Increasing Gene Editing Efficiency via CRISPR/Cas9- or Cas12a-Mediated Knock-In in Primary Human T Cells
Abstract
1. Introduction
2. T Cell Genome Editing for Therapeutic Purposes
2.1. T Cells against Tumors
2.2. T Cells against HIV
2.3. Comparison of Viral Transduction and CRISPR/Cas Technology for T Cell Genome Editing
3. Genome Editing with the CRISPR/Cas Technology
4. Approaches to Increase Knock-in Editing in Primary T Cells
4.1. Choice of CRISPR Components and Method of Their Delivery into Cells
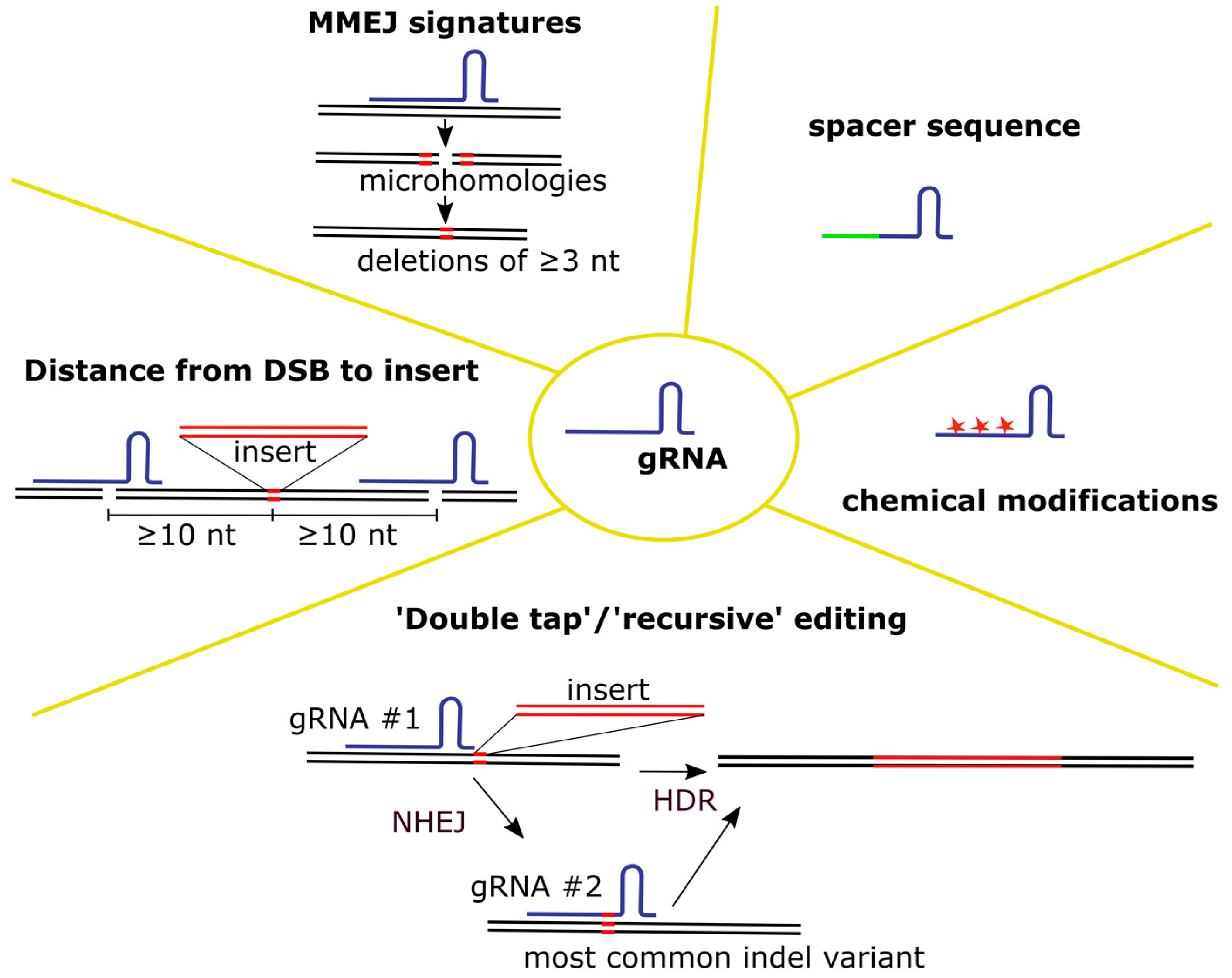
4.1.1. gRNA
- Selection of gRNA
- 2.
- Editing with multiple gRNAs
- 3.
- gRNA delivery and protection from degradation
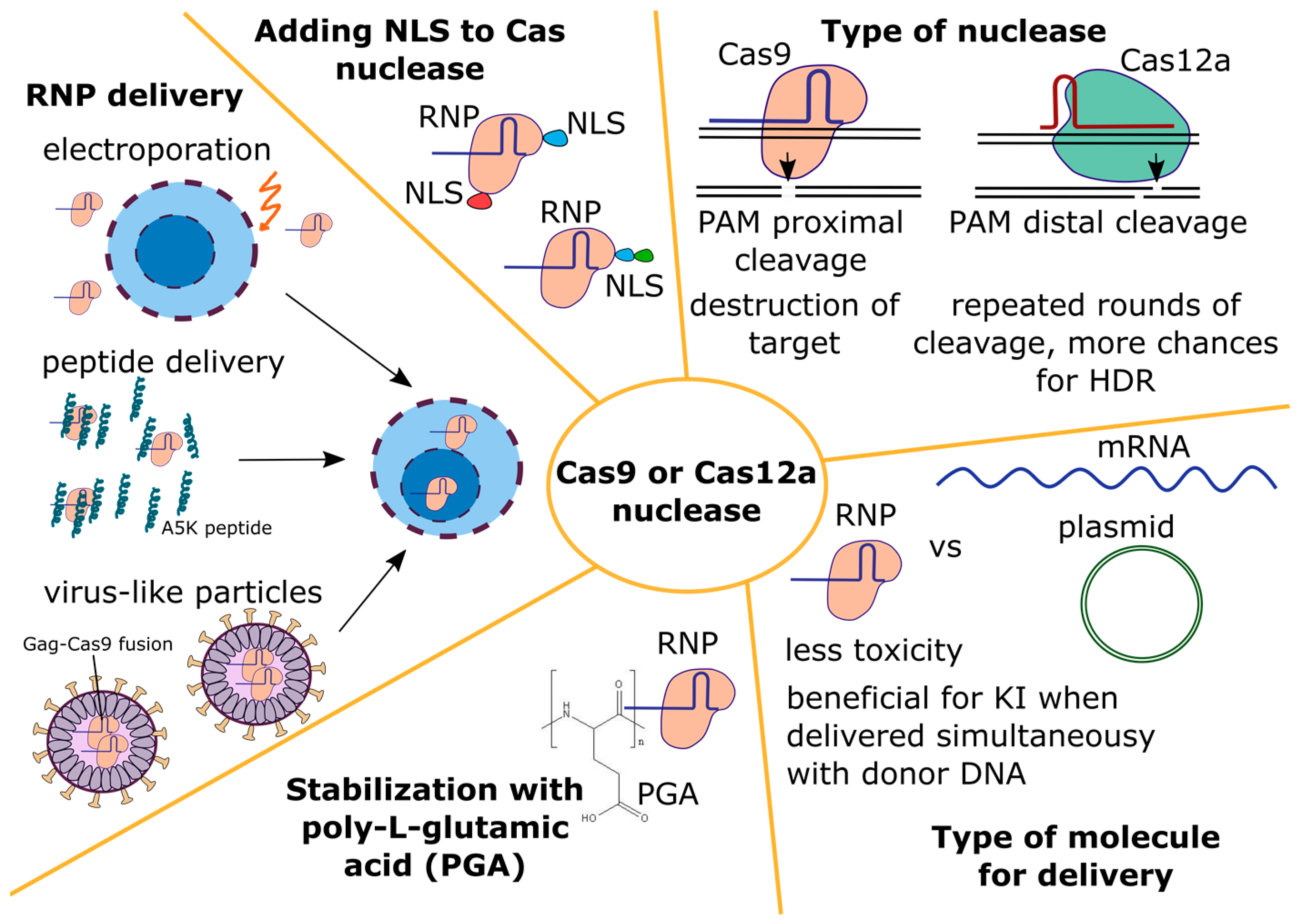
4.1.2. Cas Nuclease
- The type of nuclease
- 2.
- Type of molecule for delivery
- 3.
- Nuclease delivery into primary T cells
- 4.
