Short-Term Clinical Assessment of Treating Female Androgenetic Alopecia with Autologous Stem Cells Derived from Human Hair Follicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Patients
2.3. ACM Procedure
2.4. Clinical Evaluation of Hair Growth
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Patients
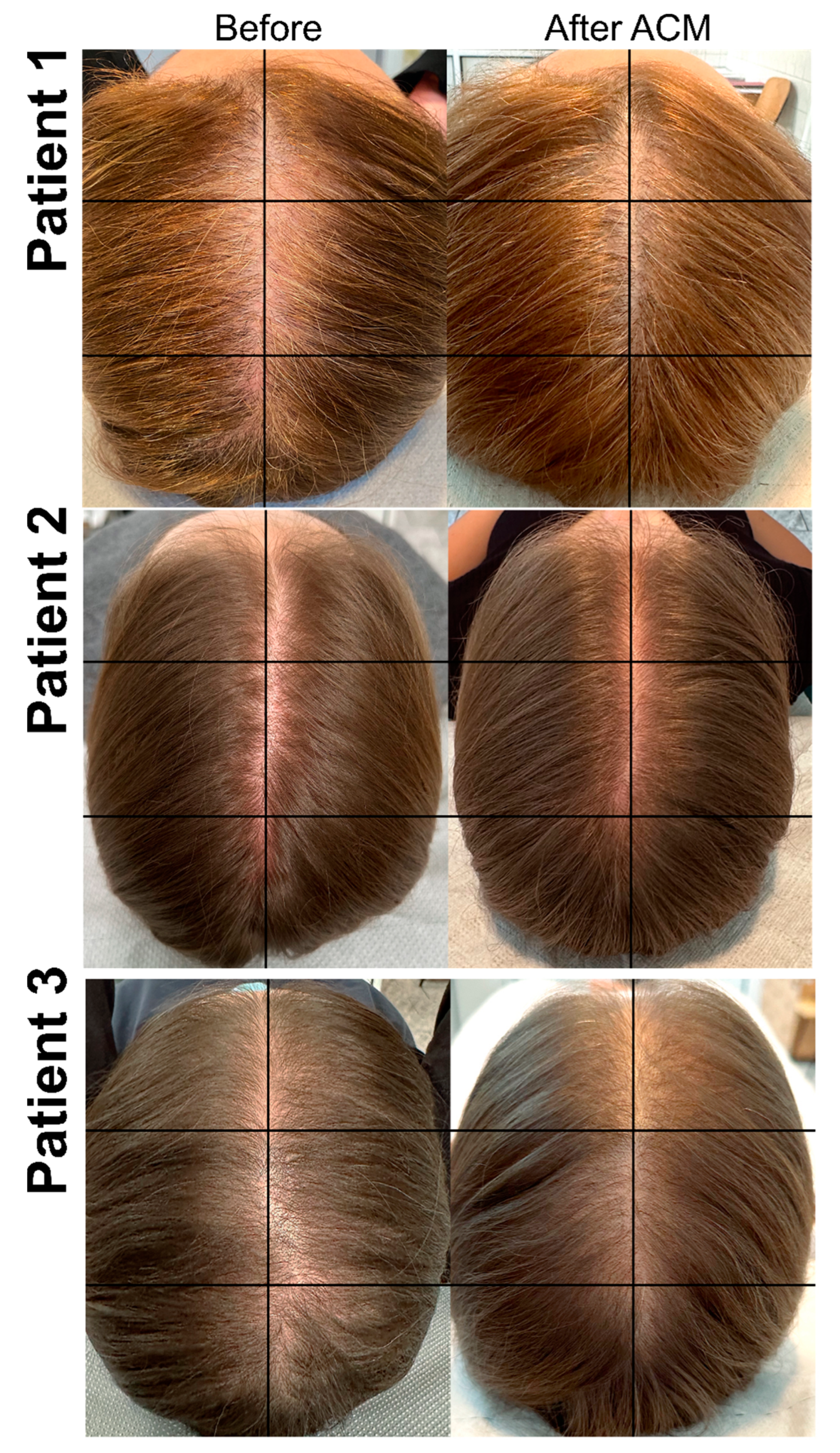
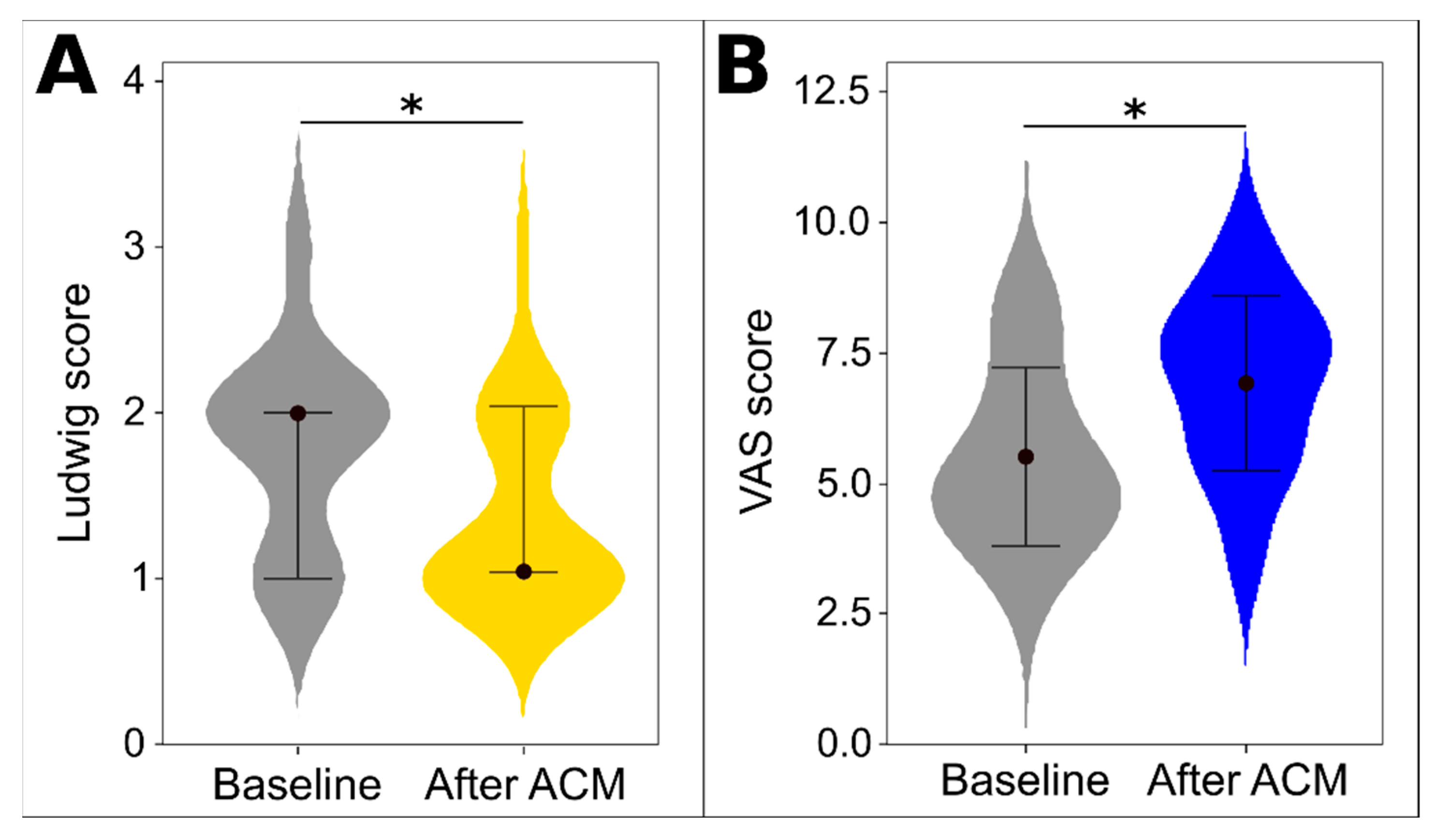
3.2. Evaluation of ACM Treatment Effectiveness
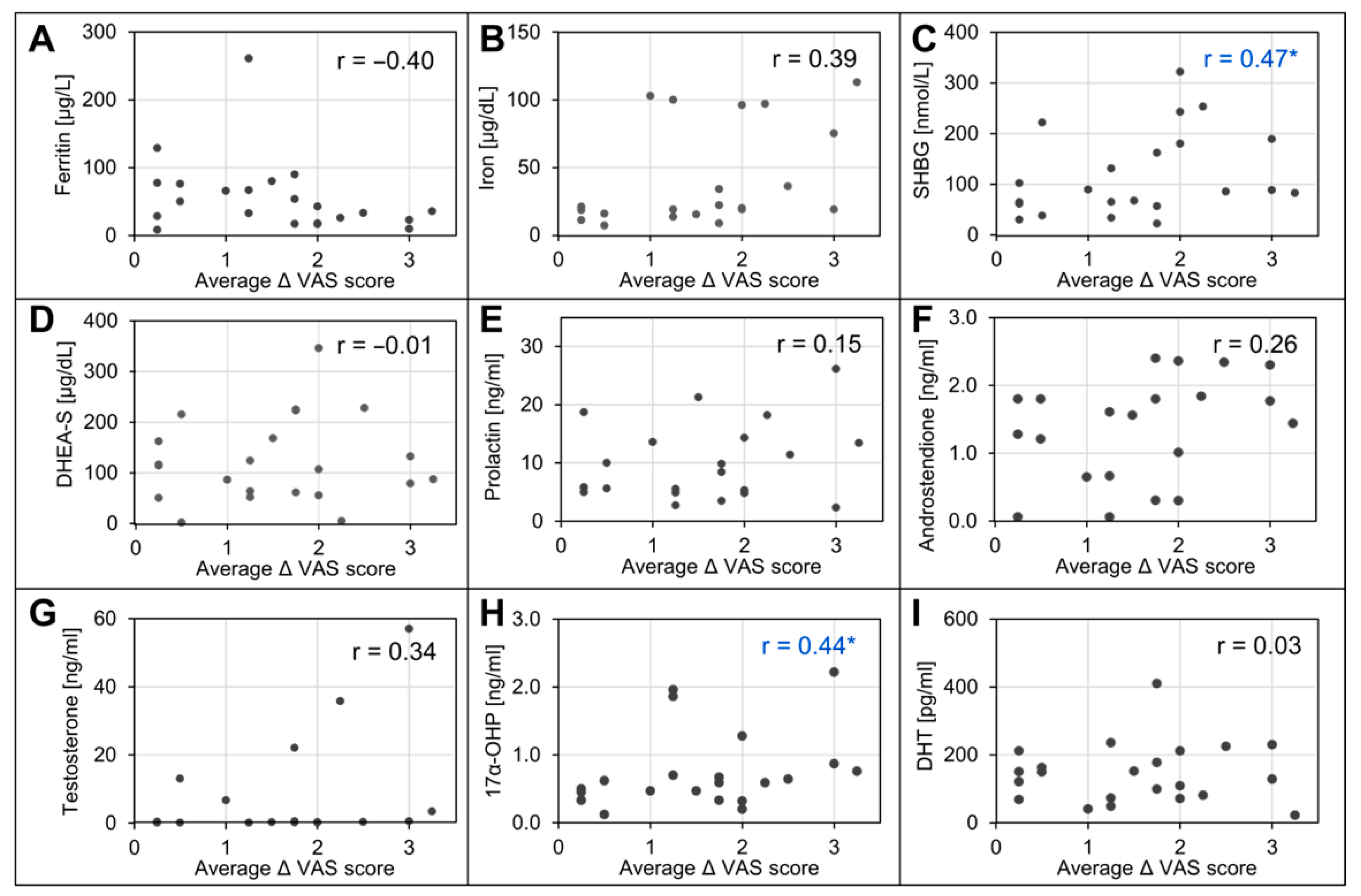
3.3. Associations between Outcomes and Baseline Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piraccini, B.M.; Alessandrini, A. Androgenetic alopecia. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2014, 149, 15–24. [Google Scholar]
- Deng, Y.; Wang, M.; He, Y.; Liu, F.; Chen, L.; Xiong, X. Cellular Senescence: Ageing and Androgenetic Alopecia. Dermatology 2023, 239, 533–541. [Google Scholar] [CrossRef]
- Küster, W.; Happle, R. The inheritance of common baldness: Two B or not two B? J. Am. Acad. Dermatol. 1984, 11, 921–926. [Google Scholar] [CrossRef]
