The Application of Ejaculate-Based Shotgun Proteomics for Male Infertility Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biospecimen Collection
2.2. Sample Preparation for LC–MS/MS
2.3. LC–MS/MS Analysis
2.4. Data Processing
3. Results and Discussion
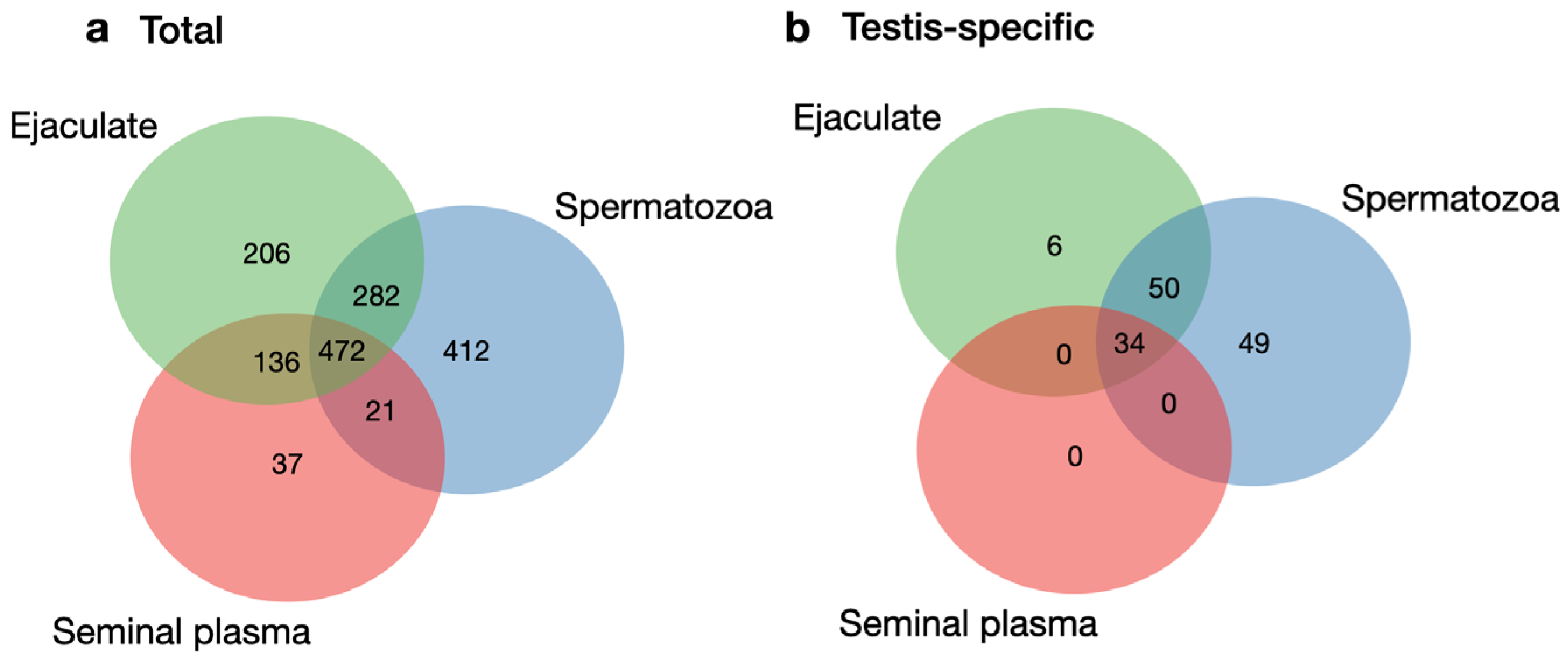
3.1. Comparison of the Ejaculate, Seminal Plasma, and Spermatozoa Proteomes
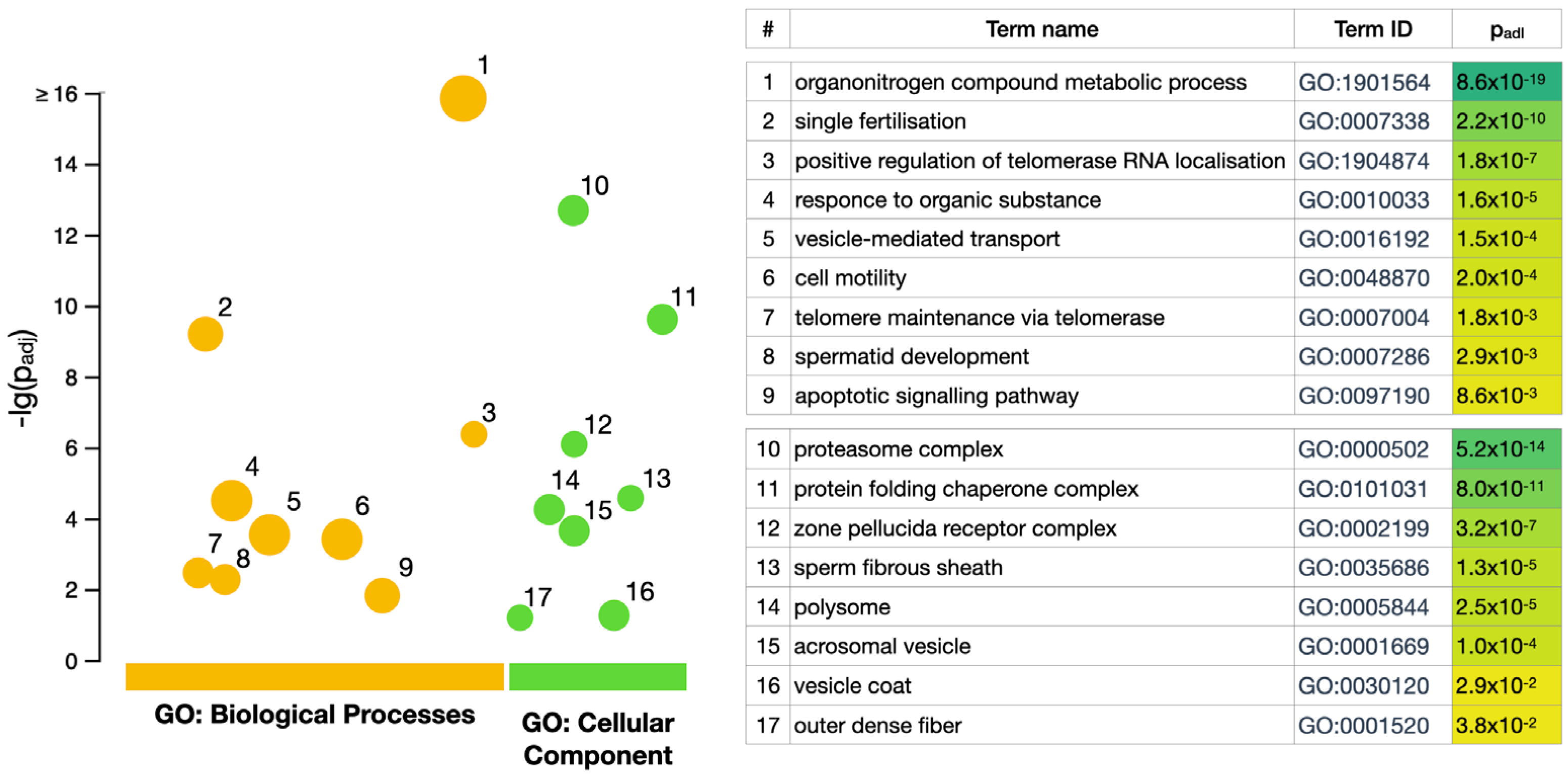
3.2. GO Enrichment Analysis
3.3. Testis-Specific Proteins
| Accession | Gene | Protein Name | Validated Unique Peptides | Spectrum Counting NSAF | Function/Disorder | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ejaculate | Plasma | Spermatozoa | Ejaculate | Plasma | Spermatozoa | ||||
| Q5JQC9 | AKAP4 | A-kinase anchor protein 4 | 53 | 33 | 58 | 1.322 | 0.655 | 2.928 | Major structural component of sperm fibrous sheath. Plays a role in sperm motility/male infertility [35,37,40] |
| O14556 | GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 13 | 10 | 32 | 1.264 | 0.747 | 3.647 | Required for sperm motility and male fertility/azoospermia [41] |
| Q9BS86 | ZPBP | Zona pellucida-binding protein 1 | 18 | 12 | 20 | 0.743 | 0.610 | 1.145 | Plays a role in acrosome compaction and sperm morphogenesis. Is implicated in sperm-oocyte interaction during fertilization/male infertility [35] |
| Q9BY14 | TEX101 | Testis-expressed protein 101 | 9 | 10 | 10 | 0.532 | 0.597 | 0.422 | Plays a role in fertilization by controlling binding of sperm to zona pellucida and migration of spermatozoa into the oviduct/azoospermia [38,42,43] |
| P54107 | CRISP1 | Cysteine-rich secretory protein 1 | 29 | 30 | 15 | 3.781 | 4.586 | 1.375 | May have a role in sperm-egg fusion and maturation [44] |
| P16562 | CRISP2 | Cysteine-rich secretory protein 2 | 11 | 30 | 8 | 0.49 | 0.383 | 0.474 | May regulate some ion channels’ activity and thereby regulate calcium fluxes during sperm capacitation [45] |
| P12273 | PIP | Prolactin-inducible protein | 65 | 62 | 47 | 28.654 | 32.801 | 14.785 | Regulation of immune system process [46] |
