High-Dose Vitamin B6 (Pyridoxine) Displays Strong Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Monocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.1.1. Culture of U937 Cells
2.1.2. Preparation of Reagents and Treatment of Cells
2.2. Cell Surface Marker Expression by Flow Cytometry
2.3. Bio-Plex Cytokine Assay
2.4. RT2 Profiler PCR Array for Human Innate and Adaptive Immune Responses
2.5. RNA Extraction from Cells
2.6. Assessing Change in Gene Expression
2.7. Analysis of Data
2.8. Statistical Analysis
3. Results
3.1. Vitamin B6 Changes Cell Surface Marker Expression in LPS-Stimulated Monocytes
3.2. Vitamin B6 Decreases Secretion of IL-1β, IL-6, IL-10, and TNF-α in LPS-Stimulated Monocytes
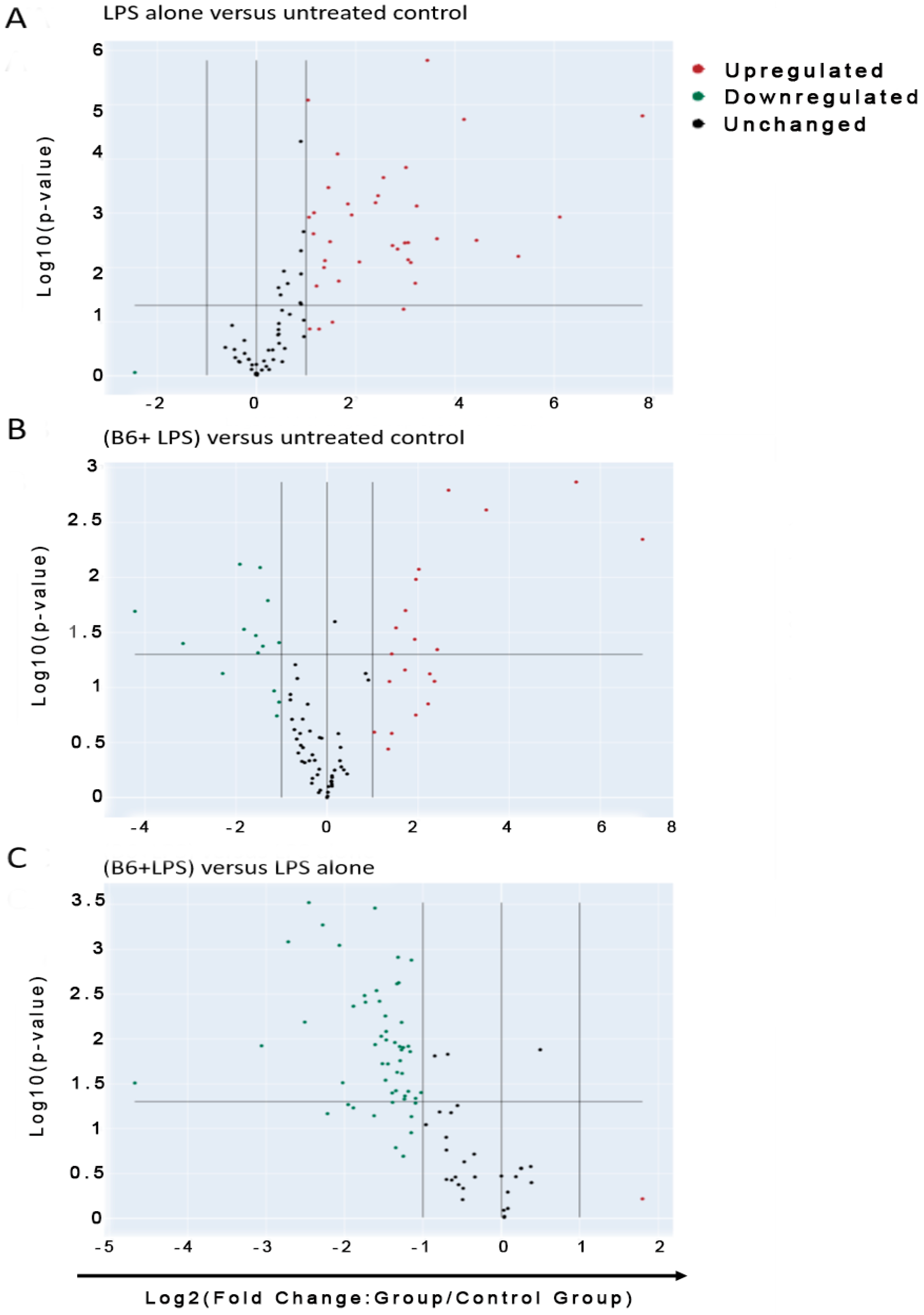
3.3. Vitamin B6 Decreases Inflammatory Gene Expression in LPS-Stimulated Monocyte Cells
3.4. Vitamin B6 Downregulates the Expression of Cytokine Genes
3.5. Vitamin B6 Downregulates Expression of Inflammatory and Defence Response Genes
3.6. Vitamin B6 Downregulates the Expression of other Genes in the Innate Immune System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Tangalakis, K.; Bosevski, M.; Apostolopoulos, V. Cognitive decline: A vitamin B perspective. Maturitas 2016, 93, 108–113. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Stojanovska, L.; Apostolopoulos, V. Be well: A potential role for vitamin B in COVID-19. Maturitas 2021, 144, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, O.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Waly, M.I.; Ali, A.; Al-Nassri, A.; Al-Mukhaini, M.; Valliatte, J.; Al-Farsi, Y. Low nourishment of B-vitamins is associated with hyperhomocysteinemia and oxidative stress in newly diagnosed cardiac patients. Exp. Biol. Med. 2015, 241, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, I.; Herrmann, W.; Kleber, M.E.; Scharnagl, H.; Hoffmann, M.M.; Winklhofer-Roob, B.M.; März, W.; Herrmann, M. Subclinical inflammation, telomere shortening, homocysteine, vitamin B6, and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. J. Nutr. 2020, 59, 1399–1411. [Google Scholar] [CrossRef]
- Lotto, V.; Choi, S.-W.; Friso, S. Vitamin B6: A challenging link between nutrition and inflammation in CVD. Br. J. Nutr. 2011, 106, 183–195. [Google Scholar] [CrossRef]
- Kumrungsee, T.; Nirmagustina, D.E.; Arima, T.; Onishi, K.; Sato, K.; Kato, N.; Yanaka, N. Novel metabolic disturbances in marginal vitamin B6-deficient rat heart. J. Nutr. Biochem. 2019, 65, 26–34. [Google Scholar] [CrossRef]
- Ji, D.; Luo, C.; Liu, J.; Cao, Y.; Wu, J.; Yan, W.; Xue, K.; Chai, J.; Zhu, X.; Wu, Y.; et al. Insufficient S-Sulfhydration of Methylenetetrahydrofolate Reductase Contributes to the Progress of Hyperhomocysteinemia. Antioxid. Redox Signal. 2022, 36, 1–14. [Google Scholar] [CrossRef]
- Sande, J.S.; Ulvik, A.; Midttun, O.; Ueland, P.M.; Hammer, H.B.; Valen, M.; Apalset, E.M.; Gjesdal, C.G. Vitamin B-6 Status Correlates with Disease Activity in Rheumatoid Arthritis Patients During Treatment with TNFα Inhibitors. J. Nutr. 2019, 149, 770–775. [Google Scholar] [CrossRef]