- Price, V.H. Androgenetic Alopecia in Women. J. Investig. Dermatol. Symp. Proc. 2003, 8, 24–27. [Google Scholar] [CrossRef]
- Uno, H.; Cappas, A.; Schlagel, C. Cyclic dynamics of hair follicles and the effect of minoxidil on the bald scalps of stumptailed macaques. Am. J. Dermatopathol. 1985, 7, 283–297. [Google Scholar] [CrossRef]
- Kaufman, K.D. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol. Clin. 1996, 14, 697–711. [Google Scholar] [CrossRef]
- Messenger, A.G. The control of hair growth: An overview. J. Investig. Dermatol. 1993, 101, 4s–9s. [Google Scholar] [CrossRef]
- Sennett, R.; Rendl, M. Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef]
- Ceruti, J.M.; Oppenheimer, F.M.; Leirós, G.J.; Balañá, M.E. Androgens downregulate BMP2 impairing the inductive role of dermal papilla cells on hair follicle stem cells differentiation. Mol. Cell. Endocrinol. 2021, 520, 111096. [Google Scholar] [CrossRef]
- Akiyama, M.; Smith, L.T.; Shimizu, H. Changing Patterns of Localization of Putative Stem Cells in Developing Human Hair Follicles. J. Investig. Dermatol. 2000, 114, 321–327. