.A.; Forslund, K.; Pauli, S.; Halldin, C.; Uppfeldt, G. Central D2-Dopamine Receptor Occupancy in Relation to Antipsychotic Drug Effects: A Double-Blind PET Study of Schizophrenic Patients. Biol. Psychiatry 1993, 33, 227–235. [Google Scholar] [CrossRef]
- De La Fuente-Sandoval, C.; León-Ortiz, P.; Azcárraga, M.; Stephano, S.; Favila, R.; Díaz-Galvis, L.; Alvarado-Alanis, P.; Ramírez-Bermúdez, J.; Graff-Guerrero, A. Glutamate Levels in the Associative Striatum Before and After 4 Weeks of Antipsychotic Treatment in First-Episode Psychosis: A Longitudinal Proton Magnetic Resonance Spectroscopy Study. JAMA Psychiatry 2013, 70, 1057. [Google Scholar] [CrossRef]
- Haaf, M.; Leicht, G.; Curic, S.; Mulert, C. Glutamatergic Deficits in Schizophrenia—Biomarkers and Pharmacological Interventions within the Ketamine Model. Curr. Pharm. Biotechnol. 2018, 19, 293. [Google Scholar] [CrossRef]
- Talati, P.; Rane, S.; Skinner, J.; Gore, J.; Heckers, S. Increased Hippocampal Blood Volume and Normal Blood Flow in Schizophrenia. Psychiatry Res. 2015, 232, 219. [Google Scholar] [CrossRef]
- Tregellas, J.R.; Smucny, J.; Harris, J.G.; Olincy, A.; Maharajh, K.; Kronberg, E.; Eichman, L.C.; Lyons, E.; Freedman, R. Intrinsic Hippocampal Activity as a Biomarker for Cognition and Symptoms in Schizophrenia. Am. J. Psychiatry 2014, 171, 549. [Google Scholar] [CrossRef] [PubMed]
- Doorduin, J.; De Vries, E.F.J.; Willemsen, A.T.M.; De Groot, J.C.; Dierckx, R.A.; Klein, H.C. Neuroinflammation in Schizophrenia-Related Psychosis: A PET Study. J. Nucl. Med. 2009, 50, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Catts, V.S.; Sheedy, D.; McCrossin, T.; Kril, J.J.; Shannon Weickert, C. Cortical Grey Matter Volume Reduction in People with Schizophrenia Is Associated with Neuro-Inflammation. Transl. Psychiatry 2016, 6, e982. [Google Scholar] [CrossRef] [PubMed]
- Aberg, K.A.; McClay, J.L.; Nerella, S.; Clark, S.; Kumar, G.; Chen, W.; Khachane, A.N.; Xie, L.; Hudson, A.; Gao, G.; et al. Methylome-Wide Association Study of Schizophrenia: Identifying Blood Biomarker Signatures of Environmental Insults. JAMA Psychiatry 2014, 71, 255. [Google Scholar] [CrossRef]
- Kano, S.; Colantuoni, C.; Han, F.; Zhou, Z.; Yuan, Q.; Wilson, A.; Takayanagi, Y.; Lee, Y.; Rapoport, J.; Eaton, W.; et al. Genome-Wide Profiling of Multiple Histone Methylations in Olfactory Cells: Further Implications for Cellular Susceptibility to Oxidative Stress in Schizophrenia. Mol. Psychiatry 2013, 18, 740. [Google Scholar] [CrossRef]
- Ren, Y.; Cui, Y.; Li, X.; Wang, B.; Na, L.; Shi, J.; Wang, L.; Qiu, L.; Zhang, K.; Liu, G.; et al. A Co-Expression Network Analysis Reveals LncRNA Abnormalities in Peripheral Blood in Early-Onset Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 63, 1–5. [Google Scholar] [CrossRef]
- Tomasik, J.; Rahmoune, H.; Guest, P.C.; Bahn, S. Neuroimmune Biomarkers in Schizophrenia. Schizophr. Res. 2016, 176, 3–13. [Google Scholar] [CrossRef]
- Sunde, R.A. MRNA Transcripts as Molecular Biomarkers in Medicine and Nutrition. J. Nutr. Biochem. 2010, 21, 665. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray Analysis Shows That Some MicroRNAs Downregulate Large Numbers of Target MRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating MicroRNAs as Potential Biomarkers for Psychiatric and Neurodegenerative Disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef]
