Exploring the Three-Dimensional Frontier: Advancements in MSC Spheroids and Their Implications for Breast Cancer and Personalized Regenerative Therapies
Abstract
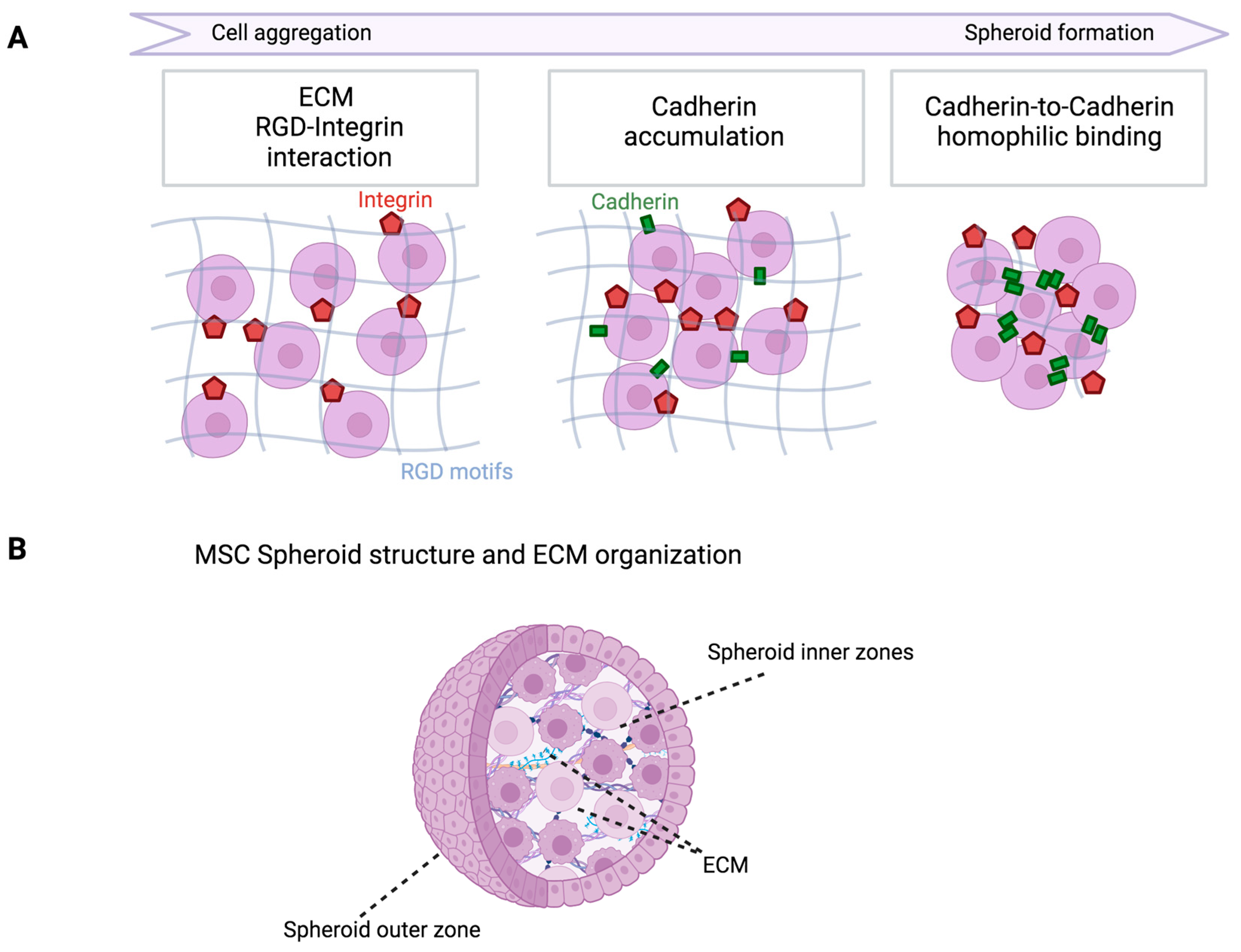
:1. Introduction
2. Three-Dimensional Models in Breast Cancer
2.1. MSCs as a Tool towards Novel Anti-Cancer Therapeutic Targets
2.2. MSC Secretome as a Co-Treatment with Traditional Therapy
2.3. Innovation of the Scaffold-Free, 3D Breast Organoid Model
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Crawford, A.; English, A.; Henshaw, K.; Mundy, J.; Corscadden, D.; Chapman, T.; Emery, P.; Hatton, P.; McGonagle, D. Synovial Fluid Mesenchymal Stem Cells in Health and Early Osteoarthritis: Detection and Functional Evaluation at the Single-Cell Level. Arthritis Rheum. 2008, 58, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Troyer, D.L. Stem Cells in the Umbilical Cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in Multicellular Spheroids Formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Bhang, S.H.; Cho, S.W.; La, W.G.; Lee, T.J.; Yang, H.S.; Sun, A.Y.; Baek, S.H.; Rhie, J.W.; Kim, B.S. Angiogenesis in Ischemic Tissue Produced by Spheroid Grafting of Human Adipose-Derived Stromal Cells. Biomaterials 2011, 32, 2734–2747. [Google Scholar] [CrossRef]
- Sarem, M.; Otto, O.; Tanaka, S.; Shastri, V.P. Cell Number in Mesenchymal Stem Cell Aggregates Dictates Cell Stiffness and Chondrogenesis. Stem Cell Res. Ther. 2019, 10, 10. [Google Scholar] [CrossRef]
- Shah, S.B.; Singh, A. Cellular Self-Assembly and Biomaterials-Based Organoid Models of Development and Diseases. Acta Biomater. 2017, 53, 29–45. [Google Scholar] [CrossRef]
- Li, M.; Izpisua Belmonte, J.C. Organoids—Preclinical Models of Human Disease. N. Engl. J. Med. 2019, 380, 569–579. [Google Scholar] [CrossRef]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell 2015, 16, 556–565. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How Mesenchymal Stem Cells Interact with Tissue Immune Responses. Art J. 2012, 59, 4. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-Inspired, Lipid-Based Boundary-Lubricated Hydrogels Counter-Surface Frictional Wear Newly-Exposed Gel Surface Counter-Surface Downloaded From. Science 2020, 370, 335–338. [Google Scholar] [CrossRef]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of Hyaline-like Cartilage Constructs Using Mesenchymal Stem Cell Sheets. Sci. Rep. 2020, 10, 20869. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kikuchi, A.; Aoyagi, T.; Okano, T. Cell Sheet Tissue Engineering: Cell Sheet Preparation, Harvesting/Manipulation, and Transplantation. J. Biomed. Mater. Res.-Part A 2019, 107, 955–967. [Google Scholar] [CrossRef]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. Cancer Cell 2015, 2, 1115–1127. [Google Scholar] [CrossRef]