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J. Dermatol. Sci. 2011, 61, 1–6. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527–539. [Google Scholar] [CrossRef]
- Zito, P.M.; Bistas, K.G.; Syed, K. Finasteride. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513329/ (accessed on 5 January 2023).
- Patel, P.; Nessel, T.A.; Kumar, D.D. Minoxidil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482378/ (accessed on 5 January 2023).
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef]
- Chien, W.Y.; Huang, H.M.; Kang, Y.N.; Chen, K.H.; Chen, C. Stem cell-derived conditioned medium for alopecia: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2024, 88, 182–192. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, Y.; Zhou, M.; Zhou, X.; Xie, Y.; Zeng, X.; Shao, F.; Zhang, C. Platelet-Rich Plasma for Androgenetic Alopecia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Cutan. Med. Surg. 2023, 27, 504–508. [Google Scholar] [CrossRef]
- Egger, A.; Tomic-Canic, M.; Tosti, A. Advances in Stem Cell-Based Therapy for Hair Loss. CellR4 Repair Replace Regen. Reprogram. 2020, 8, e2894. [Google Scholar]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Krefft-Trzciniecka, K.; Piętowska, Z.; Nowicka, D.; Szepietowski, J.C. Human Stem Cell Use in Androgenetic Alopecia: A Systematic Review. Cells 2023, 12, 951. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Cervelli, V.; Orlandi, A.; Garcovich, S. Autologous Micrografts from Scalp Tissue: Trichoscopic and Long-Term Clinical Evaluation in Male and Female Androgenetic Alopecia. BioMed Res. Int. 2020, 2020, 7397162. [Google Scholar] [CrossRef] [PubMed]
- Zari, S. Short-Term Efficacy of Autologous Cellular Micrografts in Male and Female Androgenetic Alopecia: A Retrospective Cohort Study. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1725–1736. [Google Scholar] [CrossRef]
- Chunmei, W.; Xian, H.; Yang, B.; Alkouz, Z. Clinical trial efficacy of autologous cellular micrografts in androgenic alopecia. Res. Sq. 2023. [Google Scholar] [CrossRef]
- What is Rigenera BHW? Available online: https://rigenerahbw.com/ (accessed on 5 January 2024).