- Lockwood, L.; Miller, B.; Youssef, N.A. Epigenetics and First-Episode Psychosis: A Systematic Review. Psychiatry Res. 2022, 307, 114325. [Google Scholar] [CrossRef]
- Tomassi, S.; Tosato, S. Epigenetics and Gene Expression Profile in First-Episode Psychosis: The Role of Childhood Trauma. Neurosci. Biobehav. Rev. 2017, 83, 226–237. [Google Scholar] [CrossRef]
- Dinan, T.G. MicroRNAs as a Target for Novel Antipsychotics: A Systematic Review of an Emerging Field. Int. J. Neuropsychopharmacol. 2010, 13, 395. [Google Scholar] [CrossRef]
- Srivastava, A.; Dada, O.; Qian, J.; Al-Chalabi, N.; Fatemi, A.B.; Gerretsen, P.; Graff, A.; De Luca, V. Epigenetics of Schizophrenia. Psychiatry Res. 2021, 305, 114218. [Google Scholar] [CrossRef]
- He, K.; Guo, C.; He, L.; Shi, Y. MiRNAs of Peripheral Blood as the Biomarker of Schizophrenia. Hereditas 2018, 155, 9. [Google Scholar] [CrossRef]
- Smigielski, L.; Jagannath, V.; Rössler, W.; Walitza, S.; Grünblatt, E. Epigenetic Mechanisms in Schizophrenia and Other Psychotic Disorders: A Systematic Review of Empirical Human Findings. Mol. Psychiatry 2020, 25, 1718–1748. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Med. Flum. 2021, 57, 444–465. [Google Scholar] [CrossRef]
- Gardiner, E.; Beveridge, N.J.; Wu, J.Q.; Carr, V.; Scott, R.J.; Tooney, P.A.; Cairns, M.J. Imprinted DLK1-DIO3 Region of 14q32 Defines a Schizophrenia-Associated MiRNA Signature in Peripheral Blood Mononuclear Cells. Mol. Psychiatry 2012, 17, 827. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Yu, S.L.; Hsieh, M.H.; Chen, C.H.; Chen, H.Y.; Wen, C.C.; Huang, Y.H.; Hsiao, P.C.; Hsiao, C.K.; Liu, C.M.; et al. MicroRNA Expression Aberration as Potential Peripheral Blood Biomarkers for Schizophrenia. PLoS ONE 2011, 6, e21635. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Du, J.; Qi, Y.; Liang, G.; Wang, T.; Li, S.; Xie, S.; Zeshan, B.; Xiao, Z. Aberrant Expression of Serum MiRNAs in Schizophrenia. J. Psychiatr. Res. 2012, 46, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Song, H.T.; Sun, X.Y.; Zhang, L.; Zhao, L.; Guo, Z.; Fan, H.; Zhong, A.; Niu, W.; Dai, Y.; Zhang, L.; et al. A Preliminary Analysis of Association between the Down-Regulation of MicroRNA-181b Expression and Symptomatology Improvement in Schizophrenia Patients before and after Antipsychotic Treatment. J. Psychiatr. Res. 2014, 54, 134–140. [Google Scholar] [CrossRef]
- Fan, H.M.; Sun, X.Y.; Niu, W.; Zhao, L.; Zhang, Q.L.; Li, W.; Zhong, A.; Zhang, L.; Lu, J. Altered MicroRNA Expression in Peripheral Blood Mononuclear Cells from Young Patients with Schizophrenia. J. Mol. Neurosci. 2015, 56, 562–571. [Google Scholar] [CrossRef]
