Impact of Shunt Placement on CSF Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
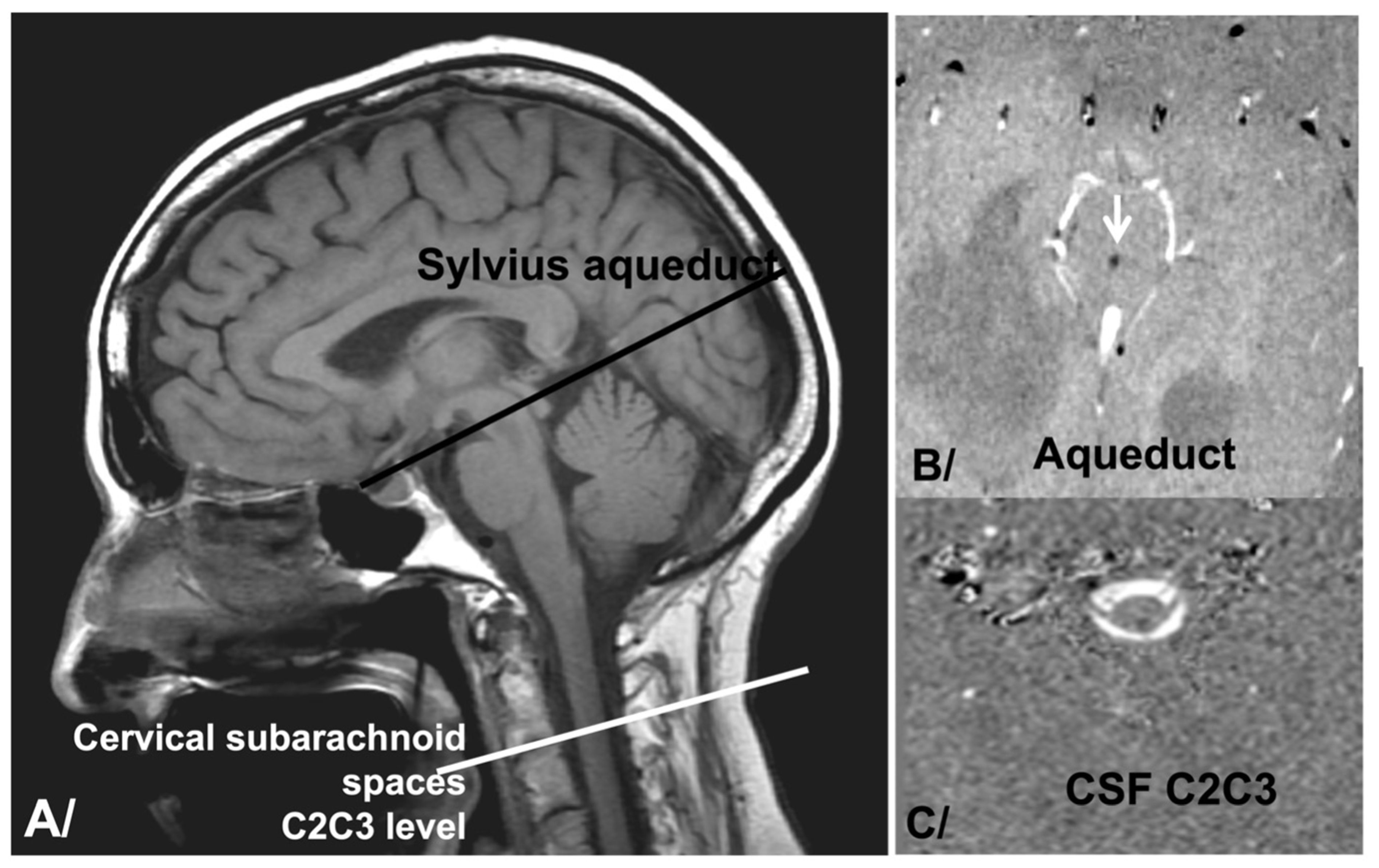
2.2. pcMRI Acquisition
2.3. Data Analysis
2.4. Statistical Analysis
2.5. IRB/Ethics
3. Results
3.1. CSF Dynamics before Shunting
3.2. CSF Dynamics Evolution after Shunt Placement
4. Discussion
4.1. Preoperative Intraventricular CSF Dynamics (Aqueductal Stroke Volume)
4.2. Preoperative Global CSF Dynamics (Aqueductal and Cervical Stroke Volumes)
4.3. Impact of Shunt Placement on Intraventricular CSF Dynamics
4.4. Impact of Shunt Placement on Cervical CSF Dynamics
4.5. Impact of Shunt Placement on Global Hydrodynamics
4.6. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anile, C.; De Bonis, P.; Albanese, A.; Di Chirico, A.; Mangiola, A.; Petrella, G.; Santini, P. Selection of patients with idiopathic normal-pressure hydrocephalus for shunt placement: A single-institution experience. J. Neurosurg. 2010, 113, 64–73. [Google Scholar] [CrossRef]
- Larsson, A.; Wikkelsö, C.; Bilting, M.; Stephensen, H. Clinical parameters in 74 consecutive patients shunt operated for normal pressure hydrocephalus. Acta Neurol. Scand. 1991, 84, 475–482. [Google Scholar] [CrossRef]
- Stein, S.C.; Langfitt, T.W. Normal-pressure hydrocephalus. Predicting the results of cerebrospinal fluid shunting. J. Neurosurg. 1974, 41, 463–470. [Google Scholar] [CrossRef]
- Hellström, P.; Klinge, P.; Tans, J.; Wikkelsø, C. A new scale for assessment of severity and outcome in iNPH. Acta Neurol. Scand. 2012, 126, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Klinge, P.; Marmarou, A.; Bergsneider, M.; Relkin, N.; Black, P.M. Outcome of shunting in idiopathic normal-pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery 2005, 57 (Suppl. S5), S40–S52. [Google Scholar] [CrossRef] [PubMed]
- Petrella, G.; Czosnyka, M.; Keong, N.; Pickard, J.D.; Czosnyka, Z. How does CSF dynamics change after shunting? Acta Neurol. Scand. 2008, 118, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.J.; Lee, S.H.; Loth, F.; Raksin, P.B.; Lichtor, T. MR-Intracranial Pressure (ICP): A method to measure intracranial elastance and pressure noninvasively by means of MR imaging: Baboon and human Study. Radiology 2000, 217, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Balédent, O.; Henry-Feugeas, M.C.; Idy-Peretti, I. Cerebrospinal fluid dynamics and relation with blood flow: A magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Investig. Radiol. 2001, 36, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Greitz, D.; Wirestam, R.; Franck, A.; Nordell, B.; Thomsen, C.; Ståhlberg, F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology 1992, 34, 370–380. [Google Scholar] [CrossRef]