- Sakakeeny, L.; Roubenoff, R.; Obin, M.; Fontes, J.D.; Benjamin, E.J.; Bujanover, Y.; Jacques, P.F.; Selhub, J. Plasma Pyridoxal-5-Phosphate Is Inversely Associated with Systemic Markers of Inflammation in a Population of U.S. Adults. J. Nutr. 2012, 142, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Ross, V.; Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Saibeni, S.; Cattaneo, M.; Vecchi, M.; Zighetti, M.L.; Lecchi, A.; Lombardi, R.; Meucci, G.; Spina, L.; de Franchis, R. Low vitamin B6 plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: Role of inflammation and correlation with acute phase reactants. Am. J. Gastroenterol. 2003, 98, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Byun, A.; Liu, Z.; Mason, J.B.; Bronson, R.T.; Crott, J.W. Dietary vitamin B6 intake modulates colonic inflammation in the IL10−/− model of inflammatory bowel disease. J. Nutr. Biochem. 2013, 24, 2138–2143. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Rogler, G. Intestinal Absorption and Vitamin Levels: Is a New Focus Needed? Dig. Dis. 2012, 30, 73–80. [Google Scholar] [CrossRef]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Oxenkrug, G.; Ratner, R.; Summergrad, P. Kynurenines and Vitamin B6: Link Between Diabetes And Depression. J. Bioinform. Diabetes 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol. Neurobiol. 2015, 52, 805–810. [Google Scholar] [CrossRef]
- Merigliano, C.; Mascolo, E.; Burla, R.; Saggio, I.; Vernì, F. The Relationship Between Vitamin B6, Diabetes and Cancer. Front. Genet. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Merigliano, C.; Mascolo, E.; La Torre, M.; Saggio, I.; Vernì, F. Protective role of vitamin B6 (PLP) against DNA damage in Drosophila models of type 2 diabetes. Sci. Rep. 2018, 8, 11432. [Google Scholar] [CrossRef] [PubMed]
- Fields, A.M.; Welle, K.; Ho, E.S.; Mesaros, C.; Susiarjo, M. Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice. Commun. Biol. 2021, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Zirwes, R.; Bangert, V.; Kaiser, R.P.; Schilling, M.; Hostalek, U.; Obeid, R. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 2015, 107, 157–165. [Google Scholar] [CrossRef]
- Marzio, A.; Merigliano, C.; Gatti, M.; Vernì, F. Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells. PLoS Genet. 2014, 10, e1004199. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kessoku, T.; Ozaki, A.; Iwaki, M.; Honda, Y.; Ogawa, Y.; Imajo, K.; Yoneda, M.; Saito, S.; Nakajima, A. Vitamin B6 efficacy in the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, single-center trial. J. Clin. Biochem. Nutr. 2021, 68, 181–186. [Google Scholar] [CrossRef]
- Abe, R.A.M.; Masroor, A.; Khorochkov, A.; Prieto, J.; Singh, K.B.; Nnadozie, M.C.; Abdal, M.; Shrestha, N.; Mohammed, L. The Role of Vitamins in Non-Alcoholic Fatty Liver Disease: A Systematic Review. Cureus 2021, 13, e16855. [Google Scholar] [CrossRef]