- Yu, H.C.; Wu, J.; Zhang, H.X.; Zhang, G.; Sui, J.; Tong, W.; Zhang, X.Y.; Nie, L.; Duan, J.; Zhang, L.; et al. Alterations of MiR-132 Are Novel Diagnostic Biomarkers in Peripheral Blood of Schizophrenia Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 63, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Lu, J.; Zhang, L.; Song, H.T.; Zhao, L.; Fan, H.M.; Zhong, A.F.; Niu, W.; Guo, Z.M.; Dai, Y.H.; et al. Aberrant MicroRNA Expression in Peripheral Plasma and Mononuclear Cells as Specific Blood-Based Biomarkers in Schizophrenia Patients. J. Clin. Neurosci. 2015, 22, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zhang, J.; Niu, W.; Guo, W.; Song, H.T.; Li, H.Y.; Fan, H.M.; Zhao, L.; Zhong, A.F.; Dai, Y.H.; et al. A Preliminary Analysis of MicroRNA as Potential Clinical Biomarker for Schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 170–178. [Google Scholar] [CrossRef]
- Wei, H.; Yuan, Y.; Liu, S.; Wang, C.; Yang, F.; Lu, Z.; Wang, C.; Deng, H.; Zhao, J.; Shen, Y.; et al. Detection of Circulating MiRNA Levels in Schizophrenia. Am. J. Psychiatry 2015, 172, 1141–1147. [Google Scholar] [CrossRef]
- Lai, C.Y.; Lee, S.Y.; Scarr, E.; Yu, Y.H.; Lin, Y.T.; Liu, C.M.; Hwang, T.J.; Hsieh, M.H.; Liu, C.C.; Chien, Y.L.; et al. Aberrant Expression of MicroRNAs as Biomarker for Schizophrenia: From Acute State to Partial Remission, and from Peripheral Blood to Cortical Tissue. Transl. Psychiatry 2016, 6, e717. [Google Scholar] [CrossRef] [PubMed]
- Camkurt, M.A.; Karababa, F.; Erdal, M.E.; Bayaz, H.; Kandemir, B.S.; Ay, M.E.; Kandemir, H.; Ay, Ö.I.; Çiçek, E.; Selek, S.; et al. Investigation of Dysregulation of Several MicroRNAs in Peripheral Blood of Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 2016, 14, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Sun, X.Y.; Niu, W.; Kong, L.; He, M.; Fan, H.; Li, W.; Zhong, A.; Zhang, L.; Lu, J. A Preliminary Analysis of MicroRNA-21 Expression Alteration after Antipsychotic Treatment in Patients with Schizophrenia. Psychiatry Res. 2016, 244, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, F.; Wang, X.; Shugart, Y.Y.; Zhao, Y.; Li, X.; Liu, Z.; Sun, N.; Yang, C.; Zhang, K.; et al. Diagnostic Value of Blood-Derived MicroRNAs for Schizophrenia: Results of a Meta-Analysis and Validation. Sci. Rep. 2017, 7, 15328. [Google Scholar] [CrossRef]
- Ma, J.; Shang, S.; Wang, J.; Zhang, T.; Nie, F.; Song, X.; Zhao, H.; Zhu, C.; Zhang, R.; Hao, D. Identification of MiR-22-3p, MiR-92a-3p, and MiR-137 in Peripheral Blood as Biomarker for Schizophrenia. Psychiatry Res. 2018, 265, 70–76. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Guo, C.; Guo, M.; Tong, S.; Zhang, Q.; Sun, H.; He, L.; Shi, Y. Identification of Serum MicroRNAs as Diagnostic Biomarkers for Schizophrenia. Hereditas 2019, 156, 23. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Guo, T.; Peng, Y.; Wang, K.; Bai, K.; Huang, Y. Screening of Schizophrenia Associated MiRNAs and the Regulation of MiR-320a-3p on Integrin Β1. Medicine 2019, 98, e14332. [Google Scholar] [CrossRef]
- Zhao, Z.; Jinde, S.; Koike, S.; Tada, M.; Satomura, Y.; Yoshikawa, A.; Nishimura, Y.; Takizawa, R.; Kinoshita, A.; Sakakibara, E.; et al. Altered Expression of MicroRNA-223 in the Plasma of Patients with First-Episode Schizophrenia and Its Possible Relation to Neuronal Migration-Related Genes. Transl. Psychiatry 2019, 9, 289. [Google Scholar] [CrossRef]