- Influence of NLS signals in the Cas nuclease on editing
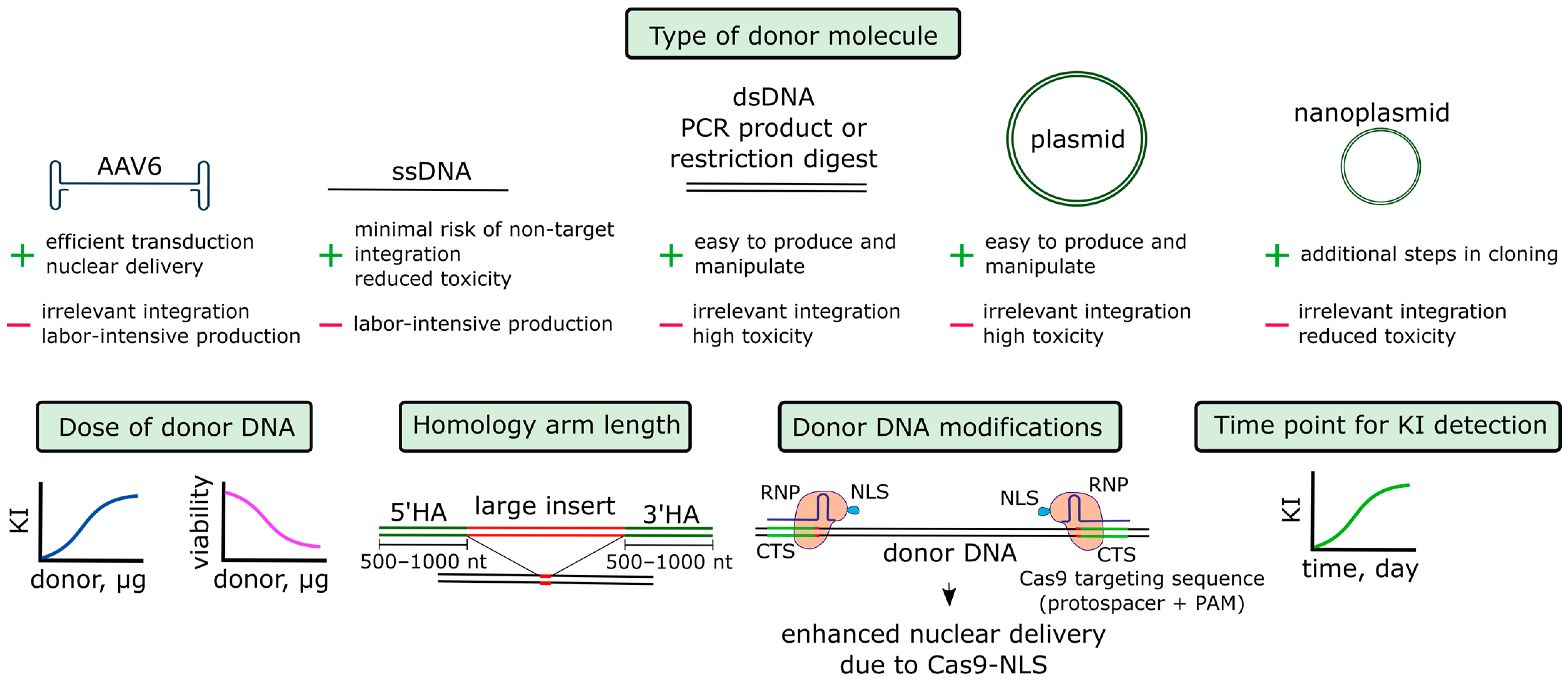
4.1.3. Donor DNA
- Donor DNA in the AAV6 vector
- 2.
- Donor DNA in the linear form
- 3.
- Additional parameters of donor DNA
- 4.
- Time point for knock-in detection
- 5.
- Binding of donor DNA to Cas9 or gRNA and targeted delivery of donor DNA into the nucleus
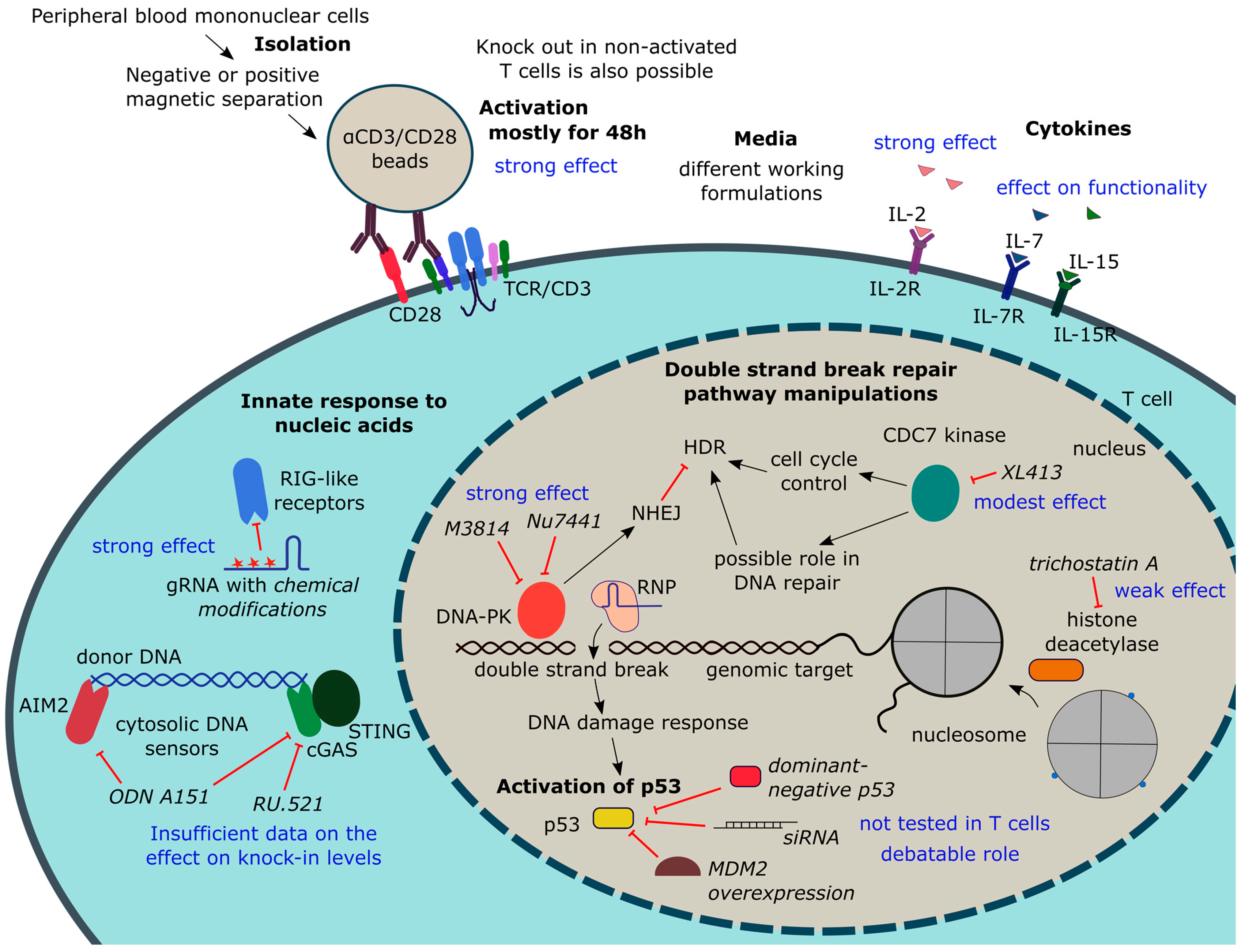
4.2. Manipulating T Cell State
4.2.1. T Cell Isolation and Activation
- The main culturing conditions affecting T cell editing efficiency
- 2.
- Knockout in non-activated T cells
- 3.
- Knock-in in non-activated T cells
4.2.2. Suppression of T Cell Response to Manipulation
- Suppression of р53 activation
- 2.
- Innate response to nucleic acids
4.2.3. Manipulating NHEJ and HDR Pathways or Cell Cycle
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Doudna, J.A. The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, T.S.; Baudrier, L.; Billon, P.; Ciccia, A. CRISPR-based genome editing through the lens of DNA repair. Mol. Cell 2022, 82, 348–388. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, K.; Tristán-Manzano, M.; Maldonado-Pérez, N.; Cortijo-Gutierrez, M.; Sánchez-Hernández, S.; Justicia-Lirio, P.; Carmona, M.D.; Herrera, C.; Martin, F.; Benabdellah, K. Using Gene Editing Approaches to Fine-Tune the Immune System. Front. Immunol. 2020, 11, 570672. [Google Scholar] [CrossRef] [PubMed]
- Cornu, T.I.; Mussolino, C.; Müller, M.C.; Wehr, C.; Kern, W.V.; Cathomen, T. HIV Gene Therapy: An Update. Hum. Gene Ther. 2021, 32, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.A.; Yao, W.; Ly, N.; Freitas, K.A.; Chen, Y.T.; Shi, Q.; Valencia, A.M.; Shifrut, E.; Kale, N.; Yost, K.E.; et al. Genome-wide CRISPR screens of T cell exhaustion identify chromatin remodeling factors that limit T cell persistence. Cancer Cell 2022, 40, 768–786.e7. [Google Scholar] [CrossRef]
- Roth, T.L.; Li, P.J.; Blaeschke, F.; Nies, J.F.; Apathy, R.; Mowery, C.; Yu, R.; Nguyen, M.L.T.; Lee, Y.; Truong, A.; et al. Pooled Knockin Targeting for Genome Engineering of Cellular Immunotherapies. Cell 2020, 181, 728–744.e21. [Google Scholar] [CrossRef]
- Dai, X.; Park, J.J.; Du, Y.; Na, Z.; Lam, S.Z.; Chow, R.D.; Renauer, P.A.; Gu, J.; Xin, S.; Chu, Z.; et al. Massively parallel knock-in engineering of human T cells. Nat. Biotechnol. 2023, 41, 1239–1255. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Feng, K.; Chen, M.; Zhang, Y.; Liu, Y.; Yang, Q.; Nie, J.; Tang, N.; Zhang, X.; et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell. Mol. Immunol. 2021, 18, 2188–2198. [Google Scholar] [CrossRef]
- Guo, Y.; Tong, C.; Su, L.; Zhang, W.; Jia, H.; Liu, Y.; Yang, Q.; Wu, Z.; Wang, Y.; Han, W. CRISPR/Cas9 genome-edited universal CAR T cells in patients with relapsed/refractory lymphoma. Blood Adv. 2022, 6, 2695–2699. [Google Scholar] [CrossRef] [PubMed]
- Foy, S.P.; Jacoby, K.; Bota, D.A.; Hunter, T.; Pan, Z.; Stawiski, E.; Ma, Y.; Lu, W.; Peng, S.; Wang, C.L.; et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature 2023, 615, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Y.; Yang, J.; Li, W.; Zhang, M.; Wang, Q.; Zhang, L.; Wei, G.; Tian, Y.; Zhao, K.; et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 2022, 609, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zu, C.; Zhang, M.; Wei, G.; Li, W.; Fu, S.; Hong, R.; Zhou, L.; Wu, W.; Cui, J.; et al. Safety and efficacy of CRISPR-based non-viral PD1 locus specifically integrated anti-CD19 CAR-T cells in patients with relapsed or refractory Non-Hodgkin’s lymphoma: A first-in-human phase I study. eClinicalMedicine 2023, 60, 102010. [Google Scholar] [CrossRef]
- Kotowski, M.; Sharma, S. CRISPR-Based Editing Techniques for Genetic Manipulation of Primary T Cells. Methods Protoc. 2020, 3, 79. [Google Scholar] [CrossRef]
- Rezalotfi, A.; Fritz, L.; Förster, R.; Bošnjak, B. Challenges of CRISPR-Based Gene Editing in Primary T Cells. Int. J. Mol. Sci. 2022, 23, 1689. [Google Scholar] [CrossRef]
- Bernard, B.E.; Landmann, E.; Jeker, L.T.; Schumann, K. CRISPR/Cas-based Human T cell Engineering: Basic Research and Clinical Application. Immunol. Lett. 2022, 245, 18–28. [Google Scholar] [CrossRef]
- Ghaffari, S.; Khalili, N.; Rezaei, N. CRISPR/Cas9 revitalizes adoptive T-cell therapy for cancer immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 269. [Google Scholar] [CrossRef]
- FDA. Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 7 December 2022).