| P10323 | ACR | Acrosin | 9 | 8 | 12 | 0.489 | 0.489 | 0.762 | The major protease of mammalian spermatozoa/globozoospermia, spermatogenic failure 9 [36] |
| P04554 | PRM2 | Protamine-2 | 6 | nd | 13 | 0.338 | nd | 2.044 | Protamines substitute for histones in the chromatin of sperm during the haploid phase of spermatogenesis/male infertility, azoospermia [47] |
| P26436 | ACRV1 | Acrosomal protein SP-10 | 4 | 3 | 7 | 0.319 | 0.139 | 1.444 | Spermatogenesis (by similarity, [48]) |
| P54652 | HSPA2 | Heat shock-related 70 kDa protein | 41 | 22 | 26 | 0.704 | 0.541 | 0.743 | Plays a role in spermatogenesis. In association with SHCBP1L may participate in the maintenance of spindle integrity during meiosis in male germ cells/varicocele [49,50] |
| P63172 | DYNLT1 | Dynein light chain Tctex-type 1 | 2 | nd | 4 | 0.16 | nd | 0.311 | Acts as a motor for the intracellular retrograde motility of vesicles and organelles along microtubules [51] |
| Q14990 | ODF1 | Outer dense fiber of sperm tails 1 | 7 | 10 | 43 | 0.235 | 0.241 | 3.341 | Component of the outer dense fibers (ODF) of spermatozoa. ODF are filamentous structures located on the outside of the axoneme in the midpiece and principal piece of the mammalian sperm tail and may help to maintain the passive elastic structures and elastic recoil of the sperm tail/spermatogenic failure 9 [52] |
| Q9NQ60 | EQTN | Equatorin | 1 | nd | 2 | 0.029 | nd | 0.033 | Acrosomal membrane-anchored protein involved in the process of fertilization and in acrosome biogenesis [53] |
| Q17RY6 | LY6K | Lymphocyte antigen 6K | 2 | nd | nd | 0.034 | nd | nd | Required for sperm migration into the oviduct and male fertility through controlling binding of sperm to zona pellucida [38,39] |
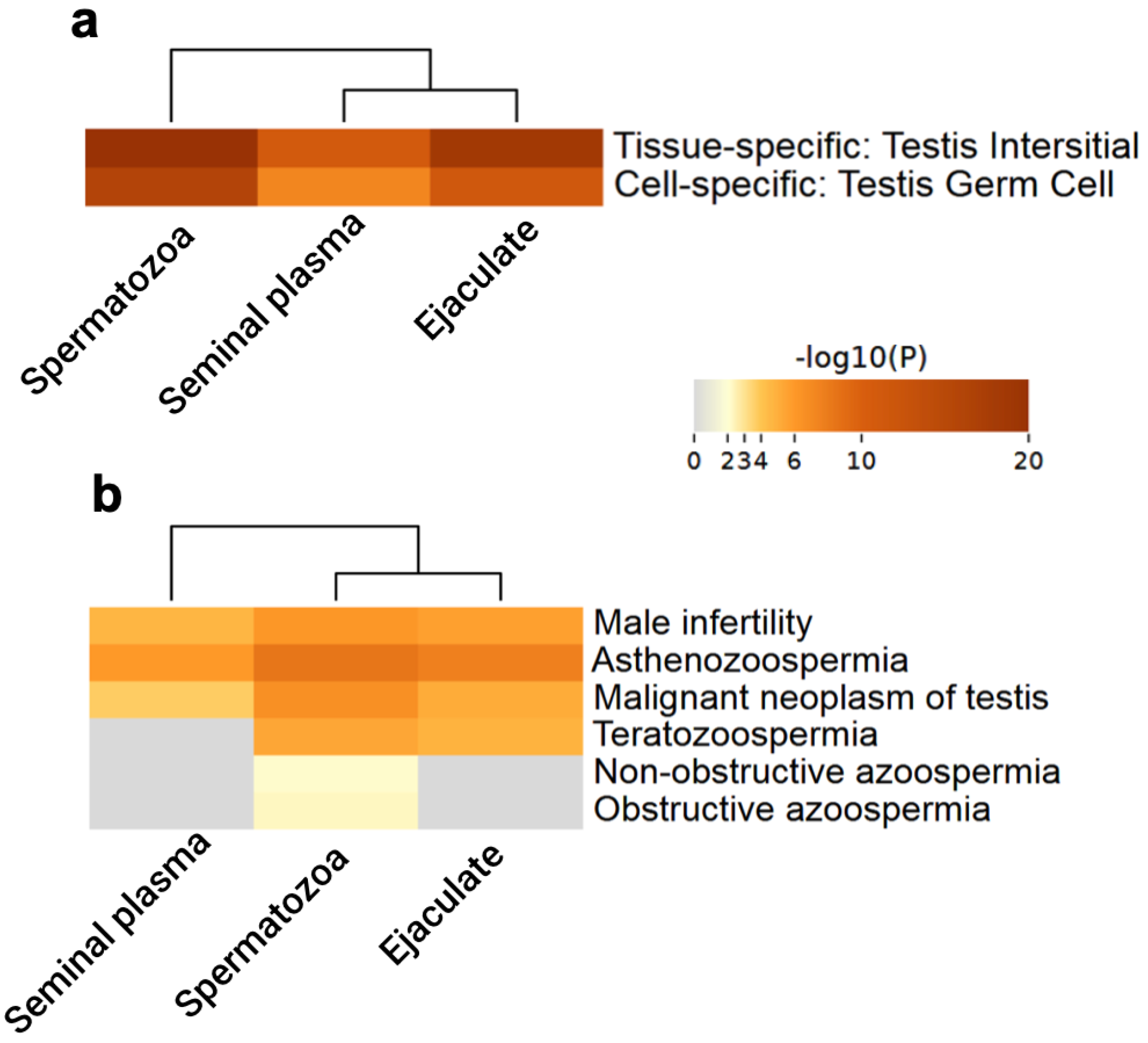
