Beneficial Effect of Faecal Microbiota Transplantation on Mild, Moderate and Severe Dextran Sodium Sulphate-Induced Ulcerative Colitis in a Pseudo Germ-Free Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Housing Conditions in Gnoto-Facilities and Feed
2.2. Pseudo Germ-Free Modelling of Animals
2.3. Obtaining the Animal Model with Induced Acute Ulcerative Colitis
2.4. Selection of Optimum Human Donor for FMT and Its Processing
2.4.1. FMT Donor Screening
2.4.2. Processing of the Faecal Microbiota Transplant
2.5. Evaluation of Clinical Colitis
2.6. Hematological Analysis
2.7. Microbiological Analysis
2.7.1. Microbiological Cultivation
2.7.2. Viability of Microorganisms in the Caecum
2.7.3. Identification of Cultivable Bacteria
2.7.4. Detection of Bacterial Microbiota Composition Based on NGS Amplicon Sequencing
2.7.5. PCR of Multiplex Protocol for Identification of Genes Encoding Factors of Pathogenicity of Escherichia coli
2.8. Histological and Immunohistochemical Analysis
Evaluation of Histopathological Finding
2.9. RNA Extraction, cDNA Synthesis and Real-Time RT-PCR
2.10. Statistical Analysis
3. Results
3.1. Next-Generation Sequencing (NGS) Analysis of Microbiological Composition of Donor′s FMT
3.2. Bacterial Composition of Faeces of Conventional SPF Mice
3.3. Antibiotic Treatment of Animals Negatively Affected the Viability of Caecal Microbiota
3.4. Clinical Evaluation of the Effects of FMT on Acute Colitis
3.4.1. FMT Alleviates Rectal Bleeding of Animals
3.4.2. FMT Administration Decreases Total Loss of Weight of Mice Following DSS-Induced Colitis
3.4.3. The Effect of FMT on Adjustment of DAI
3.4.4. FMT Causes Adjustment in the Pathological–Anatomical Findings of Mice Following DSS-Induced Colitis
3.5. Effect of FMT on Haematological Parameters of Mice with DSS-Induced Acute Colitis
3.5.1. Haematological Parameters in PGF Animal Model with Induced Acute UC
3.5.2. Positive Effect of FMT on Recovery of Haematological Parameters in Mice with DSS-Induced Acute Colitis
3.6. Effect of FMT Treatment in the Model of Acute Ulcerative Colitis from the Point of View of Light Microscopy and Histological Activity Index (HAI) of the Disease
3.6.1. Histopathological Features of DSS-Induced Acute Ulcerative Colitis of the Colon
3.6.2. FMT Alters Histological Activity Index of the Disease
3.7. FMT Modulates Selective Immunohistochemical Markers Associated with Ulcerative Colitis
3.7.1. FMT Modulates the Expression of PCNA Marker
3.7.2. FMT Modulates Expression of Anti-Apoptotic Marker Bcl-xL
3.7.3. FMT Modulates Expression of Pro-Inflammatory COX2 Marker
3.7.4. FMT Modulates Expression of Pro-Inflammatory iNOS Marker
3.8. FMT Modulated Gene Expression of Cytokines in PGF Animal Model with Induced Acute UC
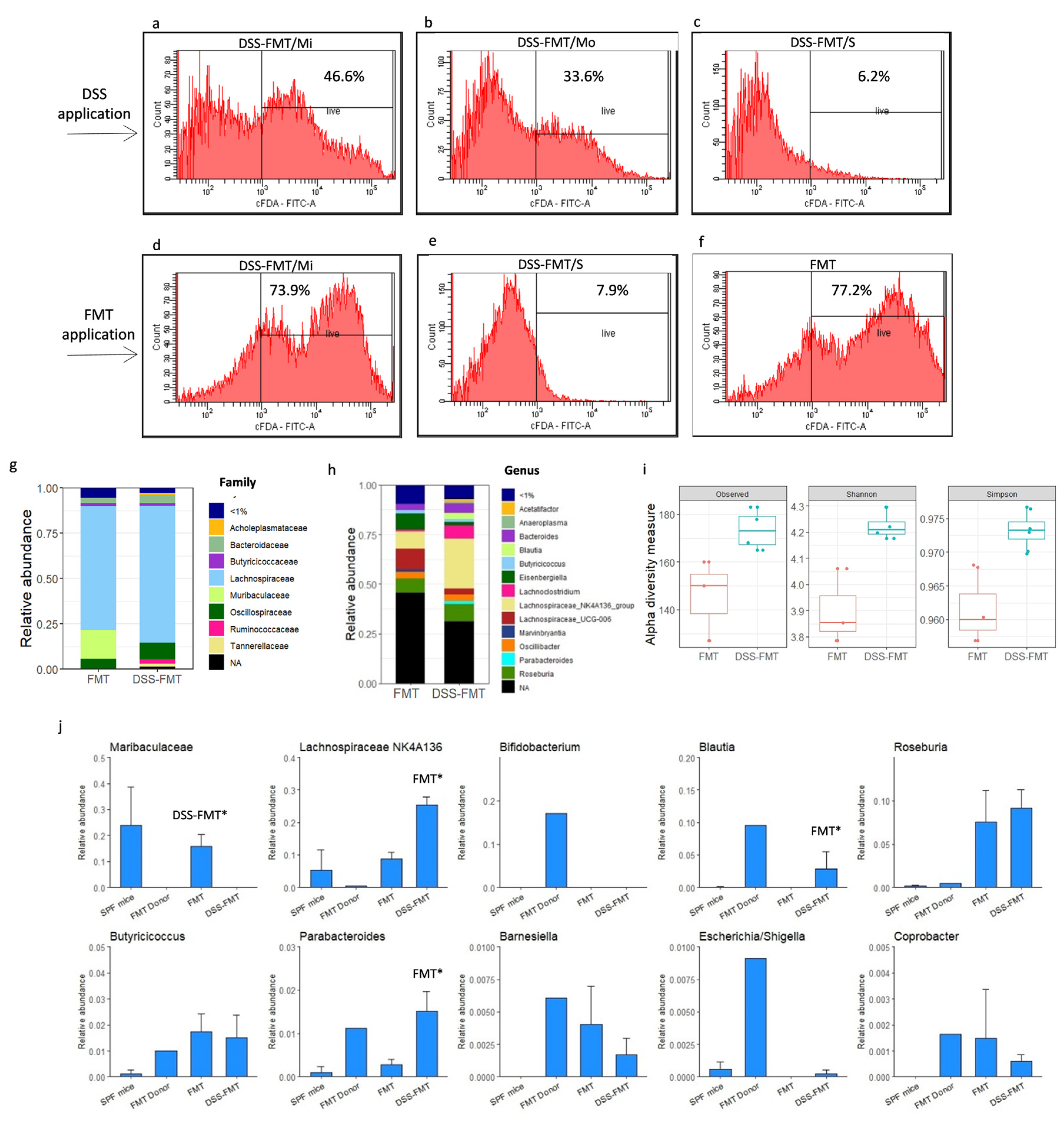
3.9. Effect of FMT on Viability of Caecal Microbiota