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 17, 147–167. [Google Scholar] [CrossRef]
- Tokarew, N.; Ogonek, J.; Endres, S.; von Bergwelt-Baildon, M.; Kobold, S. Teaching an old dog new tricks: Next-generation CAR T cells. Br. J. Cancer 2018, 120, 26–37. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Balke-Want, H.; Keerthi, V.; Gkitsas, N.; Mancini, A.G.; Kurgan, G.L.; Fowler, C.; Xu, P.; Liu, X.; Asano, K.; Patel, S.; et al. Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing. Mol. Cancer 2023, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.; Lin, Q.; Ma, G.; Yin, H.; Chen, S.; Lin, Y. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed. Pharmacother. 2020, 121, 109625. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Cheng, C.; Zhang, X.; Qiao, M.; Li, N.; Mu, W.; Wei, X.F.; Han, W.; Wang, H. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight 2020, 5, e133977. [Google Scholar] [CrossRef] [PubMed]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; Van Der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef]
- Roth, T.L.; Puig-Saus, C.; Yu, R.; Shifrut, E.; Carnevale, J.; Li, P.J.; Hiatt, J.; Saco, J.; Krystofinski, P.; Li, H.; et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 2018, 559, 405–409. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, C.; Zheng, P.; Zhang, X.; Xu, J. Current status and future development of anti-HIV chimeric antigen receptor T-cell therapy. Immunotherapy 2020, 13, 177–184. [Google Scholar] [CrossRef]
- Cohn, L.B.; Chomont, N.; Deeks, S.G. The Biology of the HIV-1 Latent Reservoir and Implications for Cure Strategies. Cell Host Microbe 2020, 27, 519–530. [Google Scholar] [CrossRef]
- Hale, M.; Mesojednik, T.; Romano Ibarra, G.S.; Sahni, J.; Bernard, A.; Sommer, K.; Scharenberg, A.M.; Rawlings, D.J.; Wagner, T.A. Engineering HIV-Resistant, Anti-HIV Chimeric Antigen Receptor T Cells. Mol. Ther. 2017, 25, 570–579. [Google Scholar] [CrossRef]
- Maldini, C.R.; Claiborne, D.T.; Okawa, K.; Chen, T.; Dopkin, D.L.; Shan, X.; Power, K.A.; Trifonova, R.T.; Krupp, K.; Phelps, M.; et al. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo. Nat. Med. 2020, 26, 1776–1787. [Google Scholar] [CrossRef]
- Liu, B.; Zou, F.; Lu, L.; Chen, C.; He, D.; Zhang, X.; Tang, X.; Liu, C.; Li, L.; Zhang, H. Chimeric Antigen Receptor T Cells Guided by the Single-Chain Fv of a Broadly Neutralizing Antibody Specifically and Effectively Eradicate Virus Reactivated from Latency in CD4+ T Lymphocytes Isolated from HIV-1-Infected Individuals Receiving Suppressive Combined Antiretroviral Therapy. J. Virol. 2016, 90, 9712–9724. [Google Scholar] [CrossRef] [PubMed]
- Anthony-Gonda, K.; Bardhi, A.; Ray, A.; Flerin, N.; Li, M.; Chen, W.; Ochsenbauer, C.; Kappes, J.C.; Krueger, W.; Worden, A.; et al. Multispecific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci. Transl. Med. 2019, 11, eaav5685. [Google Scholar] [CrossRef]
- Tebas, P.; Stein, D.; Tang, W.W.; Frank, I.; Wang, S.Q.; Lee, G.; Spratt, S.K.; Surosky, R.T.; Giedlin, M.A.; Nichol, G.; et al. Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV. N. Engl. J. Med. 2014, 370, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Schumann, K.; Lin, S.; Boyer, E.; Simeonov, D.R.; Subramaniam, M.; Gate, R.E.; Haliburton, G.E.; Ye, C.J.; Bluestone, J.A.; Doudna, J.A.; et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl. Acad. Sci. USA 2015, 112, 10437–10442. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Kaminski, R.; Yang, F.; Zhang, Y.; Cosentino, L.; Li, F.; Luo, B.; Alvarez-Carbonell, D.; Garcia-Mesa, Y.; Karn, J.; et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc. Natl. Acad. Sci. USA 2014, 111, 11461–11466. [Google Scholar] [CrossRef] [PubMed]
- Maslennikova, A.; Kruglova, N.; Kalinichenko, S.; Komkov, D.; Shepelev, M.; Golubev, D.; Siniavin, A.; Vzorov, A.; Filatov, A.; Mazurov, D. Engineering T-Cell Resistance to HIV-1 Infection via Knock-In of Peptides from the Heptad Repeat 2 Domain of gp41. MBio 2022, 13, e0358921. [Google Scholar] [CrossRef]
- Dufour, C.; Claudel, A.; Joubarne, N.; Merindol, N.; Maisonnet, T.; Masroori, N.; Plourde, M.B.; Berthoux, L. Editing of the human TRIM5 gene to introduce mutations with the potential to inhibit HIV-1. PLoS ONE 2018, 13, e0191709. [Google Scholar] [CrossRef]
- Maslennikova, A.; Mazurov, D. Application of CRISPR/Cas Genomic Editing Tools for HIV Therapy: Toward Precise Modifications and Multilevel Protection. Front. Cell. Infect. Microbiol. 2022, 12, 880030. [Google Scholar] [CrossRef]
- Sather, B.D.; Ibarra, G.S.R.; Sommer, K.; Curinga, G.; Hale, M.; Khan, I.F.; Singh, S.; Song, Y.; Gwiazda, K.; Sahni, J.; et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci. Transl. Med. 2015, 7, 307ra156. [Google Scholar] [CrossRef]
- Bushman, F.D. Retroviral Insertional Mutagenesis in Humans: Evidence for Four Genetic Mechanisms Promoting Expansion of Cell Clones. Mol. Ther. 2020, 28, 352–356. [Google Scholar] [CrossRef]
- Irving, M.; Lanitis, E.; Migliorini, D.; Ivics, Z.; Guedan, S. Choosing the Right Tool for Genetic Engineering: Clinical Lessons from Chimeric Antigen Receptor-T Cells. Hum. Gene Ther. 2021, 32, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Cornetta, K.; Lin, T.-Y.; Pellin, D.; Kohn, D.B. Meeting FDA Guidance recommendations for replication-competent virus and insertional oncogenesis testing. Mol. Ther. Methods Clin. Dev. 2023, 28, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.L.; Sherrill-Mix, S.; Everett, J.K.; Reddy, S.; Fraietta, J.A.; Porter, D.L.; Frey, N.; Gill, S.I.; Grupp, S.A.; Maude, S.L.; et al. CD19-targeting CAR T cell immunotherapy outcomes correlate with genomic modification by vector integration. J. Clin. Investig. 2020, 130, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 2005, 16, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Castella, M.; Caballero-Baños, M.; Ortiz-Maldonado, V.; González-Navarro, E.A.; Suñé, G.; Antoñana-Vidósola, A.; Boronat, A.; Marzal, B.; Millán, L.; Martín-Antonio, B.; et al. Point-of-care CAR T-cell production (ARI-0001) using a closed semi-automatic bioreactor: Experience from an academic phase i clinical trial. Front. Immunol. 2020, 11, 500967. [Google Scholar] [CrossRef] [PubMed]
- van der Walle, C.F.; Godbert, S.; Saito, G.; Azhari, Z. Formulation Considerations for Autologous T Cell Drug Products. Pharmaceutics 2021, 13, 1317. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, E.; Carnevale, E.; Prodeus, A.; Magnani, Z.I.; Camisa, B.; Merelli, I.; Politano, C.; Stasi, L.; Potenza, A.; Cianciotti, B.C.; et al. CRISPR-based gene disruption and integration of high-avidity, WT1-specific T cell receptors improve antitumor T cell function. Sci. Transl. Med. 2022, 14, eabg8027. [Google Scholar] [CrossRef] [PubMed]
- Kath, J.; Du, W.; Pruene, A.; Braun, T.; Thommandru, B.; Turk, R.; Sturgeon, M.L.; Kurgan, G.L.; Amini, L.; Stein, M.; et al. Pharmacological interventions enhance virus-free generation of TRAC-replaced CAR T cells. Mol. Ther. Methods Clin. Dev. 2022, 25, 311–330. [Google Scholar] [CrossRef]
- Müller, T.R.; Jarosch, S.; Hammel, M.; Leube, J.; Grassmann, S.; Bernard, B.; Effenberger, M.; Andrä, I.; Chaudhry, M.Z.; Käuferle, T.; et al. Targeted T cell receptor gene editing provides predictable T cell product function for immunotherapy. Cell Rep. Med. 2021, 2, 100374. [Google Scholar] [CrossRef]
- Désaulniers, K.; Ortiz, L.; Dufour, C.; Claudel, A.; Plourde, M.B.; Merindol, N.; Berthoux, L. Editing of the TRIM5 Gene Decreases the Permissiveness of Human T Lymphocytic Cells to HIV-1. Viruses 2020, 13, 24. [Google Scholar] [CrossRef]