3.4. Functional Enrichment Analysis of Testis-Specific Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calogero, A.E.; Cannarella, R.; Agarwal, A.; Hamoda, T.A.; Rambhatla, A.; Saleh, R.; Boitrelle, F.; Ziouziou, I.; Toprak, T.; Gul, M.; et al. The Renaissance of Male Infertility Management in the Golden Age of Andrology. World J. Men’s Health 2023, 41, 237–254. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Manual for the Standardized Investigation, Diagnosis and Management of the Infertile Male, 1st ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Olefir, Y.V.; Vinogradov, I.V.; Rodionov, M.A.; Zhyvulko, A.R.; Popov, D.M.; Monakov, D.M. The Sixth Edition of the WHO laboratory manual for the examination and processing of human semen: Is everything new a well-forgotten old? Urol. Her. 2023, 11, 171–176. [Google Scholar] [CrossRef]
- Ding, J.; Shang, X.; Zhang, Z.; Jing, H.; Shao, J.; Fei, Q.; Rayburn, E.R.; Li, H. FDA-approved medications that impair human spermatogenesis. Oncotarget 2017, 8, 10714–10725. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A. Update on the proteomics of male infertility: A systematic review. Arab J. Urol. 2017, 16, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Roldan, E.R.S. Male fertility overview. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: New York, NY, USA, 2018; Volume 1, pp. 408–415. [Google Scholar]
- Intasqui, P.; Agarwal, A.; Sharma, R.; Samanta, L.; Bertolla, R.P. Towards the identification of reliable sperm biomarkers for male infertility: A sperm proteomic approach. Andrologia 2018, 50, e12919. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Panner Selvam, M.K.; Baskaran, S. Proteomic Analyses of Human Sperm Cells: Understanding the Role of Proteins and Molecular Pathways Affecting Male Reproductive Health. Int. J. Mol. Sci. 2020, 21, 1621. [Google Scholar] [CrossRef] [PubMed]
- Kopf, G.S. Preparation and analysis of semen samples. In In Vitro Fertilization and Embryo Transfer, 1st ed.; Wolf, D.P., Bavister, B.D., Gerrity, M., Kopf, G.S., Eds.; Springer: Boston, MA, USA, 1988. [Google Scholar]
- Orgebin-Crist, M.-C. Studies on the function of the epididymis. Biol. Reprod. 1969, 1, 155–175. [Google Scholar] [CrossRef]
- Dacheux, J.-L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2014, 147, R27–R42. [Google Scholar] [CrossRef]