- Bird, R.P. The Emerging Role of Vitamin B6 in Inflammation and Carcinogenesis. Adv. Food Nutr. Res. 2018, 83, 151–194. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. J. Natl. Cancer Inst. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Singh, M.; Behera, J.; Theilen, N.T.; George, A.K.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Hydrogen sulfide alleviates hyperhomocysteinemia-mediated skeletal muscle atrophy via mitigation of oxidative and endoplasmic reticulum stress injury. Am. J. Physiol. Physiol. 2018, 315, C609–C622. [Google Scholar] [CrossRef] [PubMed]
- El Oudi, M.; Aouni, Z.; Mazigh, C.; Khochkar, R.; Gazoueni, E.; Haouela, H.; Machghoul, S. Homocysteine and markers of inflammation in acute coronary syndrome. Exp. Clin. Cardiol. 2010, 15, e25–e28. [Google Scholar]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Du, X.; Yang, Y.; Zhan, X.; Huang, Y.; Fu, Y.; Zhang, Z.; Liu, H.; Zhang, L.; Li, Y.; Wen, Q.; et al. Vitamin B6 prevents excessive inflammation by reducing accumulation of sphingosine-1-phosphate in a sphingosine-1-phosphate lyase–dependent manner. J. Cell. Mol. Med. 2020, 24, 13129–13138. [Google Scholar] [CrossRef]
- Chanput, W.; Peters, V.; Wichers, H. THP-1 and U937 Cells. In The Impact of Food Bioactives on Gut Health; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int. J. Nanomed. 2018, 13, 63–76. [Google Scholar] [CrossRef]
- Fu, W.J.; Hu, J.; Spencer, T.; Carroll, R.; Wu, G. Statistical models in assessing fold change of gene expression in real-time RT-PCR experiments. Comput. Biol. Chem. 2006, 30, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Suzuki, Y.; Yoshimura, K.; Yasui, H.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Macrophage Mannose Receptor CD206 Predicts Prognosis in Community-acquired Pneumonia. Sci. Rep. 2019, 9, 1875. [Google Scholar] [CrossRef]
- McGreal, E.P.; Miller, J.L.; Gordon, S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr. Opin. Immunol. 2005, 17, 18–24. [Google Scholar] [CrossRef]
- Ness, T.L.; Carpenter, K.J.; Ewing, J.L.; Gerard, C.J.; Hogaboam, C.M.; Kunkel, S.L. CCR1 and CC Chemokine Ligand 5 Interactions Exacerbate Innate Immune Responses during Sepsis. J. Immunol. 2004, 173, 6938–6948. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, E.N. Cytokines. In Encyclopedia of the Neurological Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 921–925. [Google Scholar]
- Rex, D.; Agarwal, N.; Prasad, T.S.K.; Kandasamy, R.K.; Subbannayya, Y.; Pinto, S.M. A comprehensive pathway map of IL-18-mediated signalling. J. Cell Commun. Signal. 2019, 14, 257–266. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Merriwether, E.N.; Agalave, N.M.; Dailey, D.L.; Rakel, B.A.; Kolker, S.J.; Lenert, M.E.; Spagnola, W.H.; Lu, Y.; Geasland, K.M.; Allen, L.-A.H.; et al. IL-5 mediates monocyte phenotype and pain outcomes in fibromyalgia. Pain 2021, 162, 1468–1482. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. GM-CSF-Dependent Inflammatory Pathways. Front. Immunol. 2019, 10, 2055. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Williams, L.M.; Ricchetti, G.; Sarma, U.; Smallie, T.; Foxwell, B.M.J. Interleukin-10 suppression of myeloid cell activation—A continuing puzzle. Immunology 2004, 113, 281–292. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Kuo, P.T.; Zeng, Z.; Salim, N.; Mattarollo, S.; Wells, J.W.; Leggatt, G.R. The Role of CXCR3 and Its Chemokine Ligands in Skin Disease and Cancer. Front. Med. 2018, 5, 271. [Google Scholar] [CrossRef]