- Du, Y.; Yu, Y.; Hu, Y.; Li, X.W.; Wei, Z.X.; Pan, R.Y.; Li, X.S.; Zheng, G.E.; Qin, X.Y.; Liu, Q.S.; et al. Genome-Wide, Integrative Analysis Implicates Exosome-Derived MicroRNA Dysregulation in Schizophrenia. Schizophr. Bull. 2019, 45, 1257–1266. [Google Scholar] [CrossRef]
- Horai, T.; Boku, S.; Okazaki, S.; Otsuka, I.; Ratta-apha, W.; Mouri, K.; Yamaki, N.; Hirata, T.; Hishimoto, A. MiR-19b Is Elevated in Peripheral Blood of Schizophrenic Patients and Attenuates Proliferation of Hippocampal Neural Progenitor Cells. J. Psychiatr. Res. 2020, 131, 102–107. [Google Scholar] [CrossRef]
- Gou, M.; Pan, S.; Tong, J.; Zhou, Y.; Han, J.; Xie, T.; Yu, T.; Feng, W.; Li, Y.; Chen, S.; et al. Effects of MicroRNA-181b-5p on Cognitive Deficits in First-Episode Patients with Schizophrenia: Mediated by BCL-2. J. Psychiatr. Res. 2021, 136, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Lin, J.J.; Lu, M.K.; Tan, H.P.; Jang, F.L.; Lin, S.H. Neurodevelopment Regulators MiR-137 and MiR-34 Family as Biomarkers for Early and Adult Onset Schizophrenia. NPJ Schizophr. 2021, 7, 35. [Google Scholar] [CrossRef]
- Jin, M.; Zhu, X.; Sun, Y.; Li, Z.; Li, X.; Ai, L.; He, Y.; Liu, Y.; Jia, N.; Hu, G.; et al. Identification of Peripheral Blood MiRNA Biomarkers in First-Episode Drug-Free Schizophrenia Patients Using Bioinformatics Strategy. Mol. Neurobiol. 2022, 59, 4730–4746. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, A.; Yadav, S. MiR-34 and MiR-200: Regulator of Cell Fate Plasticity and Neural Development. Neuromolecular. Med. 2019, 21, 97–109. [Google Scholar] [CrossRef]
- Jauhari, A.; Singh, T.; Singh, P.; Parmar, D.; Yadav, S. Regulation of MiR-34 Family in Neuronal Development. Mol. Neurobiol. 2018, 55, 936–945. [Google Scholar] [CrossRef]
- Kim, A.H.; Reimers, M.; Maher, B.; Williamson, V.; McMichael, O.; McClay, J.L.; van den Oord, E.J.C.G.; Riley, B.P.; Kendler, K.S.; Vladimirov, V.I. MicroRNA Expression Profiling in the Prefrontal Cortex of Individuals Affected with Schizophrenia and Bipolar Disorders. Schizophr. Res. 2010, 124, 183–191. [Google Scholar] [CrossRef]
- Bavamian, S.; Mellios, N.; Lalonde, J.; Fass, D.M.; Wang, J.; Sheridan, S.D.; Madison, J.M.; Zhou, F.; Rueckert, E.H.; Barker, D.; et al. Dysregulation of MiR-34a Links Neuronal Development to Genetic Risk Factors for Bipolar Disorder. Mol. Psychiatry 2015, 20, 573–584. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Günes, S.; Coskun, S.; Findikli, E. Peripheral Signatures of Psychiatric Disorders: MicroRNAs. Clin. Psychopharmacol. Neurosci. 2017, 15, 313. [Google Scholar] [CrossRef]
- Arora, A.; McKay, G.J.; Simpson, D.A.C. Prediction and Verification of MiRNA Expression in Human and Rat Retinas. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3962–3967. [Google Scholar] [CrossRef]
- Beveridge, N.J.; Tooney, P.A.; Carroll, A.P.; Gardiner, E.; Bowden, N.; Scott, R.J.; Tran, N.; Dedova, I.; Cairns, M.J. Dysregulation of MiRNA 181b in the Temporal Cortex in Schizophrenia. Hum. Mol. Genet. 2008, 17, 1156–1168. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, W.; Xue, Y.; Xu, H.; Li, J.; Zhao, Z.; Sun, Y.; Wang, Y.; He, J.; Huang, Y.; et al. Roles of MiR-432 and Circ_0000418 in Mediating the Anti-Depressant Action of ADAR1. Neurobiol. Stress 2021, 15, 100396. [Google Scholar] [CrossRef] [PubMed]