3.10. Effect of FMT on Composition of Caecal Microbiota in Acute UC Murine Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Wan, P.; Peng, Y.; Chen, G.; Xie, M.; Dai, Z.; Huang, K.; Dong, W.; Zeng, X.; Sun, Y. Modulation of gut microbiota by Ilex kudingcha improves dextran sulfate sodium-induced colitis. Food Res. Int. 2019, 126, 108595. [Google Scholar] [CrossRef]
- Fang, X.; Monk, J.M.; Mih, N.; Du, B.; Sastry, A.V.; Kavvas, E.; Seif, Y.; Smarr, L.; Palsson, B.O. Escherichia coli B2 strains prevalent in inflammatory bowel disease patients have distinct metabolic capabilities that enable colonization of intestinal mucosa. BMC Syst. Biol. 2018, 12, 66. [Google Scholar] [CrossRef]
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Condello Oliván, C.M.; Shiri-Sverdlov, R. Inflammatory bowel disease: A stressed “gut/feeling”. Cells 2019, 8, 659. [Google Scholar] [CrossRef]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B increases intestinal tight junction permeability by up-regulation of MIR200C-3p, which degrades occludin mRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef]
- Rosen, C.E.; Palm, N.W. Navigating the microbiota seas: Triangulation finds a way forward. Cell Host Microbe 2018, 23, 1–3. [Google Scholar] [CrossRef]
- Knox, N.C.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiome as a target for IBD treatment: Are we there yet. Curr. Treat. Options Gastroenterol. 2019, 17, 115–126. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, G.; Li, B.; Du, X.; Sun, Z.; Sun, Y.; Jiang, X. Fecal microbiota transplantation (FMT) alleviates experimental colitis in mice by gut microbiota regulation. J. Microbiol. Biotechnol. 2020, 30, 1132–1141. [Google Scholar] [CrossRef]
- Schierova, D.; Brezina, J.; Mrazek, J.; Fliegerova, K.O.; Kvasnova, S.; Bajer, L.; Drastich, P. Gut microbiome changes in patients with active left-sided ulcerative colitis after fecal microbiome transplantation and topical 5-aminosalicylic acid therapy. Cells 2020, 9, 2283. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Graef, F.A.; Healey, G.R.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Cianci, R.; Bibbò, S.; Gasbarrini, A.; Currò, D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol. Ther. 2015, 149, 191–212. [Google Scholar] [CrossRef]
- Gupta, A.; Saha, S.; Khanna, S. Therapies to modulate gut microbiota: Past, present and future. World J. Gastroenterol. 2020, 26, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Vindigni, S.M.; Surawicz, C.M. Fecal microbiota transplantation. Gastroenterol. Clin. North Am. 2017, 46, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; Feng, B.; Chen, D.; Ren, J.; Deng, M.; et al. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Tan, P.; Li, X.; Shen, J.; Feng, Q. Fecal microbiota transplantation for the treatment of inflammatory bowel disease: An update. Front. Pharmacol. 2020, 11, 574533. [Google Scholar] [CrossRef]
- Haifer, C.; Leong, R.W.; Paramsothy, S. The role of faecal microbiota transplantation in the treatment of inflammatory bowel disease. Curr. Opin. Pharmacol. 2020, 55, 8–16. [Google Scholar] [CrossRef]