- Jodar, M.; Soler-Ventura, A.; Oliva, R.; Molecular Biology of Reproduction and Development Research Group. Semen proteomics and male infertility. J. Proteom. 2017, 162, 125–134. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef]
- Barrachina, F.; Jodar, M.; Delgado-Dueñas, D.; Soler-Ventura, A.; Estanyol, J.M.; Mallofré, C.; Ballescà, J.L.; Oliva, R. Stable-protein pair analysis as a novel strategy to identify proteomic signatures: Application to seminal plasma from infertile patients. Mol. Cell. Proteom. 2019, 18, S77–S90. [Google Scholar] [CrossRef] [PubMed]
- Pilch, B.; Mann, M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006, 7, R40. [Google Scholar] [CrossRef] [PubMed]
- Zylbersztejn, D.S.; Andreoni, C.; Del Giudice, P.T.; Spaine, D.M.; Borsari, L.; Souza, G.H.M.F.; Bertolla, R.P.; Fraietta, R. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil. Steril. 2013, 99, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.W.; Thompson, H.S. Proteomics of semen and its constituents. Proteom. Clin. Appl. 2007, 1, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Durairajanayagam, D.; Sikka, S.C. Molecular Interactions Associated with Oxidative Stress-Mediated Male Infertility: Sperm and Seminal Plasma Proteomics. Adv. Exp. Med. Biol. 2022, 1358, 63–76. [Google Scholar] [PubMed]
- Ashwitha, A.; Ramesha, K.P.; Ramesh, P.; Kootimole, C.N.; Devadasan, M.J.; Ammankallu, S.; Jeyakumar, S.; Kumaresan, A.; Veerappa, V.G.; Das, D.N.; et al. Quantitative proteomics profiling of spermatozoa and seminal plasma reveals proteins associated with semen quality in Bos indicus bulls. J. Proteome Res. 2023, 273, 104794. [Google Scholar] [CrossRef] [PubMed]
- Shkrigunov, T.; Pogodin, P.; Zgoda, V.; Larina, O.; Kisrieva, Y.; Klimenko, M.; Latyshkevich, O.; Klimenko, P.; Lisitsa, A.; Petushkova, N. Protocol for Increasing the Sensitivity of MS-Based Protein Detection in Human Chorionic Villi. Curr. Issues Mol. Biol. 2022, 44, 2069–2088. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- ProteoWizard Home Page. Available online: https://proteowizard.sourceforge.io (accessed on 20 November 2023).