- Bhadelia, R.A.; Bogdan, A.R.; Kaplan, R.F.; Wolpert, S.M. Cerebrospinal fluid pulsation amplitude and its quantitative relationship to cerebral blood flow pulsations: A phase-contrast MR flow imaging study. Neuroradiology 1997, 39, 258–264. [Google Scholar] [CrossRef]
- Enzmann, D.R.; Pelc, N.J. Cerebrospinal fluid flow measured by phase-contrast cine MR. Am. J. Neuroradiol. 1993, 14, 1301–1307; discussion 1309–1310. [Google Scholar] [PubMed]
- Balédent, O.; Gondry-Jouet, C.; Meyer, M.-E.; De Marco, G.; Le Gars, D.; Henry-Feugeas, M.-C.; Idy-Peretti, I. Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Investig. Radiol. 2004, 39, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G.; Scalzo, D.; Queralt, J.; Nitz, W.N.; Atkinson, D.J.; Wong, P. Normal-pressure hydrocephalus: Evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996, 198, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dixon, G.R.; Friedman, J.A.; Luetmer, P.H.; Quast, L.M.; McClelland, R.L.; Petersen, R.C.; Maher, C.O.; Ebersold, M.J. Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin. Proc. 2002, 77, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-U.; Huh, R.; Yun, P.-H.; Kim, D.-I. Quantitative assessment of cerebrospinal fluid hydrodynamics using a phase-contrast cine MR image in hydrocephalus. Child’s Nerv. Syst. 1999, 15, 461–467. [Google Scholar] [CrossRef]
- Scollato, A.; Tenenbaum, R.; Bahl, G.; Celerini, M.; Salani, B.; Di Lorenzo, N. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. Am. J. Neuroradiol. 2008, 29, 192–197. [Google Scholar] [CrossRef]
- Capel, C.; Owashi, K.; Peltier, J.; Balédent, O. Hydrodynamic and Hemodynamic Interactions in Chronic Hydrocephalus. Biomedicines 2023, 11, 2931. [Google Scholar] [CrossRef]
- Bader, C.; Cyrille, C.; Jadwiga, Z.; Joel, D.; Fichten, A.; Catherine, G.-J.; Roger, B.; Olivier, B. Estimation of the Lateral Ventricles Volumes from a 2D Image and Its Relationship with Cerebrospinal Fluid Flow. BioMed Res. Int. 2013, 2013, 215989. [Google Scholar] [CrossRef]
- Ringstad, G.; Emblem, K.; Geier, O.; Alperin, N.; Eide, P. Aqueductal Stroke Volume: Comparisons with Intracranial Pressure Scores in Idiopathic Normal Pressure Hydrocephalus. Am. J. Neuroradiol. 2015, 36, 1623–1630. [Google Scholar] [CrossRef]