- Leung, P.C.; Cheng, K.F. Fecal microbiota transplantation: Historical review and current perspective. World J. Meta Anal. 2019, 7, 423–427. [Google Scholar] [CrossRef]
- Goldenberg, S.D.; Merrick, B. The role of faecal microbiota transplantation: Looking beyond Clostridioides difficile infection. Ther. Adv. Infect. Dis. 2021, 8, 2049936120981526. [Google Scholar]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.J.; Campbell, J. Fecal microbiota transplantation: Current status and future directions. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Gasbarrini, A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: A systematic review. J. Clin. Gastroenterol. 2014, 48, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Löwenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Therap. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.M.; Rank, K.M.; Vaughn, B.P.; Khoruts, A. Treatment of recurrent Clostridium difficile infection using fecal microbiota transplantation in patients with inflammatory bowel disease. Gut Microbes 2017, 8, 303–309. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. J. Am. Med. Assoc. 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Gancarcikova, S.; Popper, M.; Hrckova, G.; Madar, M.; Mudronova, D.; Sopkova, D.; Nemcova, R. Antibiotic-treated SPF mice as a gnotobiotic model. In Antibiotic Use in Animals; Savic, S., Ed.; InTech: Rijeka, Croatia, 2018; pp. 45–83. [Google Scholar]
- Gancarcikova, S.; Lauko, S.; Hrckova, G.; Andrejcakova, Z.; Hajduckova, V.; Madar, M.; Kolesar Fecskeova, L.; Mudronova, D.; Mravcova, K.; Strkolcova, G.; et al. Innovative animal model of DSS-induced ulcerative colitis in pseudo germ-free mice. Cells 2020, 9, 2571. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 9 September 2023).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O‘Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R8project.org/package=vegan (accessed on 9 September 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Smolen, A.J. Image Analytic Techniques for Quantification of Immunocytochemical Staining in the Nervous System; Methods in Neurosciences; Conn, P.M., Ed.; Academic Press: New York, NY, USA, 1990; pp. 208–229. [Google Scholar]
- Viennois, E.; Chen, F.; Laroui, H.; Baker, M.T.; Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: Lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res. Notes 2013, 6, 360. [Google Scholar] [CrossRef]
- Aira, A.; Arajol, C.; Casals-Pascual, C.; González-Suárez, B.; Martí, S.; Domínguez, M.Á.; Guardiola, J.; Soriano, Á. Recommendations for stool donor selection for fecal microbiota transplant. Consensus document endorsed by the Catalan Society of Digestology, Catalan Society of Infectious diseases and Clinical Microbiology and the GEMBIOTA group from Spanish Society of Infectious Diseases and Clinical Microbiology. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2022, 40, 142–146. [Google Scholar]