- Kathleen Brennan, J.Z. xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Drew, J.E. Cellular Defense System Gene Expression Profiling of Human Whole Blood: Opportunities to Predict Health Benefits in Response to Diet. Adv. Nutr. Int. Rev. J. 2012, 3, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U.; Williams, B.R.G.; Hannigan, G.E. Transcriptional Activation of Inflammatory Genes: Mechanistic Insight into Selectivity and Diversity. Biomolecules 2015, 5, 3087–3111. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.-C.; Pietersz, G.A.; Tang, C.K.; Ramsland, P.A.; Apostolopoulos, V. Reactive Oxygen Species Level Defines Two Functionally Distinctive Stages of Inflammatory Dendritic Cell Development from Mouse Bone Marrow. J. Immunol. 2010, 184, 2863–2872. [Google Scholar] [CrossRef]
- Sheng, K.C.; Wright, M.D.; Apostolopoulos, V. Inflammatory Mediators Hold the Key to Dendritic Cell Suppression and Tumor Progression. Curr. Med. Chem. 2011, 18, 5507–5518. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, B.; Zhu, W.; Zhang, R.; Li, L.; Hao, X.; Chen, S.; Hou, F. Ube2D3 and Ube2N are essential for RIG-I-mediated MAVS aggregation in antiviral innate immunity. Nat. Commun. 2017, 8, 15138. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 2009, 21, 185–193. [Google Scholar] [CrossRef]
- Campbell, G.R.; To, R.K.; Hanna, J.; Spector, S.A. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience 2021, 24, 102295. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain, Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Magro, G. SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X 2020, 2, 100029. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Edelmayer, R.; Wetter, J.; Salte, K.; Gauvin, D.; Leys, L.; Paulsboe, S.; Su, Z.; Weinberg, I.; Namovic, M.; et al. Monocytes/Macrophages play a pathogenic role in IL-23 mediated psoriasis-like skin inflammation. Sci. Rep. 2019, 9, 5310. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Bergamo, A.; Gerdol, M.; Pallavicini, A.; Greco, S.; Schepens, I.; Hamelin, R.; Armand, F.; Dyson, P.J.; Sava, G. Lysozyme-Induced Transcriptional Regulation of TNF-α Pathway Genes in Cells of the Monocyte Lineage. Int. J. Mol. Sci. 2019, 20, 5502. [Google Scholar] [CrossRef]
- Yanai, H.; Chiba, S.; Hangai, S.; Kometani, K.; Inoue, A.; Kimura, Y.; Abe, T.; Kiyonari, H.; Nishio, J.; Taguchi-Atarashi, N.; et al. Revisiting the role of IRF3 in inflammation and immunity by conditional and specifically targeted gene ablation in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 5253–5258. [Google Scholar] [CrossRef] [PubMed]
- Bajic, G.; Yatime, L.; Klos, A.; Andersen, G.R. Human C3a and C3a desArg anaphylatoxins have conserved structures, in contrast to C5a and C5a desArg. Protein Sci. 2013, 22, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tausk, F.; Gigli, I. The Human C3b Receptor: Function and Role in Human Diseases. J. Investig. Dermatol. 1990, 94, s141–s145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef]