- Honorato-Mauer, J.; Xavier, G.; Ota, V.K.; Chehimi, S.N.; Mafra, F.; Cuóco, C.; Ito, L.T.; Ormond, R.; Asprino, P.F.; Oliveira, A.; et al. Alterations in MicroRNA of Extracellular Vesicles Associated with Major Depression, Attention-Deficit/Hyperactivity and Anxiety Disorders in Adolescents. Transl. Psychiatry 2023, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G. RNA and Oxidative Stress in Alzheimer’s Disease: Focus on MicroRNAs. Oxid. Med. Cell Longev. 2020, 2020, 2638130. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.O.; Jeffries, C.D.; Jarskog, L.F.; Thomson, J.M.; Woods, K.; Newman, M.A.; Parker, J.S.; Jin, J.; Hammond, S.M. MicroRNA Expression in the Prefrontal Cortex of Individuals with Schizophrenia and Schizoaffective Disorder. Genome Biol. 2007, 8, R27. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.Z.; Puppala, S.; Cruz, D.; Neary, J.L.; Kumar, A.; Dalan, E.; Li, C.; Nathanielsz, P.; Carless, M.A. Blood-Based MiRNA Biomarkers as Correlates of Brain-Based MiRNA Expression. Front. Mol. Neurosci. 2022, 15, 817290. [Google Scholar] [CrossRef]
- Wu, K.; Li, L.; Li, S. Circulating MicroRNA-21 as a Biomarker for the Detection of Various Carcinomas: An Updated Meta-Analysis Based on 36 Studies. Tumor Biol. 2015, 36, 1973–1981. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 5. [Google Scholar] [CrossRef]
- Jenike, A.E.; Halushka, M.K. MiR-21: A Non-specific Biomarker of All Maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Beveridge, N.J.; Gardiner, E.; Carroll, A.P.; Tooney, P.A.; Cairns, M.J. Schizophrenia Is Associated with an Increase in Cortical MicroRNA Biogenesis. Mol. Psychiatry 2009, 15, 1176–1189. [Google Scholar] [CrossRef]
- Mellios, N.; Huang, H.S.; Baker, S.P.; Galdzicka, M.; Ginns, E.; Akbarian, S. Molecular Determinants of Dysregulated GABAergic Gene Expression in the Prefrontal Cortex of Subjects with Schizophrenia. Biol. Psychiatry 2009, 65, 1006–1014. [Google Scholar] [CrossRef]
- Mellios, N.; Huang, H.S.; Grigorenko, A.; Rogaev, E.; Akbarian, S. A Set of Differentially Expressed MiRNAs, Including MiR-30a-5p, Act as Post-Transcriptional Inhibitors of BDNF in Prefrontal Cortex. Hum. Mol. Genet. 2008, 17, 3030–3042. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, Y.; Guo, M.; Guo, M.; Yue, D.; Yue, D.; Chen, C.; Chen, C.; Liang, G.; Liang, G.; et al. MicroRNA-7: Expression and Function in Brain Physiological and Pathological Processes. Cell Biosci. 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.M.; Gururajan, A.; Dinan, T.G.; Kenny, P.J.; Cryan, J.F. All Roads Lead to the MiRNome: MiRNAs Have a Central Role in the Molecular Pathophysiology of Psychiatric Disorders. Trends Pharmacol. Sci. 2016, 37, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759. [Google Scholar] [CrossRef]
| Schizophrenia Spectrum and Other Psychotic Disorders |
|---|
| Schizotypal personality disorder |
| Delusional disorder |
| Brief psychotic disorder |
| Schizophreniform disorder |
| Schizophrenia |
| Schizoaffective disorder |
Psychotic disorders induced by another condition:
|
| Catatonia |
| Other specified schizophrenia spectrum and other psychotic disorder |