- Antushevich, H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta 2020, 503, 90–98. [Google Scholar] [CrossRef]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Fouhy, F.; Deane, J.; Rea, M.C.; O’Sullivan, Ó.; Ross, R.P.; O’Callaghan, G.; Plant, B.J.; Stanton, C. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PLoS ONE 2015, 10, e0119355. [Google Scholar] [CrossRef] [PubMed]
- Papanicolas, L.E.; Choo, J.M.; Wang, Y.; Leong, L.E.X.; Costello, S.P.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Bacterial viability in faecal transplants: Which bacteria survive. EBioMedicine 2019, 41, 509–516. [Google Scholar] [CrossRef] [PubMed]
- O´Reilly, S. Transplantation of Faecal Microbiota—Alternative Method of Therapy of Ulcerous Colitis. Ph.D. Thesis, The Universtity of Veterinary Medicine and Pharmacy in Kosice, Kosice, Slovakia, 2022. [Google Scholar]
- Perše, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin, C.; Xiaojun, Y. Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Yu, H.; Ge, Y.; Liu, Y.; Qin, X.; Jiang, M.; Wang, X. The functional role of fecal microbiota transplantation on dextran sulfate sodium-induced colitis in mice. Front. Cell Infect. Microbiol. 2019, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, H.G.; Zhang, M.N.; Zhang, M.H.; Wang, H.; Yang, X.Z. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J. Gastroenterol. 2021, 27, 2834–2849. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lin, S.; Yang, Y.; Gong, X.; Tong, J.; Li, K.; Li, Y. Histological and ultrastructural changes of the colon in dextran sodium sulfate-induced mouse colitis. Exp. Ther. Med. 2020, 20, 1987–1994. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef]
- Faixova, D. Study of Effect of Natural Origin Compounds of with Imunomodulatory Effect on the Model Organisms. Ph.D. Thesis, The Universtity of Veterinary Medicine and Pharmacy in Kosice, Kosice, Slovakia, 2021. [Google Scholar]
- Zhou, J.; Zhou, Z.; Ji, P.; Ma, M.; Guo, J.; Jiang, S. Effect of fecal microbiota transplantation on experimental colitis in mice. Exp. Ther. Med. 2019, 17, 2581–2586. [Google Scholar] [CrossRef]
- Xu, H.M.; Huang, H.L.; Xu, J.; He, J.; Zhao, C.; Peng, Y.; Zhao, H.L.; Huang, W.Q.; Cao, C.Y.; Zhou, Y.J.; et al. Cross-talk between butyric acid and gut microbiota in ulcerative colitis following fecal microbiota transplantation. Front. Microbiol. 2021, 12, 658292. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019, 42, 869–880. [Google Scholar] [CrossRef]
- Van Dijk, A.J.; Enthoven, P.M.; Van den Hoven, S.G.; Van Laarhoven, M.M.; Niewold, T.A.; Nabuurs, M.J.; Beynen, A.C. The effect of dietary spray-dried porcine plasma on clinical response in weaned piglets challenged with a pathogenic Escherichia coli. Vet. Microbiol. 2002, 84, 207–218. [Google Scholar] [CrossRef]
- Quigley, E.M. Leaky gut—Concept or clinical entity. Curr. Opin. Gastroenterol. 2016, 32, 74–79. [Google Scholar] [CrossRef]