- Mueller, K.P.; Piscopo, N.J.; Forsberg, M.H.; Saraspe, L.A.; Das, A.; Russell, B.; Smerchansky, M.; Cappabianca, D.; Shi, L.; Shankar, K.; et al. Production and characterization of virus-free, CRISPR-CAR T cells capable of inducing solid tumor regression. J. Immunother. Cancer 2022, 10, e004446. [Google Scholar] [CrossRef] [PubMed]
- Nahmad, A.D.; Reuveni, E.; Goldschmidt, E.; Tenne, T.; Liberman, M.; Horovitz-Fried, M.; Khosravi, R.; Kobo, H.; Reinstein, E.; Madi, A.; et al. Frequent aneuploidy in primary human T cells after CRISPR–Cas9 cleavage. Nat. Biotechnol. 2022, 40, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Shy, B.R.; Vykunta, V.S.; Ha, A.; Talbot, A.; Roth, T.L.; Nguyen, D.N.; Pfeifer, W.G.; Chen, Y.Y.; Blaeschke, F.; Shifrut, E.; et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. 2023, 41, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Senger, K.; Madireddi, S.; Akhmetzyanova, I.; Ishizuka, I.E.; Tarighat, S.; Lo, J.H.; Shaw, D.; Haley, B.; Rutz, S. High-efficiency nonviral CRISPR/Cas9-mediated gene editing of human T cells using plasmid donor DNA. J. Exp. Med. 2022, 219, e20211530. [Google Scholar] [CrossRef] [PubMed]
- Wiebking, V.; Lee, C.M.; Mostrel, N.; Lahiri, P.; Bak, R.; Bao, G.; Roncarolo, M.G.; Bertaina, A.; Porteus, M.H. Genome editing of donor-derived T-cells to generate allogenic chimeric antigen receptor-modified T cells: Optimizing αβ T cell-depleted haploidentical hematopoietic stem cell transplantation. Haematologica 2020, 106, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Haderbache, R.; Warda, W.; Hervouet, E.; da Rocha, M.N.; Trad, R.; Allain, V.; Nicod, C.; Thieblemeont, C.; Boissel, N.; Varlet, P.; et al. Droplet digital PCR allows vector copy number assessment and monitoring of experimental CAR T cells in murine xenograft models or approved CD19 CAR T cell-treated patients. J. Transl. Med. 2021, 19, 265. [Google Scholar] [CrossRef]
- Santeramo, I.; Bagnati, M.; Harvey, E.J.; Hassan, E.; Surmacz-Cordle, B.; Marshall, D.; Di Cerbo, V. Vector Copy Distribution at a Single-Cell Level Enhances Analytical Characterization of Gene-Modified Cell Therapies. Mol. Ther. Methods Clin. Dev. 2020, 17, 944–956. [Google Scholar] [CrossRef]
- Zhao, Y.; Stepto, H.; Schneider, C.K. Development of the First World Health Organization Lentiviral Vector Standard: Toward the Production Control and Standardization of Lentivirus-Based Gene Therapy Products. Hum. Gene Ther. Methods 2017, 28, 205–214. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, Y.; Yin, H. Genome Editing with mRNA Encoding ZFN, TALEN, and Cas9. Mol. Ther. 2019, 27, 735–746. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2019, 18, 67–83. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Zare, K.; Negahdaripour, M.; Barekati-Mowahed, M.; Ghasemi, Y. CRISPR Cpf1 proteins: Structure, function and implications for genome editing. Cell Biosci. 2019, 9, 36. [Google Scholar] [CrossRef]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, D.; Zhang, J.; Xu, J.; Chen, Y.E. Recent Advances in Improving Gene-Editing Specificity through CRISPR–Cas9 Nuclease Engineering. Cells 2022, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Konstantakos, V.; Nentidis, A.; Krithara, A.; Paliouras, G. CRISPR–Cas9 gRNA efficiency prediction: An overview of predictive tools and the role of deep learning. Nucleic Acids Res. 2022, 50, 3616–3637. [Google Scholar] [CrossRef]
- Jensen, K.T.; Fløe, L.; Petersen, T.S.; Huang, J.; Xu, F.; Bolund, L.; Luo, Y.; Lin, L. Chromatin accessibility and guide sequence secondary structure affect CRISPR-Cas9 gene editing efficiency. FEBS Lett. 2017, 591, 1892–1901. [Google Scholar] [CrossRef]
- Borowicz, P.; Chan, H.; Medina, D.; Gumpelmair, S.; Kjelstrup, H.; Spurkland, A. A simple and efficient workflow for generation of knock-in mutations in Jurkat T cells using CRISPR/Cas9. Scand. J. Immunol. 2020, 91, e12862. [Google Scholar] [CrossRef] [PubMed]
- Corsi, G.I.; Qu, K.; Alkan, F.; Pan, X.; Luo, Y.; Gorodkin, J. CRISPR/Cas9 gRNA activity depends on free energy changes and on the target PAM context. Nat. Commun. 2022, 13, 3006. [Google Scholar] [CrossRef]
- Graf, R.; Li, X.; Chu, V.T.; Rajewsky, K. sgRNA Sequence Motifs Blocking Efficient CRISPR/Cas9-Mediated Gene Editing. Cell Rep. 2019, 26, 1098–1103.e3. [Google Scholar] [CrossRef] [PubMed]
- Creutzburg, S.C.A.; Wu, W.Y.; Mohanraju, P.; Swartjes, T.; Alkan, F.; Gorodkin, J.; Staals, R.H.J.; van der Oost, J. Good guide, bad guide: Spacer sequence-dependent cleavage efficiency of Cas12a. Nucleic Acids Res. 2020, 48, 3228–3243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, G.; Zhang, L.; Zhao, Q. Building Potent Chimeric Antigen Receptor T Cells with CRISPR Genome Editing. Front. Immunol. 2019, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, V.; Mercuri, E.; Marcovecchio, G.E.; Castiello, M.C.; Schiroli, G.; Albano, L.; Margulies, C.; Buquicchio, F.; Fontana, E.; Beretta, S.; et al. Modeling, optimization, and comparable efficacy of T cell and hematopoietic stem cell gene editing for treating hyper-IgM syndrome. EMBO Mol. Med. 2021, 13, e13545. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Inoue, S.; Nakashima, T.; Zhang, H.; Li, Y.; Kasuya, H.; Matsukawa, T.; Wu, Z.; Yoshikawa, T.; Kataoka, M.; et al. Epigenetic profiles guide improved CRISPR/Cas9-mediated gene knockout in human T cells. Nucleic Acids Res. 2023, gkad1076. [Google Scholar] [CrossRef]
- Fu, Y.-W.; Dai, X.-Y.; Wang, W.-T.; Yang, Z.-X.; Zhao, J.-J.; Zhang, J.-P.; Wen, W.; Zhang, F.; Oberg, K.C.; Zhang, L.; et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 2021, 49, 969–985. [Google Scholar] [CrossRef]
- Tatiossian, K.J.; Clark, R.D.E.; Huang, C.; Thornton, M.E.; Grubbs, B.H.; Cannon, P.M. Rational Selection of CRISPR-Cas9 Guide RNAs for Homology-Directed Genome Editing. Mol. Ther. 2021, 29, 1057–1069. [Google Scholar] [CrossRef]
- Schubert, M.S.; Thommandru, B.; Woodley, J.; Turk, R.; Yan, S.; Kurgan, G.; McNeill, M.S.; Rettig, G.R. Optimized design parameters for CRISPR Cas9 and Cas12a homology-directed repair. Sci. Rep. 2021, 11, 19482. [Google Scholar] [CrossRef]
- Leoni, C.; Bianchi, N.; Vincenzetti, L.; Monticelli, S. An optimized workflow for CRISPR-Cas9 deletion of surface and intracellular factors in primary human T lymphocytes. PLoS ONE 2021, 16, e0247232. [Google Scholar] [CrossRef] [PubMed]
- Seki, A.; Rutz, S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med. 2018, 215, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Ruhle, A.; Mittermaier, J.; Mejías-Pérez, E.; Gapp, M.; Linder, A.; Schmacke, N.A.; Hofmann, K.; Hennrich, A.A.; Levy, D.N.; et al. Rapid, efficient and activation-neutral gene editing of polyclonal primary human resting CD4+ T cells allows complex functional analyses. Nat. Methods 2022, 19, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.R.; Lonetree, C.-L.; Kluesner, M.G.; Johnson, M.J.; Pomeroy, E.J.; Diers, M.D.; Lahr, W.S.; Draper, G.M.; Slipek, N.J.; Smeester, B.A.; et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat. Commun. 2019, 10, 5222. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lu, R.; Xin, C.; Wang, Y.; Ling, X.; Li, D.; Zhang, W.; Liu, M.; Xie, W.; Kong, L.; et al. Cas9 exo-endonuclease eliminates chromosomal translocations during genome editing. Nat. Commun. 2022, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Bodai, Z.; Bishop, A.L.; Gantz, V.M.; Komor, A.C. Targeting double-strand break indel byproducts with secondary guide RNAs improves Cas9 HDR-mediated genome editing efficiencies. Nat. Commun. 2022, 13, 2351. [Google Scholar] [CrossRef] [PubMed]