| Unspecified schizophrenia spectrum and other psychotic disorder |
| No. | Author (Year) | Diagnosis | Tissue | Method | Intervention | miRNAs Identified | Number of Patients |
|---|---|---|---|---|---|---|---|
| 1 | Gardiner et al. (2011) [43] | Schizophrenia | PBMCs | Microarray followed by qRT-PCR | All patients previously received antipsychotic treatment | mir-31 ↓ mir-431 ↓ mir-433 ↓ mir-107 ↓ mir-134 ↓ mir-99b ↓ mir-487b ↓ | 112 SCZ; 76 controls |
| 2 | Lai et al. (2011) [44] | Schizophrenia | PBMCs | qRT-PCR | All patients previously received antipsychotic treatment | miR-34a ↑ miR-449a ↑ miR-564 ↑ miR-548d ↑ miR-572 ↑ miR-652 ↑ miR-432 ↓ | 90 SCZ; 60 controls |
| 3 | Shi et al. (2012) [45] | Schizophrenia | Serum | qRT-PCR | All patients previously received antipsychotic treatment | miR-181b ↑ miR-219-2-3p ↑ miR-346 ↑ miR-1308 ↑ miR-92a ↑ miR-195 ↓ miR-17 ↓ | 115 SCZ; 40 controls |
| 4 | Song et al. (2014) [46] | Schizophrenia | Plasma | qRT-PCR | None, or at least 3 months with no psychotropic medication. | miRNA-181b ↑ miRNA-30e ↑ miRNA-34a ↑ miRNA-7 ↑ | 20 SCZ; 20 controls |
| 5 | Fan et al. (2015) [47] | Schizophrenia | PBMCs | Microarray followed by qRT-PCR | None, or at least 3 months with no psychotropic medication. | miR-1273d ↑ miR-1303 ↑ miR-21 ↑ miR-3064-5p ↑ miR-3131 ↑ miR-3687 ↑ miR-4428 ↑ miR-4725-3p ↑ miR-5096 ↑ | 55 SCZ; 28 controls |
| 6 | Yu et al. (2015) [48] | Schizophrenia | PBMCs | Microarray followed by qRT-PCR | 105 treatment-naive patients | miR-132 ↓ miR-134 ↓ miR-1271 ↓ miR-664 ↓ miR-200c ↓ miR-432 ↓ | 105 SCZ; 130 control |
| 7 | Sun et al. (2015a) [49] | Schizophrenia | Plasma and PBMCs | qRT-PCR | No antipsychotic treatment, or at least 3 months with no psychotropic medication. | miR-132 ↑ plasma miR-195 ↑ plasma miR-30e ↑ plasma miR-7 ↑ plasma miR-212 ↑ PBMC miR-34a ↑ PBMC miR-30e ↑ PBMC | 25 SCZ; 13 control |
| 8 | Sun et al. (2015b) [50] | Schizophrenia | Plasma | qRT-PCR | No antipsychotic treatment, or at least 3 months with no psychotropic medication. | miR-30e ↑ miR-181b ↑ miR-34a ↑ miR-346 ↑ miR-7 ↑ | 61 SCZ; 62 control |
| 9 | Wei et al. (2015) [51] | Schizophrenia | Plasma | RNA Sequencing followed by qRT-PCR | 164 drug-naive patients; 400 patients with previous antipsychotic treatment | miR-130b ↑ miR-193a-3p ↑ | 564 SCZ; 400 control |
| 10 | Lai et al. (2016) [52] | Schizophrenia (acute state) | PBMCs | qRT-PCR | 4 drug-naive patients; 44 patients with previous antipsychotic treatment | miR-34a ↑ miR-564 ↑ miR-548d ↑ miR-449a ↑ | 48 SCZ; 37 control |
| 11 | Camkurt et al. (2016) [53] | Schizophrenia (Active psychotic episode) | Whole blood | qRT-PCR | 3 drug-naive patients; 13 patients with previous antipsychotic treatment | miR-9-5p ↑ miR-29a-3p ↑ miR-106b-5p ↑ miR-125a-3p ↑ miR-125b-3p ↑ | 16 SCZ; 16 control |
| 12 | Chen et al. (2016) [54] | Schizophrenia | PBMCs | Microarray followed by qRT-PCR | None, or at least 3 months with no psychotropic medication. | miR-1273d ↑ miR-1303 ↑ miR-21 ↑ miR-3064-5p ↑ miR-3131 ↑ miR-3687 ↑ miR-4428 ↑ miR-4725-3p ↑ miR-5096 ↑ | 82 SCZ; 43 controls |