- Centola, M.; Abbruzzese, F.; Scotti, C.; Barbero, A.; Vadalà, G.; Denaro, V.; Martin, I.; Trombetta, M.; Rainer, A.; Marsano, A. Scaffold-Based Delivery of a Clinically Relevant Anti-Angiogenic Drug Promotes the Formation of in Vivo Stable Cartilage. Tissue Eng.-Part A 2013, 19, 1960–1971. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing.Pdf. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Nguyen, D.; Hgg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef]
- Tsimbouri, P.M.; Childs, P.G.; Pemberton, G.D.; Yang, J.; Jayawarna, V.; Orapiriyakul, W.; Burgess, K.; González-García, C.; Blackburn, G.; Thomas, D.; et al. Stimulation of 3D Osteogenesis by Mesenchymal Stem Cells Using a Nanovibrational Bioreactor. Nat. Biomed. Eng. 2017, 1, 758–770. [Google Scholar] [CrossRef]
- Jacobi, N.; Smolinska, V.; Stierschneider, A.; Klein, C.; Önder, K.; Lechner, P.; Kaiser, H.; Hundsberger, H.; Eger, A.; Gesundheitsforschung, T. Development of Organotypic Cancer Models for the Identification of Individualized Cancer Therapies. In Proceedings of the Forschungsforum der Österreichischen Fachhochschulen 2016, Vienna, Austria, 2 October–2 November 2015; pp. 1–9. [Google Scholar]
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer Micrometastases. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef]
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F. Concise Review: Dissecting a Discrepancy in the Literature: Do Mesenchymal Stem Cells Support or Suppress Tumor Growth? Stem Cells 2011, 29, 11–19. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef]
- Saleh, F.A.; Genever, P.G. Turning Round: Multipotent Stromal Cells, a Three-Dimensional Revolution? Cytotherapy 2011, 13, 903–912. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paul Solomon, F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Aggeler, J.; Ram, T.G.; Bissell, M.J. Functional Differentiation and Alveolar Morphogenesis of Primary Mammary Cultures on Reconstituted Basement Membrane. Bone 1989, 23, 223–235. [Google Scholar] [CrossRef]
- Petersen, O.W.; Ronnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef]
- Simian, M.; Hirai, Y.; Navre, M.; Werb, Z.; Lochter, A.; Bissell, M.J. The Interplay of Matrix Metalloproteinases, Morphogens and Growth Factors Is Necessary for Branching of Mammary Epithelial Cells. Bone 2001, 23, 3117–3131. [Google Scholar] [CrossRef]
- Debnath, J.; Muthuswamy, S.K.; Brugge, J.S. Morphogenesis and Oncogenesis of MCF-10A Mammary Epithelial Acini Grown in Three-Dimensional Basement Membrane Cultures. Methods 2003, 30, 256–268. [Google Scholar] [CrossRef]
- Mirabdollahi, M.; Sadeghi-Aliabadi, H.; Javanmard, S.H. Human Wharton’s Jelly Mesenchymal Stem Cells-Derived Secretome Could Inhibit Breast Cancer Growth In Vitro and In Vivo. Iran. J. Basic Med. Sci. 2020, 23, 945–953. [Google Scholar] [CrossRef]