- Wang, A.; Keita, Å.V.; Phan, V.; McKay, C.M.; Schoultz, I.; Lee, J.; Murphy, M.P.; Fernando, M.; Ronaghan, N.; Balce, D.; et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am. J. Pathol. 2014, 184, 2516–2527. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Nakase, H. OPeNing the epithelial barrier: Osteopontin preserves gut barrier function during intestinal inflammation. Dig. Dis. Sci. 2019, 64, 294–296. [Google Scholar] [CrossRef]
- Elgamrani, Y.; Errami, A.A.; Krati, K. A rare association: Celiac disease and ulcerative colitis. J. Med. Diagn. Meth. 2016, 5, 3. [Google Scholar]
- Girbovan, A.; Sur, G.; Samasca, G.; Lupan, I. Dysbiosis a risk factor for celiac disease. Med. Microbiol. Immunol. 2017, 206, 83–91. [Google Scholar] [CrossRef]
- Shah, A.; Walker, M.; Burger, D.; Martin, N.; von Wulffen, M.; Koloski, N.; Jones, M.; Talley, N.J.; Holtmann, G.J. Link between celiac disease and inflammatory bowel disease. J. Clin. Gastroenterol. 2019, 53, 514–522. [Google Scholar] [CrossRef]
- Perathoner, A.; Kogler, P.; Denecke, C.; Pratschke, J.; Kafka-Ritsch, R.; Zitt, M. Enterolithiasis-associated ileus in Crohn’s disease. World J. Gastroenterol. 2012, 18, 6160–6163. [Google Scholar] [CrossRef]
- Brinkman, D.J.; Ten Hove, A.S.; Vervoordeldonk, M.J.; Luyer, M.D.; de Jonge, W.J. Neuroimmune interactions in the gut and their significance for intestinal immunity. Cells 2019, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef] [PubMed]
- Strowski, M.Z.; Wiedenmann, B. Probiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseases. Gut 2009, 58, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- De Leon, L.M.; Watson, J.B.; Kelly, C.R. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2013, 11, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Quera, R.; Espinoza, R.; Estay, C.; Rivera, D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J. Crohns Colitis 2014, 8, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Xu, B.; Wang, X.; Zhang, Y.; Wang, H.; Kong, X.; Zhu, H.; Wu, K. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 245–251. [Google Scholar] [CrossRef]
- Fujimoto, T.; Imaeda, H.; Takahashi, K.; Kasumi, E.; Bamba, S.; Fujiyama, Y.; Andoh, A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J. Gastroenterol. Hepatol. 2013, 28, 613–619. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn‘s disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Li, J.; Butcher, J.; Mack, D.; Stintzi, A. Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 139–153. [Google Scholar] [CrossRef]
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm forming Lactobacillus: New challenges for the development of probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef]