- Vaudel, M.; Barsnes, H.; Berven, F.; Sickmann, A.; Martens, L. SearchGUI: An open-source graphical user interface for simultaneous OMSSA and X!Tandem searches. Proteomics 2011, 11, 996–999. [Google Scholar] [CrossRef]
- Vaudel, M.; Burkhart, J.; Zahedi, R.; Oveland, E.; Berven, F.; Sickmann, A.; Martens, L.; Barsnes, H. PeptideShaker enables reanalysis of MS-derived proteomics data sets. Nat. Biotechnol. 2015, 33, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, P.; Pathan, M.; Chitti, S.V.; Kang, T.; Mathivanan, S. FunRich enables enrichment analysis of OMICs datasets. J. Mol. Biol. 2021, 433, 166747. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Kull, M.; Peterson, H.; Hansen, J.; Vilo, J. g:Profiler—A web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007, 35, W193–W200. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas: Tissue Atlas, Placenta-Specific Proteome. Available online: https://www.proteinatlas.org/humanproteome/tissue/placenta (accessed on 20 November 2023).
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.K.; Amjad, S.; Ryu, D.Y.; Adegoke, E.O.; Rahman, M.S.; Park, Y.J.; Pang, M.G. Establishment of a male fertility prediction model with sperm RNA markers in pigs as a translational animal model. J. Anim. Sci. Biotechnol. 2022, 13, 84. [Google Scholar] [CrossRef]
- Greither, T.; Schumacher, J.; Dejung, M.; Behre, H.M.; Zischler, H.; Butter, F.; Herlyn, H. Fertility Relevance Probability Analysis Shortlists Genetic Markers for Male Fertility Impairment. Cytogenet. Genome Res. 2020, 160, 506–522. [Google Scholar] [CrossRef]
- Corda, P.O.; Moreira, J.; Howl, J.; Oliveira, P.F.; Fardilha, M.; Silva, J.V. Differential Proteomic Analysis of Human Sperm: A Systematic Review to Identify Candidate Targets to Monitor Sperm Quality. World J. Men’s Health 2023. [Google Scholar] [CrossRef]