| 13 | Liu et al. (2017) [55] | Schizophrenia | PBMCs | qRT-PCR | None, or at least 3 months with no psychotropic medication. | miR-181b-5p ↑ miR-21-5p ↑ miR-195-5p ↑ miR-137 ↑ miR-34a-5p ↑ miR-346 ↓ | 20 first-episode schizophrenia 19 schizophrenia 50 controls |
| 14 | Ma et al. (2018) [56] | First-onset schizophrenia | Whole blood | RNA sequencing followed by qRT-PCR | All patients were drug-naive. | miR-22-3p ↑ miR148b-5p ↑ miR-181a-5p ↑ miR-181b-5p ↑ miR-199b-5p ↑ miR-92a-3p ↑ | 10 first-onset SCZ; 10 control (RNA sequencing) and 44 SCZ; 44 controls (qRT-PCR) |
| 15 | He et al. (2019) [57] | Schizophrenia | Serum | qRT-PCR | All patients had previously received antipsychotic treatment | miR-34a-5p ↑ miR-449a ↑ miR-432-5p ↓ | 40 SCZ; 40 control |
| 16 | Wang et al. (2019) [58] | Schizophrenia | Serum | Microarray followed by qRT-PCR | 59 treatment-naive patients 3 clinically cured patients | miR-320a-3p ↓ miR-320b ↓ | 3 treatment-naive SCZ, 3 clinically cured SCZ and 3 control (Microarray analysis); 59 SCZ and 60 control (qRT-PCR validation) |
| 17 | Zhao et al. (2019) [59] | First-episode psychosis and Schizophrenia | Plasma | Microarray followed by qRT-PCR | 17 FEP patients with no history of antipsychotic treatment for longer than 16 weeks 21 SCZ patients with previous antipsychotic treatment | miR-223-3p ↑ FEP, SCZ miR-6131 ↑FEP | 17 FEP 17 control and 21 SCZ; 21 control |
| 18 | Du et al. (2019) [60] | Schizophrenia | Serum-derived exosomes | RNA sequencing followed by qRT-PCR | 106 drug-free patients 43 patients with previous antipsychotic treatment | miR-206 ↑ miR-619 ↑ miR-144-3p ↓ | 49 drug-free first-episode SCZ; 46 controls (RNA sequencing) 100 SCZ (57 first-episode, drug-free patients and 43 chronically treated patients); 100 controls (qRT-PCR) |
| 19 | Horai et al. (2020) [61] | Schizophrenia | Whole blood | qRT-PCR | All patients were under chronic antipsychotic treatment | miR-19b ↑ | 22 SCZ; 19 control |
| 20 | Gou et al. (2021) [62] | First-episode schizophrenia | Whole blood | qRT-PCR | 10 drug-naive patients; 113 patients had previously received antipsychotic medication | miR-181b-5p ↑ | 123 first-episode SCZ; 50 controls |
| 21 | Chen et al. (2021) [63] | Schizophrenia Bipolar disorder | Plasma | qRT-PCR | Medically stabilized | miR-137 ↑ SCZ, relatives miR-34b ↑ SCZ, relatives miR-34c ↑ SCZ, relatives | 215 SCZ; 72 unaffected first-degree relatives of SCZ patients 31 BD; 100 controls |
| 22 | Jin et al. (2022) [64] | First-episode Schizophrenia | Whole blood | RNA sequencing followed by qRT-PCR | None | miR-9-5p ↓ miR-4467 ↑ | 35 FES; 60 control |
| No. | miRNAs | Study (Author, Year)/Tissue | Expression |
| 1. | miR-34a | Lai et al. (2011)/PBMC [44] Song et al. (2014)/Plasma [46] Sun et al. (2015a)/PBMC [49] Sun et al. (2015b)/Plasma [50] Lai et al. (2016)/PBMC [52] Liu et al. (2017)/PBMC [55] He et al. (2019)/Serum [57] | ↑ 7 studies (4 PBMC; 2 Plasma; 1 Serum) |
| 2. | miR-181b | Shi et al. (2012)/Serum [45] Song et al. (2014)/Plasma [46] Sun et al. (2015b)/Plasma [50] Liu et al. (2017)/PBMC [55] Ma et al. (2018)/Whole blood [56] Gou et al. (2021)/Whole blood [62] | ↑ 6 studies (1 PBMC; 2 Plasma; 1 Serum; 2 Whole Blood) |
| 3. | miR-432 | Lai et al. (2011)/PBMC [44] Yu et al. (2015)/PBMC [48] He et al. (2019)/Serum [57] | ↓ 3 studies (2 PBMC; 1 Serum) |