- Clarke, M.R.; Imhoff, F.M.; Baird, S.K. Mesenchymal Stem Cells Inhibit Breast Cancer Cell Migration and Invasion through Secretion of Tissue Inhibitor of Metalloproteinase-1 and -2. Mol. Carcinog. 2015, 54, 1214–1219. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ullah, M.; Zeitouni, S.; Beaver, J.; Prockop, D.J. Cancer Cells Enter Dormancy after Cannibalizing Mesenchymal Stem/Stromal Cells (MSCs). Proc. Natl. Acad. Sci. USA 2016, 113, E6447–E6456. [Google Scholar] [CrossRef]
- He, M.F.; Wang, S.; Wang, Y.; Wang, X.N. Modeling Cell-in-Cell Structure into Its Biological Significance. Cell Death Dis. 2013, 4, e630. [Google Scholar] [CrossRef]
- Overholtzer, M.; Mailleux, A.A.; Mouneimne, G.; Normand, G.; Schnitt, S.J.; King, R.W.; Cibas, E.S.; Brugge, J.S. A Nonapoptotic Cell Death Process, Entosis, That Occurs by Cell-in-Cell Invasion. Cell 2007, 131, 966–979. [Google Scholar] [CrossRef]
- Rastogi, V.; Sharma, R.; Misra, S.R.; Yadav, L.; Sharma, V. Emperipolesis–A Review. J. Clin. Diagn. Res. 2014, 8, ZM01–ZM02. [Google Scholar] [CrossRef]
- Pérez-Mancera, P.A.; Young, A.R.J.; Narita, M. Inside and out: The Activities of Senescence in Cancer. Nat. Rev. Cancer 2014, 14, 547–558. [Google Scholar] [CrossRef]
- Hoare, M.; Narita, M. Transmitting Senescence to the Cell Neighbourhood. Nat. Cell Biol. 2013, 15, 887–889. [Google Scholar] [CrossRef]
- Adinolfi, E.; Callegari, M.G.; Cirillo, M.; Pinton, P.; Giorgio, C.; Cavagna, D.; Rizzuto, R.; Di Virgilio, F. Expression of the P2X7 Receptor Increases the Ca2+ Content of the Endoplasmic Reticulum, Activates NFATc1, and Protects from Apoptosis. J. Biol. Chem. 2009, 284, 10120–10128. [Google Scholar] [CrossRef]
- Adinolfi, E.; Pizzirani, C.; Idzko, M.; Panther, E.; Norgauer, J.; Di Virgilio, F.; Ferrari, D. P2X7 Receptor: Death or Life? Purinergic Signal. 2005, 1, 219–227. [Google Scholar] [CrossRef]
- Maffey, A.; Storini, C.; Diceglie, C.; Martelli, C.; Sironi, L.; Calzarossa, C.; Tonna, N.; Lovchik, R.; Delamarche, E.; Ottobrini, L.; et al. Mesenchymal Stem Cells from Tumor Microenvironment Favour Breast Cancer Stem Cell Proliferation, Cancerogenic and Metastatic Potential, via Ionotropic Purinergic Signalling. Sci. Rep. 2017, 7, 13162. [Google Scholar] [CrossRef]
- McAndrews, K.M.; McGrail, D.J.; Ravikumar, N.; Dawson, M.R. Mesenchymal Stem Cells Induce Directional Migration of Invasive Breast Cancer Cells through TGF-β. Sci. Rep. 2015, 5, 16941. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Yi, J.; McGrail, D.J.; Dawson, M.R. Enhanced Adhesion of Stromal Cells to Invasive Cancer Cells Regulated by Cadherin 11. ACS Chem. Biol. 2015, 10, 1932–1938. [Google Scholar] [CrossRef]