- Möller, L.; Aird, E.J.; Schröder, M.S.; Kobel, L.; Kissling, L.; van de Venn, L.; Corn, J.E. Recursive Editing improves homology-directed repair through retargeting of undesired outcomes. Nat. Commun. 2022, 13, 4550. [Google Scholar] [CrossRef]
- Ma, H.; Tu, L.-C.; Naseri, A.; Huisman, M.; Zhang, S.; Grunwald, D.; Pederson, T. CRISPR-Cas9 nuclear dynamics and target recognition in living cells. J. Cell Biol. 2016, 214, 529–537. [Google Scholar] [CrossRef]
- Hendel, A.; Bak, R.O.; Clark, J.T.; Kennedy, A.B.; Ryan, D.E.; Roy, S.; Steinfeld, I.; Lunstad, B.D.; Kaiser, R.J.; Wilkens, A.B.; et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015, 33, 985–989. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. 2017, 23, 2255–2266. [Google Scholar] [CrossRef]
- Allen, D.; Rosenberg, M.; Hendel, A. Using Synthetically Engineered Guide RNAs to Enhance CRISPR Genome Editing Systems in Mammalian Cells. Front. Genome Ed. 2021, 2, 617910. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-programmed genome editing in human cells. Elife 2013, 2013, e00471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Roth, T.L.; Li, P.J.; Chen, P.A.; Apathy, R.; Mamedov, M.R.; Vo, L.T.; Tobin, V.R.; Goodman, D.; Shifrut, E.; et al. Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat. Biotechnol. 2020, 38, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Danner, E.; Li, X.; Graf, R.; Lebedin, M.; de la Rosa, K.; Kühn, R.; Rajewsky, K.; Chu, V.T. Precise CRISPR-Cas–mediated gene repair with minimal off-target and unintended on-target mutations in human hematopoietic stem cells. Sci. Adv. 2022, 8, eabm9106. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Park, J.J.; Du, Y.; Kim, H.R.; Wang, G.; Errami, Y.; Chen, S. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat. Methods 2019, 16, 247–254. [Google Scholar] [CrossRef]
- Glaser, V.; Flugel, C.; Kath, J.; Du, W.; Drosdek, V.; Franke, C.; Stein, M.; Pruß, A.; Schmueck-Henneresse, M.; Volk, H.-D.; et al. Combining different CRISPR nucleases for simultaneous knock-in and base editing prevents translocations in multiplex-edited CAR T cells. Genome Biol. 2023, 24, 89. [Google Scholar] [CrossRef]
- Allen, A.G.; Khan, S.Q.; Margulies, C.M.; Viswanathan, R.; Lele, S.; Blaha, L.; Scott, S.N.; Izzo, K.M.; Gerew, A.; Pattali, R.; et al. A highly efficient transgene knock-in technology in clinically relevant cell types. Nat. Biotechnol. 2023, 1–12. [Google Scholar] [CrossRef]
- Foss, D.V.; Muldoon, J.J.; Nguyen, D.N.; Carr, D.; Sahu, S.U.; Hunsinger, J.M.; Wyman, S.K.; Krishnappa, N.; Mendonsa, R.; Schanzer, E.V.; et al. Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes. Nat. Biomed. Eng. 2023, 7, 647–660. [Google Scholar] [CrossRef]
- Zhang, L.; Zuris, J.A.; Viswanathan, R.; Edelstein, J.N.; Turk, R.; Thommandru, B.; Rube, H.T.; Glenn, S.E.; Collingwood, M.A.; Bode, N.M.; et al. AsCas12a ultra nuclease facilitates the rapid generation of therapeutic cell medicines. Nat. Commun. 2021, 12, 3908. [Google Scholar] [CrossRef]
- Mohr, M.; Damas, N.; Gudmand-Høyer, J.; Zeeberg, K.; Jedrzejczyk, D.; Vlassis, A.; Morera-Gómez, M.; Pereira-Schoning, S.; Puš, U.; Oliver-Almirall, A.; et al. The CRISPR-Cas12a Platform for Accurate Genome Editing, Gene Disruption, and Efficient Transgene Integration in Human Immune Cells. ACS Synth. Biol. 2023, 12, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Alzubi, J.; Lock, D.; Rhiel, M.; Schmitz, S.; Wild, S.; Mussolino, C.; Hildenbeutel, M.; Brandes, C.; Rositzka, J.; Lennartz, S.; et al. Automated generation of gene-edited CAR T cells at clinical scale. Mol. Ther. Methods Clin. Dev. 2021, 20, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Jasin, M.; Haber, J.E. The democratization of gene editing: Insights from site-specific cleavage and double-strand break repair. DNA Repair 2016, 44, 6–16. [Google Scholar] [CrossRef]
- MacLeod, D.T.; Antony, J.; Martin, A.J.; Moser, R.J.; Hekele, A.; Wetzel, K.J.; Brown, A.E.; Triggiano, M.A.; Hux, J.A.; Pham, C.D.; et al. Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Mol. Ther. 2017, 25, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; DeClercq, J.J.; Hayward, S.B.; Li, P.W.-L.; Shivak, D.A.; Gregory, P.D.; Lee, G.; Holmes, M.C. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 2016, 44, e30. [Google Scholar] [CrossRef]
- Martin-Sancho, L.; Lewinski, M.K.; Pache, L.; Stoneham, C.A.; Yin, X.; Becker, M.E.; Pratt, D.; Churas, C.; Rosenthal, S.B.; Liu, S.; et al. Functional Landscape of SARS-CoV-2 Cellular Restriction. Mol. Cell 2021, 81, 2656–2668. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, M.; Das, A.T.; Herrera-Carrillo, E.; Berkhout, B. Extinction of all infectious HIV in cell culture by the CRISPR-Cas12a system with only a single crRNA. Nucleic Acids Res. 2020, 48, 5527–5539. [Google Scholar] [CrossRef]
- Kamali, E.; Rahbarizadeh, F.; Hojati, Z.; Frödin, M. CRISPR/Cas9-mediated knockout of clinically relevant alloantigenes in human primary T cells. BMC Biotechnol. 2021, 21, 9. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, X.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017, 8, 17002–17011. [Google Scholar] [CrossRef]
- Yang, M.; Tkach, D.; Boyne, A.; Kazancioglu, S.; Duclert, A.; Poirot, L.; Duchateau, P.; Juillerat, A. Optimized two-step electroporation process to achieve efficient nonviral-mediated gene insertion into primary T cells. FEBS Open Bio 2022, 12, 38–50. [Google Scholar] [CrossRef]
- Kagita, A.; Lung, M.S.Y.; Xu, H.; Kita, Y.; Sasakawa, N.; Iguchi, T.; Ono, M.; Wang, X.H.; Gee, P.; Hotta, A. Efficient ssODN-Mediated Targeting by Avoiding Cellular Inhibitory RNAs through Precomplexed CRISPR-Cas9/sgRNA Ribonucleoprotein. Stem Cell Rep. 2021, 16, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Pruene, A.; Darguzyte, M.; vom Stein, A.F.; Nguyen, P.-H.; Wagner, D.L.; Kath, J.; Roig-Merino, A.; Heuser, M.; Riehm, L.L.; et al. Non-viral TRAC-knocked-in CD19KICAR-T and gp350KICAR-T cells tested against Burkitt lymphomas with type 1 or 2 EBV infection: In vivo cellular dynamics and potency. Front. Immunol. 2023, 14, 1086433. [Google Scholar] [CrossRef] [PubMed]
- VanderBurgh, J.A.; Corso, T.N.; Levy, S.L.; Craighead, H.G. Scalable continuous-flow electroporation platform enabling T cell transfection for cellular therapy manufacturing. Sci. Rep. 2023, 13, 6857. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Electroporation. Cold Spring Harb. Protoc. 2019, 2019, pdb.top096271. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.R.; Tsuchida, C.A.; Nguyen, D.N.; Shy, B.R.; McGarrigle, E.R.; Sandoval Espinoza, C.R.; Carr, D.; Blaeschke, F.; Marson, A.; Doudna, J.A. Targeted delivery of CRISPR-Cas9 and transgenes enables complex immune cell engineering. Cell Rep. 2021, 35, 109207. [Google Scholar] [CrossRef]
- Mangeot, P.E.; Risson, V.; Fusil, F.; Marnef, A.; Laurent, E.; Blin, J.; Mournetas, V.; Massouridès, E.; Sohier, T.J.M.; Corbin, A.; et al. Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nat. Commun. 2019, 10, 45. [Google Scholar] [CrossRef]
- Gee, P.; Lung, M.S.Y.; Okuzaki, Y.; Sasakawa, N.; Iguchi, T.; Makita, Y.; Hozumi, H.; Miura, Y.; Yang, L.F.; Iwasaki, M.; et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020, 11, 1334. [Google Scholar] [CrossRef]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265.e16. [Google Scholar] [CrossRef]