- Marcarelli, M.; Trovato, L.; Novarese, E.; Riccio, M.; Graziano, A. Rigenera protocol in the treatment of surgical wound dehiscence. Int. Wound J. 2017, 14, 277–281. [Google Scholar] [CrossRef]
- Giaccone, M.; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A New Medical Device, Based on Rigenera Protocol, in the Management of Complex Wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 1013. [Google Scholar]
- Aliberti, F.; Paolin, E.; Benedetti, L.; Cusella, G.; Ceccarelli, G. 3D bioprinting and Rigenera® micrografting technology: A possible countermeasure for wound healing in spaceflight. Front. Bioeng. Biotechnol. 2022, 10, 937709. [Google Scholar] [CrossRef]
- Álvarez, X.; Valenzuela, M.; Tuffet, J. Clinical and Histological Evaluation of the Regenera® Method for the Treatment of Androgenetic Alopecia. Int. Educ. Appl. Sci. J. 2018, 3, 8–9. [Google Scholar]
- McCormack, H.M.; Horne, D.J.D.L.; Sheather, S. Clinical applications of visual analogue scales: A critical review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef]
- Gupta, M.; Mysore, V. Classifications of Patterned Hair Loss: A Review. J. Cutan. Aesthetic Surg. 2016, 9, 3–12. [Google Scholar] [CrossRef]
- Kasumagic-Halilovic, E. Trichoscopic Findings in Androgenetic Alopecia. Med. Arch. 2021, 75, 109–111. [Google Scholar] [CrossRef]
- Kumar, Y.; Bhatia, A.; Minz, R.W. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: A journey revisited. Diagn. Pathol. 2009, 4, 1. [Google Scholar] [CrossRef]
- Muro, Y. Antinuclear antibodies. Autoimmunity 2005, 38, 3–9. [Google Scholar] [CrossRef]
- Nosal, R.S.; Superville, S.S.; Amraei, R.; Varacallo, M. Biochemistry, Antinuclear Antibodies (ANA). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537071/ (accessed on 4 January 2023).
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Stem cells from human hair follicles: First mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017, 4, 58. [Google Scholar] [CrossRef]
- Ruiz, R.G.; Rosell, J.M.C.; Ceccarelli, G.; De Sio, C.; De Angelis, G.C.; Pinto, H.; Astarita, C.; Graziano, A. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. J. Cell. Physiol. 2020, 235, 4587–4593. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Wang, Y. Definition, Prevalence, and Risk Factors of Low Sex Hormone-Binding Globulin in US Adults. J. Clin. Endocrinol. Metab. 2021, 106, e3946–e3956. [Google Scholar] [CrossRef]
- Laurent, M.R.; Hammond, G.L.; Blokland, M.; Jardí, F.; Antonio, L.; Dubois, V.; Khalil, R.; Sterk, S.S.; Gielen, E.; Decallonne, B.; et al. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: Validation of the free hormone hypothesis. Sci. Rep. 2016, 6, 35539. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, Q.; Zhu, Q.; Zhu, J.; Du, X. Association Between Trichoscopic Features and Serum Hormone Levels and Vitamin D Concentration in Patients with Androgenetic Alopecia in Eastern China: A Cross-Sectional Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2547–2555. [Google Scholar] [CrossRef]
- Urysiak-Czubatka, I.; Kmieć, M.L.; Broniarczyk-Dyła, G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Postep. Dermatol. I Alergol. 2014, 31, 207–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Jing, J.; Wu, X.; Lv, Z. Serum Levels of Androgen-Associated Hormones Are Correlated with Curative Effect in Androgenic Alopecia in Young Men. Med. Sci. Monit. 2018, 24, 7770–7777. [Google Scholar] [CrossRef]