- Alagón Fernández Del Campo, P.; De Orta Pando, A.; Straface, J.I.; López Vega, J.R.; Toledo Plata, D.; Niezen Lugo, S.F.; Alvarez Hernández, D.; Barrientos Fortes, T.; Gutiérrez-Kobeh, L.; Solano-Gálvez, S.G.; et al. The use of probiotic therapy to modulate the gut microbiota and dendritic cell responses in inflammatory bowel diseases. Med. Sci. 2019, 7, 33. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core altered microorganisms in colitis mouse model: A comprehensive time-point and fecal microbiota transplantation analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Vermeiren, J.; Van den Abbeele, P.; Laukens, D.; Vigsnaes, L.K.; De Vos, M.; Boon, N.; Van de Wiele, T. Decreased colonization of fecal Clostridium coccoides/Eubacterium rectale species from ulcerative colitis patients in an in vitro dynamic gut model with mucin environment. FEMS Microbiol. Ecol. 2012, 79, 685–696. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhou, R.; Ng, S.C.; Li, J.; Huang, M.; Zhou, F.; Wang, X.; Shen, B.; Kamm, M.A.; et al. Characteristics of fecal and mucosa-associated microbiota in Chinese patients with inflammatory bowel disease. Medicine 2014, 93, e51. [Google Scholar] [CrossRef]
- Lauko, S. The Use of Gnotobiotic Animals in Research of Targeted Modulation of Intestinal Ecosystem in Orevention and Treatment of Inflammatory Bowel Diseases (IBD). Ph.D. Thesis, The Universtity of Veterinary Medicine and Pharmacy in Kosice, Kosice, Slovakia, 2022. [Google Scholar]








| Score | Weight Loss | Stool Consistency | Bleeding | Maximum Score |
|---|---|---|---|---|
| 0 1 | No weight loss 5–10% | Formed Mild soft | No bleeding Few blood-tinged stools | 10 |
| 2 3 | 11–15% 16–20% | Very soft Watery stool | Slight bleeding Gross bleeding | |
| 4 | >20% | – | – |
| Grade (Score) | Epithelial Erosion | Crypt Damage | Infiltration of Inflammatory Cells | Depletion/Loss of Goblet Cell Mucin-Positive Area |
|---|---|---|---|---|
| 0 | Morphologically normal | None | Absence of infiltrate | None |
| 1 | Focal destruction | Some crypt damage, spaces between crypts | Infiltrate at the subepithelial and lamina propria | Minimal (<20%) |
| 2 | Zonal destruction | Large spaces between crypts | Infiltrate reaches muscularis mucosae | Mild (21–35%) |
| 3 | Diffuse and mucosal ulcerations | Large spaces without crypts, surrounded by normal crypts | Severe and extensive infiltrate reaching submocosa and involving muscularis propria | Moderate (36–50%) |
| 4 | – | No crypts | – | Marked (>50%) |
| Forms | Score | ||
|---|---|---|---|
| Mild (Mi) | Moderate (Mo) | Severe (S) | |
| Weight loss Bleeding | 0–0.3 0–1.25 | 0.3–0.5 1.0–1.5 | 0.5–1.0 1.5–2.0 |
| Group | FMT (n = 18) | DSS-FMT/Mi (n = 6) | DSS-FMT/Mo (n = 9) | DSS-FMT/S (n = 12) | Ref BALB/c | |
|---|---|---|---|---|---|---|
| after DSS | WBC (×109/L) | 3.18 ± 0.15 | 6.26 ± 0.03, *** FMT, Y | 8.28 ± 0.77 *** FMT | 9.92 ± 3.12 * FMT | 3.59–6.40 |
| after FMT | WBC (×109/L) | 3.28 ± 0.28 | 5.75 ± 0.05 ** FMT, Y | 6.96 ± 0.76 *** FMT | 7.30 ± 0.23 * Mi, *** FMT | |
| DSS | Ly (×109/L) | 2.24 ± 0.01 | 4.20 ± 0.17 *** FMT | 5.30 ± 0.24 * Mi, *** FMT | 6.86 ± 1.91 * FMT | 2.29–3.59 |
| FMT | Ly (×109/L) | 2.03 ± 0.18 | 3.90 ± 0.05 ** FMT | 4.90 ± 1.00 | 3.85 ± 0.54 *** FMT | |
| DSS | Mo (×109/L) | 0.28 ± 0.03 | 0.63 ± 0.08 ** FMT | 0.71 ± 0.14 ** FMT, X | 0.26 ± 0.08 * Mi, X | 0.06–0.62 |