- Chaudhury, K.; Das, T.; Chakravarty, B.; Bhattacharyya, A.K. Acrosin activity as a potential marker for sperm membrane characteristics in unexplained male infertility. Fertil. Steril. 2005, 83, 104–109. [Google Scholar] [CrossRef]
- Miki, K.; Willis, W.D.; Brown, P.R.; Goulding, E.H.; Fulcher, K.D.; Eddy, E.M. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev. Biol. 2002, 248, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Yoshitake, H.; Tsukamoto, H.; Matsuura, H.; Kato, K.; Sakuraba, M.; Takamori, K.; Fujiwara, H.; Takeda, S.; Araki, Y. TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells. Sci. Rep. 2016, 6, 23616. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, Z.; Shen, C. An update of the regulatory factors of sperm migration from the uterus into the oviduct by genetically manipulated mice. Mol. Reprod. Dev. 2019, 86, 935–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, D.; Tu, C.; Meng, L.; Tan, Y.; Ji, Z.; Cheng, J.; Lu, G.; Lin, G.; Zhang, H.; et al. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum. Mol. Genet. 2021, 31, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Margaryan, H.; Dorosh, A.; Capkova, J.; Manaskova-Postlerova, P.; Philimonenko, A.; Hozak, P.; Peknicova, J. Characterization and possible function of glyceraldehyde-3-phosphate dehydrogenase-spermatogenic protein GAPDHS in mammalian sperm. Reprod. Biol. Endocrinol. 2015, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Drabovich, A.P.; Dimitromanolakis, A.; Saraon, P.; Soosaipillai, A.; Batruch, I.; Mullen, B.; Jarvi, K.; Diamandis, E.P. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci. Transl. Med. 2013, 5, 212ra160. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, K.A.; Schlegel, P.; Drabovich, A.; Lau, S.; Soosaipillai, A.; Korbakis, D.; Brinc, D.; Mullen, B.; Diamandis, E. Semen biomarker TEX101 predicts sperm retrieval success for men with testicular failure. F1000Research 2021, 10, 569. [Google Scholar] [CrossRef]