- Gutierrez-Guerrero, A.; Abrey Recalde, M.J.; Mangeot, P.E.; Costa, C.; Bernadin, O.; Périan, S.; Fusil, F.; Froment, G.; Martinez-Turtos, A.; Krug, A.; et al. Baboon Envelope Pseudotyped “Nanoblades” Carrying Cas9/gRNA Complexes Allow Efficient Genome Editing in Human T, B, and CD34+ Cells and Knock-in of AAV6-Encoded Donor DNA in CD34+ Cells. Front. Genome Ed. 2021, 3, 604371. [Google Scholar] [CrossRef]
- Bothmer, A.; Gareau, K.W.; Abdulkerim, H.S.; Buquicchio, F.; Cohen, L.; Viswanathan, R.; Zuris, J.A.; Marco, E.; Fernandez, C.A.; Myer, V.E.; et al. Detection and Modulation of DNA Translocations During Multi-Gene Genome Editing in T Cells. Cris. J. 2020, 3, 177–187. [Google Scholar] [CrossRef]
- Maggio, I.; Zittersteijn, H.A.; Wang, Q.; Liu, J.; Janssen, J.M.; Ojeda, I.T.; van der Maarel, S.M.; Lankester, A.C.; Hoeben, R.C.; Gonçalves, M.A.F.V. Integrating gene delivery and gene-editing technologies by adenoviral vector transfer of optimized CRISPR-Cas9 components. Gene Ther. 2020, 27, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, R.; Martinez-Lage, M.; Martin, M.C.; Garcia, A.; Bueno, C.; Castaño, J.; Ramirez, J.C.; Menendez, P.; Cigudosa, J.C.; Rodriguez-Perales, S. Efficient Recreation of t(11;22) EWSR1-FLI1 + in Human Stem Cells Using CRISPR/Cas9. Stem Cell Rep. 2017, 8, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.; Liu, P.; Zeng, J.; Wang, Y.; Maitland, S.A.; Idrizi, F.; Ponnienselvan, K.; Zhu, L.J.; Luban, J.; Bauer, D.E.; et al. Optimization of Nuclear Localization Signal Composition Improves CRISPR-Cas12a Editing Rates in Human Primary Cells. GEN Biotechnol. 2022, 1, 271–284. [Google Scholar] [CrossRef]
- Liu, P.; Luk, K.; Shin, M.; Idrizi, F.; Kwok, S.; Roscoe, B.; Mintzer, E.; Suresh, S.; Morrison, K.; Frazão, J.B.; et al. Enhanced Cas12a editing in mammalian cells and zebrafish. Nucleic Acids Res. 2019, 47, 4169–4180. [Google Scholar] [CrossRef]
- Gier, R.A.; Budinich, K.A.; Evitt, N.H.; Cao, Z.; Freilich, E.S.; Chen, Q.; Qi, J.; Lan, Y.; Kohli, R.M.; Shi, J. High-performance CRISPR-Cas12a genome editing for combinatorial genetic screening. Nat. Commun. 2020, 11, 3455. [Google Scholar] [CrossRef]
- Liu, J.; Srinivasan, S.; Li, C.-Y.; Ho, I.-L.; Rose, J.; Shaheen, M.; Wang, G.; Yao, W.; Deem, A.; Bristow, C.; et al. Pooled library screening with multiplexed Cpf1 library. Nat. Commun. 2019, 10, 3144. [Google Scholar] [CrossRef]
- Staahl, B.T.; Benekareddy, M.; Coulon-Bainier, C.; Banfal, A.A.; Floor, S.N.; Sabo, J.K.; Urnes, C.; Munares, G.A.; Ghosh, A.; Doudna, J.A. Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol. 2017, 35, 431–434. [Google Scholar] [CrossRef]
- Richardson, C.D.; Ray, G.J.; DeWitt, M.A.; Curie, G.L.; Corn, J.E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 2016, 34, 339–344. [Google Scholar] [CrossRef]
- Odé, Z.; Condori, J.; Peterson, N.; Zhou, S.; Krenciute, G. CRISPR-Mediated Non-Viral Site-Specific Gene Integration and Expression in T Cells: Protocol and Application for T-Cell Therapy. Cancers 2020, 12, 1704. [Google Scholar] [CrossRef]
- Wienert, B.; Nguyen, D.N.; Guenther, A.; Feng, S.J.; Locke, M.N.; Wyman, S.K.; Shin, J.; Kazane, K.R.; Gregory, G.L.; Carter, M.A.M.; et al. Timed inhibition of CDC7 increases CRISPR-Cas9 mediated templated repair. Nat. Commun. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Davidsson, M.; Negrini, M.; Hauser, S.; Svanbergsson, A.; Lockowandt, M.; Tomasello, G.; Manfredsson, F.P.; Heuer, A. A comparison of AAV-vector production methods for gene therapy and preclinical assessment. Sci. Rep. 2020, 10, 21532. [Google Scholar] [CrossRef] [PubMed]
- Hastie, E.; Samulski, R.J. Adeno-Associated Virus at 50: A Golden Anniversary of Discovery, Research, and Gene Therapy Success—A Personal Perspective. Hum. Gene Ther. 2015, 26, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, K.S.; Chen, Z.; Mir, A.; Dokshin, G.A.; Krishnamurthy, P.M.; Yoon, Y.; Gallant, J.; Xu, P.; Zhang, X.-O.; Ozturk, A.R.; et al. 5′-Modifications improve potency and efficacy of DNA donors for precision genome editing. Elife 2021, 10, e72216. [Google Scholar] [CrossRef] [PubMed]
- Dhungel, B.P.; Bailey, C.G.; Rasko, J.E.J. Journey to the Center of the Cell: Tracing the Path of AAV Transduction. Trends Mol. Med. 2021, 27, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Koujin, T.; Shindo, T.; Bilir, Ş.; Osakada, H.; Nishimura, K.; Hirano, Y.; Asakawa, H.; Mori, C.; Kobayashi, S.; et al. Transfected plasmid DNA is incorporated into the nucleus via nuclear envelope reformation at telophase. Commun. Biol. 2022, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Carlson-Stevermer, J.; Abdeen, A.A.; Kohlenberg, L.; Goedland, M.; Molugu, K.; Lou, M.; Saha, K. Assembly of CRISPR ribonucleoproteins with biotinylated oligonucleotides via an RNA aptamer for precise gene editing. Nat. Commun. 2017, 8, 1711. [Google Scholar] [CrossRef]
- Shams, F.; Bayat, H.; Mohammadian, O.; Mahboudi, S.; Vahidnezhad, H.; Soosanabadi, M.; Rahimpour, A. Advance trends in targeting homology-directed repair for accurate gene editing: An inclusive review of small molecules and modified CRISPR-Cas9 systems. BioImpacts 2022, 12, 371–391. [Google Scholar] [CrossRef]
- Mandal, P.K.; Ferreira, L.M.R.; Collins, R.; Meissner, T.B.; Boutwell, C.L.; Friesen, M.; Vrbanac, V.; Garrison, B.S.; Stortchevoi, A.; Bryder, D.; et al. Efficient Ablation of Genes in Human Hematopoietic Stem and Effector Cells using CRISPR/Cas9. Cell Stem Cell 2014, 15, 643–652. [Google Scholar] [CrossRef]
- Chen, X.; Rinsma, M.; Janssen, J.M.; Liu, J.; Maggio, I.; Gonçalves, M.A.F.V. Probing the impact of chromatin conformation on genome editing tools. Nucleic Acids Res. 2016, 44, 6482–6492. [Google Scholar] [CrossRef]
- Amirache, F.; Lévy, C.; Costa, C.; Mangeot, P.-E.; Torbett, B.E.; Wang, C.X.; Nègre, D.; Cosset, F.-L.; Verhoeyen, E. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 2014, 123, 1422–1424. [Google Scholar] [CrossRef]
- Ghassemi, S.; Nunez-Cruz, S.; O’Connor, R.S.; Fraietta, J.A.; Patel, P.R.; Scholler, J.; Barrett, D.M.; Lundh, S.M.; Davis, M.M.; Bedoya, F.; et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunol. Res. 2018, 6, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- López-Cantillo, G.; Urueña, C.; Camacho, B.A.; Ramírez-Segura, C. CAR-T Cell Performance: How to Improve Their Persistence? Front. Immunol. 2022, 13, 878209. [Google Scholar] [CrossRef] [PubMed]
- Stroncek, D.F.; Ren, J.; Lee, D.W.; Tran, M.; Frodigh, S.E.; Sabatino, M.; Khuu, H.; Merchant, M.S.; Mackall, C.L. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy 2016, 18, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Koya, R.C.; Chodon, T.; Graham, N.A.; Graeber, T.G.; Comin-Anduix, B.; Ribas, A. The Impact of Ex Vivo Clinical Grade Activation Protocols on Human T-Cell Phenotype and Function for the Generation of Genetically Modified Cells for Adoptive Cell Transfer Therapy. J. Immunother. 2010, 33, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339. [Google Scholar] [CrossRef] [PubMed]
- Besser, M.J.; Schallmach, E.; Oved, K.; Treves, A.J.; Markel, G.; Reiter, Y.; Schachter, J. Modifying interleukin-2 concentrations during culture improves function of T cells for adoptive immunotherapy. Cytotherapy 2009, 11, 206–217. [Google Scholar] [CrossRef]
- Kaartinen, T.; Luostarinen, A.; Maliniemi, P.; Keto, J.; Arvas, M.; Belt, H.; Koponen, J.; Mäkinen, P.I.; Loskog, A.; Mustjoki, S.; et al. Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy 2017, 19, 1130. [Google Scholar] [CrossRef]