| FMT | Mo (×109/L) | 0.30 ± 0.03 | 0.45 ± 0.15 | 0.33 ± 0.03 X | 0.83 ± 0.17 * Mo, ** FMT, X | |
| DSS | Gran (×109/L) | 0.83 ± 0.08 | 1.43 ± 0.06 ** FMT | 2.42 ± 0.56 ** FMT | 2.26 ± 0.96 | 0.74–1.78 |
| FMT | Gran (×109/L) | 0.95 ± 0.12 | 1.40 ± 0.10 | 1.73 ± 0.28 * FMT | 2.95 ± 0.68 ** FMT | |
| DSS | RBC (×1012/L) | 9.53 ± 0.07 | 9.56 ± 0.17 | 10.51 ± 0.16 ** Mi, *** FMT | 7.69 ± 0.89 ** Mi, *** Mo, FMT | 8.16–9.98 |
| FMT | RBC (×1012/L) | 9.41 ± 0.10 | 9.34 ± 0.10 | 10.34 ± 0.30 ** Mi, FMT | 6.41 ± 0.53 *** Mo, Mi, FMT | |
| DSS | HGB (g/dL) | 15.26 ± 0.22 | 15.28 ± 0.32 | 17.09 ± 0.23 ** Mi, *** FMT | 11.53 ± 0.43 *** Mi, Mo, FMT | 12.4–15.4 |
| FMT | HGB (g/dL) | 15.24 ± 0.21 | 15.02 ± 0.20 | 16.66 ± 0.37 ** FMT, Mi | 12.01 ± 0.62 ** Mi, *** FMT, Mo | |
| DSS | HCT (%) | 46.35 ± 0.29 | 47.00 ± 1.02 | 58.10 ± 2.17 ** Mi, *** FMT | 39.83 ± 1.07 ** Mi,*** Mo, FMT | 43.5–55.4 |
| FMT | HCT (%) | 44.38 ± 1.62 | 44.73 ± 0.78 | 55.90 ± 2.41 ** FMT, Mi | 36.54 ± 1.75 * FMT, ** Mi, *** Mo | |
| DSS | PLT (×109/L) | 650.5 ± 51.05 | 1192.00 ± 70.41 *** FMT | 1565 ± 32.89 *** FMT, Mi, Y | 2048 ± 323.00 *** FMT,** Mi, * Mo | 600–960 |
| FMT | PLT (×109/L) | 791.50 ± 18.75 | 1108 ± 178.40 ** FMT | 1210 ± 83.49 *** FMT, Y | 1607 ± 232.7 *** FMT | |
| DSS | MCV (fL) | 48.82 ± 0.51 | 48.65 ± 0.28 | 53.36 ± 0.64 *** FMT, Mi | 51.90 ± 1.06 * Mi, FMT, | 50.8–55.6 |
| FMT | MCV (fL) | 50.01 ± 0.64 | 47.88 ± 0.41 | 54.32 ± 2.36 * FMT, Mi | 53.41 ± 0.21 *** Mi, FMT | |
| DSS | MCH (pg) | 15.52 ± 0.24 | 15.73 ± 0.13 | 16.35 ± 0.08 ** Mi, *** FMT | 16.00 ± 0.40 | 13–15.5 |
| FMT | MCH (pg) | 15.94 ± 0.09 | 16.20 ± 0.10 | 16.88 ± 0.31 ** FMT | 16.88 ± 0.61 ** FMT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauko, S.; Gancarcikova, S.; Hrckova, G.; Hajduckova, V.; Andrejcakova, Z.; Fecskeova, L.K.; Bertkova, I.; Hijova, E.; Kamlarova, A.; Janicko, M.; et al. Beneficial Effect of Faecal Microbiota Transplantation on Mild, Moderate and Severe Dextran Sodium Sulphate-Induced Ulcerative Colitis in a Pseudo Germ-Free Animal Model. Biomedicines 2024, 12, 43. https://doi.org/10.3390/biomedicines12010043
Lauko S, Gancarcikova S, Hrckova G, Hajduckova V, Andrejcakova Z, Fecskeova LK, Bertkova I, Hijova E, Kamlarova A, Janicko M, et al. Beneficial Effect of Faecal Microbiota Transplantation on Mild, Moderate and Severe Dextran Sodium Sulphate-Induced Ulcerative Colitis in a Pseudo Germ-Free Animal Model. Biomedicines. 2024; 12(1):43. https://doi.org/10.3390/biomedicines12010043
Chicago/Turabian StyleLauko, Stanislav, Sona Gancarcikova, Gabriela Hrckova, Vanda Hajduckova, Zuzana Andrejcakova, Livia Kolesar Fecskeova, Izabela Bertkova, Emilia Hijova, Anna Kamlarova, Martin Janicko, and et al. 2024. "Beneficial Effect of Faecal Microbiota Transplantation on Mild, Moderate and Severe Dextran Sodium Sulphate-Induced Ulcerative Colitis in a Pseudo Germ-Free Animal Model" Biomedicines 12, no. 1: 43. https://doi.org/10.3390/biomedicines12010043