- Firooz, A.; Rajabi-Estarabadi, A.; Zartab, H.; Hassanzadeh, H.; Dowlati, Y. Classification and Scoring of Androgenetic Alopecia (Male and Female Pattern). In Agache’s Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants; Humbert, P., Fanian, F., Maibach, H.I., Agache, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1437–1442. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Azziz, R.; Rafi, A.; Smith, B.R.; Bradley, E.L.; Zacur, H.A. On the Origin of the Elevated 17-Hydroxyprogesterone Levels after Adrenal Stimulation in Hyperandrogenism. J. Clin. Endocrinol. Metab. 1990, 70, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Abdin, R.; Gaumond, S.I.; Issa, N.T.; Jimenez, J.J. Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1387–1406. [Google Scholar] [CrossRef] [PubMed]

| Variable | Format | Value | Min–Max Value |
|---|---|---|---|
| Age | Mean (SD) | 40.1 (12) | 25.0–68.0 |
| Vitamin D3 (ng/mL) | Mean (SD) | 38.4 (19) | 8.00–81.0 |
| Vitamin B12 (pg/mL) | Median (IQR) | 260 (181) | 123–763 |
| Ferritin (µg/L) | Median (IQR) | 43.0 (47) | 8.00–261 |
| Iron (µg/dL) | Median (IQR) | 20.4 (39) | 7.00–113 |
| Folic acid (ng/mL) | Median (IQR) | 5.40 (4.5) | 3.00–68.0 |
| TSH (µIU/mL) | Median (IQR) | 1.41 (1.0) | 0.00–3.00 |
| Anti-TPO (IU/mL) | No. over/under norm † | 1/22 | N/A |
| Anti-TG (IU/mL) | No. over/under norm ‡ | 1/22 | N/A |
| SHBG (nmol/L) | Median (IQR) | 85.4 (112) | 22.0–322 |
| ACTH (pg/mL) | Median (IQR) | 9.64 (6.9) | 1.00–35.0 |
| Cortisol (µg/L) | Median (IQR) | 11.4 (10) | 3.00–35.0 |
| DHEA-S (μg/dL) | Mean (SD) | 131 (91) | 1.00–346 |
| Prolactin (ng/mL) | Median (IQR) | 5.84 (8.5) | 2.00–26.0 |
| Androstenedione (ng/mL) | Mean (SD) | 1.43 (1) | 0.00–4.00 |
| Testosterone (ng/mL) | Median (IQR) | 0.30 (3.36) | 0.00–56.0 |
| Hb (g/dL) | Mean (SD) | 13.3 (0.9) | 11.0–14.0 |
| 17α-hydroxyprogesterone (ng/mL) | Median (IQR) | 0.59 (0.4) | 0.00–2.00 |
| DHT (pg/mL) | Median (IQR) | 134 (118) | 22.0–409 |
| ANA | No. positive/negative | 7/16 | N/A |
| Ludwig scale score | 1/2/3 | 7/14/2 | N/A |
| Variable | Format | After | p Value † | Value |
|---|---|---|---|---|
| Specialist 1 | 5 (2) | 7 (2) | <0.001 | 40.1 (12) |
| Specialist 2 | 6 (2.5) | 8 (2.5) | <0.001 | 38.4 (19) |
| Specialist 3 | 5 (2.5) | 7 (2) | <0.001 | 260 (181) |
| Specialist 4 | 5 (2) | 7 (2) | <0.001 | 43.0 (47) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krefft-Trzciniecka, K.; Piętowska, Z.; Pakiet, A.; Nowicka, D.; Szepietowski, J.C. Short-Term Clinical Assessment of Treating Female Androgenetic Alopecia with Autologous Stem Cells Derived from Human Hair Follicles. Biomedicines 2024, 12, 153. https://doi.org/10.3390/biomedicines12010153
Krefft-Trzciniecka K, Piętowska Z, Pakiet A, Nowicka D, Szepietowski JC. Short-Term Clinical Assessment of Treating Female Androgenetic Alopecia with Autologous Stem Cells Derived from Human Hair Follicles. Biomedicines. 2024; 12(1):153. https://doi.org/10.3390/biomedicines12010153
Chicago/Turabian StyleKrefft-Trzciniecka, Katarzyna, Zuzanna Piętowska, Alicja Pakiet, Danuta Nowicka, and Jacek C. Szepietowski. 2024. "Short-Term Clinical Assessment of Treating Female Androgenetic Alopecia with Autologous Stem Cells Derived from Human Hair Follicles" Biomedicines 12, no. 1: 153. https://doi.org/10.3390/biomedicines12010153
APA StyleKrefft-Trzciniecka, K., Piętowska, Z., Pakiet, A., Nowicka, D., & Szepietowski, J. C. (2024). Short-Term Clinical Assessment of Treating Female Androgenetic Alopecia with Autologous Stem Cells Derived from Human Hair Follicles. Biomedicines, 12(1), 153. https://doi.org/10.3390/biomedicines12010153