- Alvarez-Fernández, C.; Escribà-Garcia, L.; Vidal, S.; Sierra, J.; Briones, J. A short CD3/CD28 costimulation combined with IL-21 enhance the generation of human memory stem T cells for adoptive immunotherapy. J. Transl. Med. 2016, 14, 214. [Google Scholar] [CrossRef]
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef]
- Tebas, P.; Jadlowsky, J.K.; Shaw, P.A.; Tian, L.; Esparza, E.; Brennan, A.L.; Kim, S.; Naing, S.Y.; Richardson, M.W.; Vogel, A.N.; et al. CCR5-edited CD4+ T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Investig. 2021, 131, e144486. [Google Scholar] [CrossRef]
- Doyon, Y.; Vo, T.D.; Mendel, M.C.; Greenberg, S.G.; Wang, J.; Xia, D.F.; Miller, J.C.; Urnov, F.D.; Gregory, P.D.; Holmes, M.C. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods 2011, 8, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Mintier, G.; Ma-Edmonds, M.; Storton, D.; Wang, X.; Xiao, X.; Kienzle, B.; Zhao, D.; Feder, J.N. ‘Cold shock’ increases the frequency of homology directed repair gene editing in induced pluripotent stem cells. Sci. Rep. 2018, 8, 2080. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Zhang, X.; An, C.; Cheng, C.; Wang, H. Temperature effect on CRISPR-Cas9 mediated genome editing. J. Genet. Genom. 2017, 44, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, P.; Aksoy, B.A.; Czech, E.; Hammerbacher, J. Viable and efficient electroporation-based genetic manipulation of unstimulated human T cells. bioRxiv 2019, 466243. [Google Scholar] [CrossRef]
- Oh, S.A.; Seki, A.; Rutz, S. Ribonucleoprotein Transfection for CRISPR/Cas9-Mediated Gene Knockout in Primary T Cells. Curr. Protoc. Immunol. 2019, 124, e69. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, E.; Botla, S.; Persson, J.; Schmierer, B.; Taipale, J. CRISPR–Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018, 24, 927–930. [Google Scholar] [CrossRef]
- Ihry, R.J.; Worringer, K.A.; Salick, M.R.; Frias, E.; Ho, D.; Theriault, K.; Kommineni, S.; Chen, J.; Sondey, M.; Ye, C.; et al. P53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat. Med. 2018, 24, 939–946. [Google Scholar] [CrossRef]
- Schiroli, G.; Conti, A.; Ferrari, S.; della Volpe, L.; Jacob, A.; Albano, L.; Beretta, S.; Calabria, A.; Vavassori, V.; Gasparini, P.; et al. Precise Gene Editing Preserves Hematopoietic Stem Cell Function following Transient p53-Mediated DNA Damage Response. Cell Stem Cell 2019, 24, 551–565.e8. [Google Scholar] [CrossRef]
- Enache, O.M.; Rendo, V.; Abdusamad, M.; Lam, D.; Davison, D.; Pal, S.; Currimjee, N.; Hess, J.; Pantel, S.; Nag, A.; et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat. Genet. 2020, 52, 662–668. [Google Scholar] [CrossRef]
- Haideri, T.; Howells, A.; Jiang, Y.; Yang, J.; Bao, X.; Lian, X.L. Robust genome editing via modRNA-based Cas9 or base editor in human pluripotent stem cells. Cell Rep. Methods 2022, 2, 100290. [Google Scholar] [CrossRef]
- Jiang, L.; Ingelshed, K.; Shen, Y.; Boddul, S.V.; Iyer, V.S.; Kasza, Z.; Sedimbi, S.; Lane, D.P.; Wermeling, F. CRISPR/Cas9-Induced DNA Damage Enriches for Mutations in a p53-Linked Interactome: Implications for CRISPR-Based Therapies. Cancer Res. 2022, 82, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhong, A.; Wu, Y.; Sidharta, M.; Beaury, M.; Zhao, X.; Studer, L.; Zhou, T. Transient inhibition of p53 enhances prime editing and cytosine base-editing efficiencies in human pluripotent stem cells. Nat. Commun. 2022, 13, 6354. [Google Scholar] [CrossRef]
- Kurup, S.P.; Moioffer, S.J.; Pewe, L.L.; Harty, J.T. p53 Hinders CRISPR/Cas9-Mediated Targeted Gene Disruption in Memory CD8 T Cells In Vivo. J. Immunol. 2020, 205, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G. Nucleic Acid Immunity. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–169. [Google Scholar]
- Kim, S.; Koo, T.; Jee, H.-G.; Cho, H.-Y.; Lee, G.; Lim, D.-G.; Shin, H.S.; Kim, J.-S. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 2018, 28, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S.; Cedrone, E.; Neun, B.; Behlke, M.A.; Dobrovolskaia, M.A. Chemical Modification of CRISPR gRNAs Eliminate type I Interferon Responses in Human Peripheral Blood Mononuclear Cells. J. Cytokine Biol. 2018, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Wienert, B.; Shin, J.; Zelin, E.; Pestal, K.; Corn, J.E. In vitro–transcribed guide RNAs trigger an innate immune response via the RIG-I pathway. PLoS Biol. 2018, 16, e2005840. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Azizian, K.T.; Haque, A.K.M.A.; Henderson, J.M.; Hendel, A.; Shore, S.; Antony, J.S.; Hogrefe, R.I.; Kormann, M.S.D.; Porteus, M.H.; et al. Uridine Depletion and Chemical Modification Increase Cas9 mRNA Activity and Reduce Immunogenicity without HPLC Purification. Mol. Ther. Nucleic Acids 2018, 12, 530–542. [Google Scholar] [CrossRef]
- Semenova, N.; Bosnjak, M.; Markelc, B.; Znidar, K.; Cemazar, M.; Heller, L. Multiple cytosolic DNA sensors bind plasmid DNA after transfection. Nucleic Acids Res. 2019, 47, 10235–10246. [Google Scholar] [CrossRef]
- Cerboni, S.; Jeremiah, N.; Gentili, M.; Gehrmann, U.; Conrad, C.; Stolzenberg, M.-C.; Picard, C.; Neven, B.; Fischer, A.; Amigorena, S.; et al. Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes. J. Exp. Med. 2017, 214, 1769–1785. [Google Scholar] [CrossRef]
- Smirnikhina, S.A.; Zaynitdinova, M.I.; Sergeeva, V.A.; Lavrov, A.V. Improving Homology-Directed Repair in Genome Editing Experiments by Influencing the Cell Cycle. Int. J. Mol. Sci. 2022, 23, 5992. [Google Scholar] [CrossRef]
- Wen, W.; Quan, Z.-J.; Li, S.-A.; Yang, Z.-X.; Fu, Y.-W.; Zhang, F.; Li, G.-H.; Zhao, M.; Yin, M.-D.; Xu, J.; et al. Effective control of large deletions after double-strand breaks by homology-directed repair and dsODN insertion. Genome Biol. 2021, 22, 236. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qu, Y.; Cheng, J.K.W.; Hughes, N.W.; Zhang, Q.; Wang, M.; Cong, L. dCas9-based gene editing for cleavage-free genomic knock-in of long sequences. Nat. Cell Biol. 2022, 24, 268–278. [Google Scholar] [CrossRef] [PubMed]
| Factor | Parameters | |
|---|---|---|
| CRISPR components and method of their delivery into cells | gRNA | Spacer sequence |
| Chemical modifications | ||
| Using several gRNAs per gene | ||
| Delivery method | ||
| Protection from degradation | ||
| Cas nuclease | Type of nuclease | |
| Type of delivered molecule | ||
| Delivery method | ||
| Nuclear localization signal (NLS) | ||
| Donor DNA | Type of molecule | |
| Dose | ||
| Length of homology arms | ||
| Delivery method | ||
| Modifications | ||
| Manipulating T cell state | Culturing conditions | Isolation |
| Activation | ||
| Media | ||
| Activation time | ||
| Cytokines | ||
| T cell response to manipulation | Activation of p53 | |
| Innate response to nucleic acids | ||
| DSB repair pathway choice | Inhibition of NHEJ | |
| Stimulation of HDR | ||
| The Type of a Donor DNA Molecule Delivered to T Cells | Reference |
|---|---|
| ssDNA | Roth et al., 2018 [27] |
| Shy et al., 2023 [54] | |
| dsDNA PCR product | Roth et al., 2018 [27] |
| Nguyen et al., 2020 [95] | |
| Ode et al., 2020 [130] | |
| Kath et al., 2022 [49] | |
| Oh et al., 2022 [55] | |
| Shy et al., 2023 [54] | |
| Mueller et al., 2022 [52] | |
| Mohr et al., 2023 [102] | |
| Braun et al., 2023 [113] | |
| Glaser et al., 2023 [98] | |
| dsDNA restriction digest | Zhang et al., 2022 [13] |
| Mohr et al., 2023 [102] | |
| Plasmid | Wienert et al., 2020 [131] |
| Oh et al., 2022 [55] | |
| Foy et al., 2022 [12] | |
| nanoplasmid | Oh et al., 2022 [55] |