- Ernesto, J.I.; Weigel Muñoz, M.; Battistone, M.A.; Vasen, G.; Martínez-López, P.; Orta, G.; Figueiras-Fierro, D.; De la Vega-Beltran, J.L.; Moreno, I.A.; Guidobaldi, H.A.; et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell Biol. 2015, 210, 1213–1224. [Google Scholar] [CrossRef]
- Lim, S.; Kierzek, M.; O’Connor, A.E.; Brenker, C.; Merriner, D.J.; Okuda, H.; Volpert, M.; Gaikwad, A.; Bianco, D.; Potter, D.; et al. CRISP2 Is a Regulator of Multiple Aspects of Sperm Function and Male Fertility. Endocrinology 2019, 160, 915–924. [Google Scholar] [CrossRef]
- Chiu, W.W.; Chamley, L.W. Human seminal plasma prolactin-inducible protein is an immunoglobulin G-binding protein. J. Rep. Immunol. 2003, 60, 97–111. [Google Scholar] [CrossRef]
- Torregrosa, N.; Domínguez-Fandos, D.; Camejo, M.I.; Shirley, C.R.; Meistrich, M.L.; Ballescà, J.L.; Oliva, R. Protamine 2 precursors, protamine 1/protamine 2 ratio, DNA integrity and other sperm parameters in infertile patients. Hum. Reprod. 2006, 21, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Lalmansingh, A.S.; Urekar, C.J.; Reddi, P.P. TDP-43 is a transcriptional repressor: The testis-specific mouse acrv1 gene is a TDP-43 target in vivo. J. Biol. Chem. 2011, 286, 10970–10982. [Google Scholar] [CrossRef]
- Dix, D.J.; Allen, J.W.; Collins, B.W.; Mori, C.; Nakamura, N.; Poorman-Allen, P.; Goulding, E.H.; Eddy, E.M. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc. Natl. Acad. Sci. USA. 1996, 93, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Heat Shock Protein Family A (Hsp70) Member 2, Gene Cards—The Human Gene Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=HSPA2&keywords=Hspa2 (accessed on 6 December 2023).
- Indu, S.; Sekhar, S.C.; Sengottaiyan, J.; Kumar, A.; Pillai, S.M.; Laloraya, M.; Kumar, P.G. Aberrant Expression of Dynein light chain 1 (DYNLT1) is Associated with Human Male Factor Infertility. Mol. Cell. Proteom. 2015, 14, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Outer Dense Fiber of Sperm Tails 1, Gene Cards—The Human Gene Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ODF1&keywords=ODF1 (accessed on 6 December 2023).