| 4. | miR-30e | Song et al. (2014)/Plasma [46] Sun et al. (2015a)/Plasma and PBMC [49] Sun et al. (2015b)/Plasma [50] | ↑ 3 studies (1 PBMC; 3 Plasma) |
| 5. | miR-21 | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] Liu et al. (2017)/PBMC [55] | ↑ 3 studies (3 PBMC) |
| 6. | miR-137 | Liu et al. (2017)/PBMC [55] Chen et al. (2021)/Plasma [63] | ↑ 2 studies (1 PBMC; 1 Plasma) |
| 7. | miR-134 | Gardiner et al. (2011)/PBMC [43] Yu et al. (2015)/PBMC [48] | ↓ 2 studies (2 PBMC) |
| 8. | miR-7 | Sun et al. (2015a)/Plasma [49] Sun et al. (2015b)/Plasma [50] | ↑ 2 studies (2 Plasma) |
| 9. | miR-92a | Shi et al. (2012)/Serum [45] Ma et al. (2018)/Whole Blood [56] | ↑ 2 studies (1 Serum; 1 Whole Blood) |
| 10. | miR-1273d | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 11. | miR-1303 | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 12. | miR-3064-5p | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 13. | miR-3131 | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 14. | miR-3687 | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 15. | miR-4428 | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 16. | miR-4725-3p | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 17. | miR-5096 | Fan et al. (2015)/PBMC [47] Chen et al. (2016)/PBMC [54] | ↑ 2 studies (2 PBMC) |
| 18. | miR-195 | Shi et al. (2012)/Serum [45] Sun et al. (2015a)/Plasma [49] Liu et al. (2017)/PBMC [55] | ↑ 2 studies (1 PBMC: 1 Plasma) ↓ 1 study (1 Serum) |
| 19. | miR-346 | Shi et al. (2012)/Serum [45] Sun et al. (2015b)/Plasma [50] Liu et al. (2017)/PBMC [55] | ↑ 2 studies (1 Plasma; 1 Serum) ↓ 1 study (1 PBMC) |
| 20. | miR-132 | Sun et al. (2015a)/Plasma [49] Yu et al. (2015)/PBMC [48] | ↑ 1 study (1 Plasma) ↓ 1 study (1 PBMC) |
| No. | Study (Author, Year) | miRNA/Tissue | Correlation on the PANSS Scale |
|---|---|---|---|
| 1. | Lai et al. (2011) [44] | miR-449/PBMCs | Positively correlated with negative symptoms |
| 2. | Song et al. (2014) [46] | miR-181b/Plasma | Positively correlated with the amelioration of negative symptoms |
| 3. | Chen et al. (2016) [54] | miR-21/PBMCs | Negatively correlated with the amelioration of positive symptoms, general psychopathology, and aggressiveness symptoms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, Ș.-A.; Dobre, M.; Milanesi, E.; Hinescu, M.E. Blood-Based MicroRNAs in Psychotic Disorders—A Systematic Review. Biomedicines 2023, 11, 2536. https://doi.org/10.3390/biomedicines11092536
Grosu Ș-A, Dobre M, Milanesi E, Hinescu ME. Blood-Based MicroRNAs in Psychotic Disorders—A Systematic Review. Biomedicines. 2023; 11(9):2536. https://doi.org/10.3390/biomedicines11092536
Chicago/Turabian StyleGrosu, Ștefania-Alexandra, Maria Dobre, Elena Milanesi, and Mihail Eugen Hinescu. 2023. "Blood-Based MicroRNAs in Psychotic Disorders—A Systematic Review" Biomedicines 11, no. 9: 2536. https://doi.org/10.3390/biomedicines11092536
APA StyleGrosu, Ș.-A., Dobre, M., Milanesi, E., & Hinescu, M. E. (2023). Blood-Based MicroRNAs in Psychotic Disorders—A Systematic Review. Biomedicines, 11(9), 2536. https://doi.org/10.3390/biomedicines11092536