- Leavy, O. Immune regulation: Macrophages join the FOXP3 suppressor gang. Nat. Rev. Immunol. 2011, 11, 438. [Google Scholar] [CrossRef]
- Gao, H.; A Ward, P. STAT3 and suppressor of cytokine signaling 3: Potential targets in lung inflammatory responses. Expert Opin. Ther. Targets 2007, 11, 869–880. [Google Scholar] [CrossRef]
- Andersen, M.N.; Etzerodt, A.; Graversen, J.H.; Holthof, L.C.; Moestrup, S.K.; Hokland, M.; Møller, H.J. STAT3 inhibition specifically in human monocytes and macrophages by CD163-targeted corosolic acid-containing liposomes. Cancer Immunol. Immunother. 2019, 68, 489–502. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef]
- Owen, G.R.; Le, D.; Stoychev, S.; Cerutti, N.M.; Papathanasopoulos, M. Redox exchange of the disulfides of human two-domain CD4 regulates the conformational dynamics of each domain, providing insight into its mechanisms of control. Biochem. Biophys. Res. Commun. 2018, 497, 811–817. [Google Scholar] [CrossRef]
- Camilli, G.; Cassotta, A.; Battella, S.; Palmieri, G.; Santoni, A.; Paladini, F.; Fiorillo, M.T.; Sorrentino, R. Regulation and trafficking of the HLA-E molecules during monocyte-macrophage differentiation. J. Leukoc. Biol. 2015, 99, 121–130. [Google Scholar] [CrossRef]
- Guan, K.-L. The mitogen activated protein kinase signal transduction pathway: From the cell surface to the nucleus. Cell. Signal. 1994, 6, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J.H. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Oyenuga, A.O.; Couper, D.; Matsushita, K.; Boerwinkle, E.; Folsom, A.R. Association of monocyte myeloperoxidase with incident cardiovascular disease: The Atherosclerosis Risk in Communities Study. PLoS ONE 2018, 13, e0205310. [Google Scholar] [CrossRef] [PubMed]
- Best, K.T.; Lee, F.K.; Knapp, E.; Awad, H.A.; Loiselle, A.E. Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing. Sci. Rep. 2019, 9, 10926. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.E.K.; Hopkins, R.A.; Lim, C.K.; Jamuar, S.S.; Ong, C.; Thoon, K.C.; Koh, M.J.; Shin, E.M.; Lian, D.W.Q.; Weerasooriya, M.; et al. Dominant-negative NFKBIA mutation promotes IL-1β production causing hepatic disease with severe immunodeficiency. J. Clin. Investig. 2020, 130, 5817–5832. [Google Scholar] [CrossRef]
- Boisson-Dupuis, S.; Kong, X.-F.; Okada, S.; Cypowyj, S.; Puel, A.; Abel, L.; Casanova, J.-L. Inborn errors of human STAT1: Allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr. Opin. Immunol. 2012, 24, 364–378. [Google Scholar] [CrossRef]
- Ramana, C.V.; Chatterjee-Kishore, M.; Nguyen, H.; Stark, G.R. Complex roles of Stat1 in regulating gene expression. Oncogene 2000, 19, 2619–2627. [Google Scholar] [CrossRef]
- Montaser Shaheen, H.E.B. Hematology, 7th ed.; Hoffman, R.E.J.B., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yu, T.; Gan, S.; Zhu, Q.; Dai, D.; Li, N.; Wang, H.; Chen, X.; Hou, D.; Wang, Y.; Pan, Q.; et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019, 10, 4353. [Google Scholar] [CrossRef]
- Zanoni, I.; Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef]