- Chiang, W.W.B.; Takoudis, C.G.; Lee, S.H.; Weis-McNulty, A.; Glick, R.; Alperin, N. Relationship between ventricular morphology and aqueductal cerebrospinal fluid flow in healthy and communicating hydrocephalus. Investig. Radiol. 2009, 44, 192–199. [Google Scholar] [CrossRef]
- Bradley, W.G. Cerebrospinal Fluid Dynamics and Shunt Responsiveness in Patients with Normal-Pressure Hydrocephalus. Mayo Clin. Proc. 2002, 77, 507–508. [Google Scholar] [CrossRef]
- Greitz, D. Radiological assessment of hydrocephalus: New theories and implications for therapy. Neurosurg. Rev. 2004, 27, 145–165; discussion 166–167. [Google Scholar] [CrossRef] [PubMed]
- Luetmer, P.H.; Huston, J.; Friedman, J.A.; Dixon, G.R.; Petersen, R.C.; Jack, C.R.; McClelland, R.L.; Ebersold, M.J. Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: Technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2002, 50, 534–543; discussion 543–544. [Google Scholar] [PubMed]
- Kahlon, B.; Annertz, M.; Ståhlberg, F.; Rehncrona, S. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery 2007, 60, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A. Toward a better understanding of normal pressure hydrocephalus. Am. J. Neuroradiol. 2001, 22, 596. [Google Scholar]
- Hill, M.E.; Lougheed, W.M.; Barnett, H.J. A treatable form of dementia due to normal-pressure, communicating hydrocephalus. Can. Med. Assoc. J. 1967, 97, 1309–1320. [Google Scholar] [PubMed]
- Scollato, A.; Gallina, P.; Gautam, B.; Pellicanò, G.; Cavallini, C.; Tenenbaum, R.; Di Lorenzo, N. Changes in aqueductal CSF stroke volume in shunted patients with idiopathic normal-pressure hydrocephalus. Am. J. Neuroradiol. 2009, 30, 1580–1586. [Google Scholar] [CrossRef]
- Egeler-Peerdeman, S.M.; Barkhof, F.; Walchenbach, R.; Valk, J. Cine phase-contrast MR imaging in normal pressure hydrocephalus patients: Relation to surgical outcome. Acta Neurochir. Suppl. 1998, 71, 340–342. [Google Scholar]
- Bradley, W.G. MR prediction of shunt response in NPH: CSF morphology versus physiology. Am. J. Neuroradiol. 1998, 19, 1285–1286. [Google Scholar]
- Boon, A.J.W.; Tans, J.T.J.; Delwel, E.J.; Egeler-Peerdeman, S.M.; Hanlo, P.W.; Wurzer, H.A.L.; Avezaat, C.J.J.; de Jong, D.A.; Gooskens, R.H.J.M.; Hermans, J. Dutch Normal-Pressure Hydrocephalus Study: Prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J. Neurosurg. 1997, 87, 687–693. [Google Scholar] [CrossRef]