- Equatorin, Gene Cards—The Human Gene Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=EQTN&keywords=EQTN (accessed on 6 December 2023).
- Pan, J.B.; Hu, S.C.; Shi, D.; Cai, M.C.; Li, Y.B.; Zou, Q.; Ji, Z.L. PaGenBase: A pattern gene database for the global and dynamic understanding of gene function. PLoS ONE 2013, 8, e80747. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; DeFalco, T. Essential roles of interstitial cells in testicular development and function. Andrology 2020, 8, 903–914. [Google Scholar] [CrossRef]
- Dobrinski, I. Germ cell transplantation. Semin. Reprod. Med. 2005, 23, 257–265. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Amer, M.K.; Mostafa, R.M.; Fathy, A.; Saad, H.M.; Mostafa, T. Ropporin gene expression in infertile asthenozoospermic men with varicocele before and after repair. Urology 2015, 85, 805–808. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, T.; Guo, J.; Zhou, Q.; Gu, Y.; Zhang, J.; Hu, L.; Zong, Y.; Song, J.; Zhang, S.; et al. Homozygous pathogenic variants in ACTL9 cause fertilization failure and male infertility in humans and mice. Am. J. Hum. Genet. 2021, 108, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Peng, J.; Long, X.; Li, J.; Wu, L.; Huang, K.; Zhu, X. Sperm Autoantigenic Protein 17 Predicts the Prognosis and the Immunotherapy Response of Cancers: A Pan-Cancer Analysis. Front. Immunol. 2022, 13, 844736. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, L.; Wei, H.; Chen, L.; Demir, Ö.; Sandusky, G.; Sun, E.; Wang, J.; Mo, J.; Zeng, L.; Fishel, M. Adapting AlphaLISA high throughput screen to discover a novel small-molecule inhibitor targeting protein arginine methyltransferase 5 in pancreatic and colorectal cancers. Oncotarget 2017, 8, 39963–39977. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Kurth, F.; Kappert, K.; Horst, D.; Mülleder, M.; Hartmann, G.; Ralser, M. Quantitative protein biomarker panels: A path to improved clinical practice through proteomics. EMBO Mol. Med. 2023, 15, e16061. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkrigunov, T.; Zgoda, V.; Klimenko, P.; Kozlova, A.; Klimenko, M.; Lisitsa, A.; Kurtser, M.; Petushkova, N. The Application of Ejaculate-Based Shotgun Proteomics for Male Infertility Screening. Biomedicines 2024, 12, 49. https://doi.org/10.3390/biomedicines12010049
Shkrigunov T, Zgoda V, Klimenko P, Kozlova A, Klimenko M, Lisitsa A, Kurtser M, Petushkova N. The Application of Ejaculate-Based Shotgun Proteomics for Male Infertility Screening. Biomedicines. 2024; 12(1):49. https://doi.org/10.3390/biomedicines12010049
Chicago/Turabian StyleShkrigunov, Timur, Victor Zgoda, Peter Klimenko, Anna Kozlova, Maria Klimenko, Andrey Lisitsa, Mark Kurtser, and Natalia Petushkova. 2024. "The Application of Ejaculate-Based Shotgun Proteomics for Male Infertility Screening" Biomedicines 12, no. 1: 49. https://doi.org/10.3390/biomedicines12010049
APA StyleShkrigunov, T., Zgoda, V., Klimenko, P., Kozlova, A., Klimenko, M., Lisitsa, A., Kurtser, M., & Petushkova, N. (2024). The Application of Ejaculate-Based Shotgun Proteomics for Male Infertility Screening. Biomedicines, 12(1), 49. https://doi.org/10.3390/biomedicines12010049