- Nolan, A.; Kobayashi, H.; Naveed, B.; Kelly, A.; Hoshino, Y.; Hoshino, S.; Karulf, M.R.; Rom, W.N.; Weiden, M.D.; Gold, J.A. Differential Role for CD80 and CD86 in the Regulation of the Innate Immune Response in Murine Polymicrobial Sepsis. PLoS ONE 2009, 4, e6600. [Google Scholar] [CrossRef]
- Forde, E.A.; Dogan, R.-N.E.; Karpus, W.J. CCR4 contributes to the pathogenesis of experimental autoimmune encephalomyelitis by regulating inflammatory macrophage function. J. Neuroimmunol. 2011, 236, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Jinquan, T.; Quan, S.; Jacobi, H.H.; Jing, C.; Millner, A.; Jensen, B.; Madsen, H.O.; Ryder, L.P.; Svejgaard, A.; Malling, H.J.; et al. CXC chemokine receptor 3 expression on CD34(+) hematopoietic progenitors from human cord blood induced by granulocyte-macrophage colony-stimulating factor: Chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood 2000, 96, 1230–1238. [Google Scholar]
- Takashima, K.; Oshiumi, H.; Matsumoto, M.; Seya, T. TICAM-1 is dispensable in STING-mediated innate immune responses in myeloid immune cells. Biochem. Biophys. Res. Commun. 2018, 499, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Seya, T.; Shime, H.; Takaki, H.; Azuma, M.; Oshiumi, H.; Matsumoto, M. TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. OncoImmunology 2012, 1, 917–923. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tournier, C.; Hess, P.; Yang, D.D.; Xu, J.; Turner, T.K.; Nimnual, A.; Bar-Sagi, D.; Jones, S.N.; Flavell, R.A.; Davis, R.J. Requirement of JNK for Stress- Induced Activation of the Cytochrome c-Mediated Death Pathway. Science 2000, 288, 870–874. [Google Scholar] [CrossRef]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009, 8, 480–499. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Plevin, R.E.; Knoll, M.; McKay, M.; Arbabi, S.; Cuschieri, J. The Role of Lipopolysaccharide Structure in Monocyte Activation and Cytokine Secretion. Shock 2016, 45, 22–27. [Google Scholar] [CrossRef]
- Huang, S.-C.; Wei, J.C.-C.; Wu, D.J.; Huang, Y.-C. Vitamin B6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur. J. Clin. Nutr. 2010, 64, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tsuchiya, K.; Kinoshita, T.; Kushiyama, H.; Suidasari, S.; Hatakeyama, M.; Imura, H.; Kato, N.; Suda, T. Vitamin B6 Prevents IL-1β Protein Production by Inhibiting NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 24517–24527. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, N.; Koyama, T.A.; Komatsu, S.; Nakamura, E.; Kanda, M.; Kato, N. Vitamin B6 suppresses NF-kappaB activation in LPS-stimulated mouse macrophages. Int. J. Mol. Med. 2005, 16, 1071–1075. [Google Scholar] [PubMed]
- Yi, S.-J.; Wu, Y.; Li, L.-L.; Liang, Q.-K.; Xiao, Y. Compound amino acid combined with high-dose vitamin B6 attenuate traumatic coagulopathy via inhibiting inflammation by HMGB1/TLR4/NF-κB pathway. J. Inflamm. 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Y.; Fuchsberger, M.; Xiang, S.D.; Apostolopoulos, V.; Plebanski, M. Myeloid Derived Suppressor Cells and Their Role in Diseases. Curr. Med. Chem. 2013, 20, 1437–1444. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Wilson, K.; Apostolopoulos, V.; Plebanski, M. Dendritic Cells and Myeloid Derived Suppressor Cells Fully Responsive to Stimulation via Toll-Like Receptor 4 Are Rapidly Induced from Bone-Marrow Cells by Granulocyte-Macrophage Colony-Stimulating Factor. Vaccines 2020, 8, 522. [Google Scholar] [CrossRef]