- Malm, J.; Kristensen, B.; Karlsson, T.; Fagerlund, M.; Elfverson, J.; Ekstedt, J. The predictive value of cerebrospinal fluid dynamic tests in patients with the idiopathic adult hydrocephalus syndrome. Arch. Neurol. 1995, 52, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.; Metanbou, S.; Gondry-Jouet, C.; Balédent, O. Extracranial versus intracranial hydro-hemodynamics during aging: A PC-MRI pilot cross-sectional study. Fluids Barriers CNS 2020, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Tipton, P.W.; Elder, B.D.; Cogswell, P.M.; Graff-Radford, N. Normal pressure hydrocephalus, or Hakim syndrome: Review and update. Neurol. Neurochir. Pol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A. Vascular Compliance in Normal Pressure Hydrocephalus. Am. J. Neuroradiol. 2000, 21, 1574–1585. [Google Scholar]
- Czosnyka, M.; Pickard, J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Eide, P.K.; Sorteberg, W. Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: A 6-year review of 214 patients. Neurosurgery 2010, 66, 80–91. [Google Scholar] [CrossRef]
- Marmarou, A.; Bergsneider, M.; Klinge, P.; Relkin, N.; Black, P.M. The Value of Supplemental Prognostic Tests for the Preoperative Assessment of Idiopathic Normal-pressure Hydrocephalus. Neurosurgery 2005, 57, S2–S17. [Google Scholar] [CrossRef]



| Preoperative PCMRI (T1) | PCMRI 6 Months after Surgery (T2) | PCMRI 12 Months after Surgery (T3) | p (Comparison of T1 and T2) | p (Comparison of T2 and T3) | p (Comparison of T1 and T3) | |
|---|---|---|---|---|---|---|
| SVAQU | 240 ± 114 μL/cc | 214 ± 157 μL/cc | 193 ± 145 μL/cc | 0.003 | 0.12 | 0.001 |
| SVCERV | 627 ± 229 μL/cc | 557 ± 234 μL/cc | 496 ± 234 μL/cc | 0.007 | 0.001 | 0.001 |
| CSFRATIO | 40 ± 20% | 40 ± 27% | 42 ± 32% | 0.52 | 0.09 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capel, C.; Owashi, K.; Metanbou, S.; Peltier, J.; Balédent, O. Impact of Shunt Placement on CSF Dynamics. Biomedicines 2024, 12, 20. https://doi.org/10.3390/biomedicines12010020
Capel C, Owashi K, Metanbou S, Peltier J, Balédent O. Impact of Shunt Placement on CSF Dynamics. Biomedicines. 2024; 12(1):20. https://doi.org/10.3390/biomedicines12010020
Chicago/Turabian StyleCapel, Cyrille, Kimi Owashi, Serge Metanbou, Johann Peltier, and Olivier Balédent. 2024. "Impact of Shunt Placement on CSF Dynamics" Biomedicines 12, no. 1: 20. https://doi.org/10.3390/biomedicines12010020
APA StyleCapel, C., Owashi, K., Metanbou, S., Peltier, J., & Balédent, O. (2024). Impact of Shunt Placement on CSF Dynamics. Biomedicines, 12(1), 20. https://doi.org/10.3390/biomedicines12010020