| AAV6 | Eyquem et al., 2017 [26] |
| Dai et al., 2019 [97] | |
| Wiebking et al., 2020 [56] | |
| Fu et al., 2021 [79] | |
| Tran et al., 2022 [96] | |
| Foss et al., 2023 [100] | |
| Allen et al., 2023 [99] |
| Parameter | Conditions | Reference |
|---|---|---|
| T cell isolation | No isolation from PBMC | Nahmad et al., 2022 [53] |
| Kath et al., 2022 [49] | ||
| Negative separation | Schuman et al., 2015 [35] | |
| Ren et al., 2017 [91] | ||
| Eyquem et al., 2017 [26] | ||
| Roth et al., 2018 [27] | ||
| Webber et al., 2019 [85] | ||
| Dai et al., 2019 [97] | ||
| Nguyen et al., 2020 [95] | ||
| Wienert et al., 2020 [131] | ||
| Tran et al., 2022 [96] | ||
| Mueller et al., 2022 [52] | ||
| Balke-Want et al., 2023 [23] | ||
| Shy et al., 2023 [54] | ||
| Foss et al., 2023 [100] | ||
| Mohr et al., 2023 [102] | ||
| Positive separation | Ode et al., 2020 [130] | |
| Fu et al., 2021 [79] | ||
| Kath et al., 2022 [49] | ||
| Oh et al., 2022 [55] | ||
| Foy et al., 2022 [12] | ||
| Zhang et al., 2022 [13] | ||
| Shy et al., 2023 [54] | ||
| Glaser et al., 2023 [98] | ||
| Activation | Dynabeads Human T-Activator CD3/CD28 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) at a 1:1 or 2:1 bead:cell ratio | Ren et al., 2017 [91] |
| Eyquem et al., 2017 [26] | ||
| Roth et al., 2018 [27] | ||
| Dai et al., 2019 [97] | ||
| Webber et al., 2019 [85] | ||
| Ode et al., 2020 [130] * | ||
| Wienert et al., 2020 [131] | ||
| Nguyen et al., 2020 [95] | ||
| Wiebking et al., 2020 [56] | ||
| Fu et al., 2021 [79] | ||
| Zhang et al., 2021 [101] | ||
| Kath et al., 2022 [49] | ||
| Tran et al., 2022 [96] | ||
| Shy et al., 2023 [54] | ||
| Balke-Want et al., 2023 [23] | ||
| Allen et al., 2023 [99] | ||
| Foss et al., 2023 [100] | ||
| αCD3/αCD28 soluble antibodies | Nahmad et al., 2022 [53] | |
| αCD3/αCD28-coated tissue culture plates | Schuman et al., 2015 [35] | |
| Kath et al., 2022 [49] | ||
| Glaser et al., 2023 [98] | ||
| T Cell TransAct (Miltenyi Biotec, Bergisch Gladbach, Germany) | Oh et al., 2022 [55] | |
| Foy et al., 2022 [12] | ||
| Zhang et al., 2022 [13] | ||
| Braun et al., 2023 [113] | ||
| Human CD3/CD28/CD2 T cell Activator (STEMCELL Technologies, Vancouver, BC, Canada) | Mueller et al., 2022 [52] | |
| Mohr et al., 2023 [102] | ||
| Medium | OpTmizer CTS T cell Expansion SFM + 2.5% CTS Immune Cell SR (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) + L-Glutamine + Penicillin/Streptomycin + N-Acetyl-L-cysteine (10 mM) | Webber et al., 2019 [85] |
| RPMI 1640 + 10% FCS | Kath et al., 2022 [49] | |
| Glaser et al., 2023 [98] | ||
| Braun et al., 2023 [113] | ||
| RPMI 1640 + 10% FCS + 1× GlutaMAX (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) | Tran et al., 2022 [96] | |
| RPMI-1640 + supplemented with 5 mmol/L Hepes + 2 mmol/L GlutaMAX (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) + 50 μg/mL penicillin/streptomycin+ 50 μmol/L 2-mercaptoethanol + 5 mmol/L nonessential amino acids + 5 mmol/L sodium pyruvate + 10% FBS | Schuman et al., 2015 [35] | |
| X-VIVO 10 medium (Lonza, Basel, Switzerland) + 5% human serum + 1.6 mg mL−1 N-acetylcysteine + 2 mM GlutaMAX (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) | Allen et al., 2023 [99] | |
| X-VIVO 15 medium (Lonza, Basel, Switzerland) + 5% FBS + 50 µM 2-mercaptoethanol+ 10 mM N-acetyl L-cysteine | Roth et al., 2018 [27] | |
| Wienert et al., 2020 [131] | ||
| Nguyen et al., 2020 [95] | ||
| Shy et al., 2023 [54] | ||
| Foss et al., 2023 [100] | ||
| X-VIVO 15 medium (Lonza, Basel, Switzerland)+ CTS Immune Cell Serum Replacement (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) | Zhang et al., 2022 [13] | |
| X-VIVO 15 medium (Lonza, Basel, Switzerland) + 5% human serum | Eyquem et al., 2017 [26] | |
| Dai et al., 2019 [97] | ||
| Wiebking et al., 2020 [56] | ||
| MEM-Alpha (Biological Industries, Sartorius, Göttingen, Germany) + 10% FCS | Nahmad et al., 2022 [53] | |
| For activation: PRIME-XV T Cell CDM media (Irvine Scientific, FUJIFILM Corporation, Santa Ana, CA, USA) For culturing: RPMI 1640 + 10% FBS + 2 mM GlutaMAX (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) + 1 mM sodium pyruvate + 0.1 mM nonessential amino acids + 55 μM 2-mercaptoethanol + 100 U/mL penicillin + 100 μg/mLs streptomycin + 10 mM Hepes (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) | Oh et al., 2022 [55] | |
| ImmunoCult™-XF T Cell Expansion Medium (STEMCELL Technologies, Vancouver, BC, Canada) | Fu et al., 2021 [79] | |
| Mueller et al., 2022 [52] | ||
| Mohr et al., 2023 [102] | ||
| TexMACS media (Miltenyi Biotec, Bergisch Gladbach, Germany) + 3% human male AB Serum | Balke-Want et al., 2023 [23] | |
| Foy et al., 2022 [12] | ||
| PRIME-XV (Irvine Scientific, FUJIFILM Corporation, Santa Ana, CA, USA) | Foy et al., 2022 [12] | |
| cytokines | IL-2 | Schuman et al., 2015 [35] |
| Eyquem et al., 2017 [26] | ||
| Dai et al., 2019 [97] | ||
| Wiebking et al., 2020 [56] | ||
| Fu et al., 2021 [79] | ||
| Nahmad et al., 2022 [53] | ||
| Tran et al., 2022 [96] | ||
| Mueller et al., 2022 [52] | ||
| IL-7,15 | Ode et al., 2020 [130] | |
| Oh et al., 2022 [55] | ||
| Foy et al., 2022 [12] | ||
| Balke-Want et al., 2023 [23] | ||
| Glaser et al., 2023 [98] | ||
| Braun et al., 2023 [113] | ||
| IL-2,7,15 | Roth et al., 2018 [27] | |
| Webber et al., 2019 [85] | ||
| Wienert et al., 2020 [131] | ||
| Nguyen et al., 2020 [95] | ||
| Zhang et al., 2021 [101] | ||
| Oh et al., 2022 [55] | ||
| Kath et al., 2022 [49] | ||
| Shy et al., 2023 [54] | ||
| Zhang et al., 2022 [13] | ||
| Allen et al., 2023 [99] | ||
| Foss et al., 2023 [100] | ||
| Mohr et al., 2023 [102] | ||
| Culturing time interval from isolation (day 0) until delivery of CRISPR/Cas (the next day after this interval) | 1 d | Nahmad et al., 2022 [53] |
| Balke-Want et al., 2023 [23] | ||
| Allen et al., 2023 [99] | ||
| 2 d | Schuman et al., 2015 [35] | |
| Eyquem et al., 2017 [26] | ||
| Ren et al., 2017 [91] | ||
| Roth et al., 2018 [27] | ||
| Webber et al., 2019 [85] | ||
| Dai et al., 2019 [97] | ||
| Wienert et al., 2020 [131] | ||
| Ode et al., 2020 [130] | ||
| Nguyen et al., 2020 [95] | ||
| Wiebking et al., 2020 [56] | ||
| Fu et al., 2021 [79] | ||
| Kath et al., 2022 [49] | ||
| Oh et al., 2022 [55] | ||
| Foy et al., 2022 [12] | ||
| Zhang et al., 2022 [13] | ||
| Nahmad et al., 2022 [53] | ||
| Mueller et al., 2022 [52] | ||
| Shy et al., 2023 [54] | ||
| Glaser et al., 2023 [98] | ||
| Foss et al., 2023 [100] | ||
| Braun et al., 2023 [113] | ||
| Mohr et al., 2023 [102] | ||
| 3 d | Wiebking et al., 2020 [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruglova, N.; Shepelev, M. Increasing Gene Editing Efficiency via CRISPR/Cas9- or Cas12a-Mediated Knock-In in Primary Human T Cells. Biomedicines 2024, 12, 119. https://doi.org/10.3390/biomedicines12010119
Kruglova N, Shepelev M. Increasing Gene Editing Efficiency via CRISPR/Cas9- or Cas12a-Mediated Knock-In in Primary Human T Cells. Biomedicines. 2024; 12(1):119. https://doi.org/10.3390/biomedicines12010119
Chicago/Turabian StyleKruglova, Natalia, and Mikhail Shepelev. 2024. "Increasing Gene Editing Efficiency via CRISPR/Cas9- or Cas12a-Mediated Knock-In in Primary Human T Cells" Biomedicines 12, no. 1: 119. https://doi.org/10.3390/biomedicines12010119
APA StyleKruglova, N., & Shepelev, M. (2024). Increasing Gene Editing Efficiency via CRISPR/Cas9- or Cas12a-Mediated Knock-In in Primary Human T Cells. Biomedicines, 12(1), 119. https://doi.org/10.3390/biomedicines12010119