- Kawai, T.; Sato, S.; Ishii, K.J.; Coban, C.; Hemmi, H.; Yamamoto, M.; Terai, K.; Matsuda, M.; Inoue, J.-I.; Uematsu, S.; et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004, 5, 1061–1068. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Franquesa, M.; Sarrias, M.-R.; Borràs, F.E. Low doses of LPS exacerbate the inflammatory response and trigger death on TLR3-primed human monocytes. Cell Death Dis. 2018, 9, 499. [Google Scholar] [CrossRef]
- Tan, B.; Wong, J.J.-M.; Sultana, R.; Koh, J.C.J.W.; Jit, M.; Mok, Y.H.; Lee, J.H. Global Case-Fatality Rates in Pediatric Severe Sepsis and Septic Shock: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 352–362. [Google Scholar] [CrossRef]
- Rudd, K.E.J.S.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R. Global, regional, and national sepsis incidence and mortality, 1990-2017. Anal. Glob. Burd. Dis. Study 2020, 395, 200–211. [Google Scholar]
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation Is a Necessary Evil. Front. Cell Dev. Biol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Morris, P.E.; Paz, H.L.; Russell, J.A.; Edens, T.R.; Bernard, G.R. Safety and efficacy of affinity-purified, anti–tumor necrosis factor-α, ovine fab for injection (CytoFab) in severe sepsis*. Crit. Care Med. 2006, 34, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 589095. [Google Scholar] [CrossRef] [PubMed]
- Wald, E.L.; Badke, C.M.; Hintz, L.K.; Spewak, M.; Sanchez-Pinto, L.N. Vitamin therapy in sepsis. Pediatr. Res. 2021, 91, 328–336. [Google Scholar] [CrossRef]
- Giustina, A.D.; Danielski, L.G.; Novochadlo, M.M.; Goldim, M.P.; Joaquim, L.; Metzker, K.L.; DE Carli, R.J.; Denicol, T.; Cidreira, T.; Vieira, T.; et al. Vitamin B6 reduces oxidative stress in lungs and liver in experimental sepsis. An. Acad. Bras. Cienc. 2019, 91, e20190434. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikkelsen, K.; Dargahi, N.; Fraser, S.; Apostolopoulos, V. High-Dose Vitamin B6 (Pyridoxine) Displays Strong Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Monocytes. Biomedicines 2023, 11, 2578. https://doi.org/10.3390/biomedicines11092578
Mikkelsen K, Dargahi N, Fraser S, Apostolopoulos V. High-Dose Vitamin B6 (Pyridoxine) Displays Strong Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Monocytes. Biomedicines. 2023; 11(9):2578. https://doi.org/10.3390/biomedicines11092578
Chicago/Turabian StyleMikkelsen, Kathleen, Narges Dargahi, Sarah Fraser, and Vasso Apostolopoulos. 2023. "High-Dose Vitamin B6 (Pyridoxine) Displays Strong Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Monocytes" Biomedicines 11, no. 9: 2578. https://doi.org/10.3390/biomedicines11092578
APA StyleMikkelsen, K., Dargahi, N., Fraser, S., & Apostolopoulos, V. (2023). High-Dose Vitamin B6 (Pyridoxine) Displays Strong Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Monocytes. Biomedicines, 11(9), 2578. https://doi.org/10.3390/biomedicines11092578