- Cufí, S.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Martin-Castillo, B.; Joven, J.; Menendez, J.A. Metformin against TGFβ-Induced Epithelial-to-Mesenchymal Transition (EMT): From Cancer Stem Cells to Aging-Associated Fibrosis. Cell Cycle 2010, 9, 4461–4468. [Google Scholar] [CrossRef]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential Role of Mesenchymal Stem Cells (MSCs) in the Breast Tumour Microenvironment: Stimulation of Epithelial to Mesenchymal Transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]
- Chaffer, C.; Marjanovic, N.; Reinhardt, F.; Alessio, A.C.D.; Young, R.A.; Weinberg, R.A. Poised Chromatin at the ZEB1 Promoter Enables Cell Plasticity and Enhances Tumorigenicity. Cell 2013, 154, 61–74. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Otterbein, H.; Ungefroren, H.; Hass, R. Changes in uPA, PAI-1, and TGF-β Production during Breast Cancer Cell Interaction with Human Mesenchymal Stroma/Stem-like Cells (MSC). Int. J. Mol. Sci. 2019, 20, 2630. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Influence of Shaking Culture on the Biological Functions of Cell Aggregates Incorporating Gelatin Hydrogel Microspheres. J. Biosci. Bioeng. 2019, 128, 606–612. [Google Scholar] [CrossRef]
- Hayashi, K.; Tabata, Y. Preparation of Stem Cell Aggregates with Gelatin Microspheres to Enhance Biological Functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef]
- Barcellos-de-Souza, P.; Gori, V.; Bambi, F.; Chiarugi, P. Tumor Microenvironment: Bone Marrow-Mesenchymal Stem Cells as Key Players. Biochim. Et Biophys. Acta-Rev. Cancer 2013, 1836, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Tabata, Y. Immunosuppressive Mesenchymal Stem Cells Aggregates Incorporating Hydrogel Microspheres Promote an in Vitro Invasion of Cancer Cells. Regen. Ther. 2021, 18, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Henkle, S.L.; Betancourt, A.M. Mesenchymal Stem Cell 1 (MSC1)-Based Therapy Attenuates Tumor Growth Whereas MSC2-Treatment Promotes Tumor Growth and Metastasis. PLoS ONE 2012, 7, e45590. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-Dimensional Spheroid Cell Culture of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Leads to Enhanced Paracrine Induction of Wound Healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.; Liao, S.; Wang, W.; Wang, J.; Li, X.; Ding, Y.; Liang, Y.; Gao, F.; Yang, M.; et al. Potent Paracrine Effects of Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Attenuate Doxorubicin-Induced Cardiomyopathy. Sci. Rep. 2015, 5, 90. [Google Scholar] [CrossRef]
- Serras, A.S.; Camões, S.P.; Antunes, B.; Costa, V.M.; Dionísio, F.; Yazar, V.; Vitorino, R.; Remião, F.; Castro, M.; Oliveira, N.G.; et al. The Secretome of Human Neonatal Mesenchymal Stem Cells Modulates Doxorubicin-Induced Cytotoxicity: Impact in Non-Tumor Cells. Int. J. Mol. Sci. 2021, 22, 13072. [Google Scholar] [CrossRef]
- Djomehri, S.I.; Burman, B.; Gonzalez, M.E.; Takayama, S.; Kleer, C.G. A Reproducible Scaffold-Free 3D Organoid Model to Study Neoplastic Progression in Breast Cancer. J. Cell Commun. Signal. 2019, 13, 129–143. [Google Scholar] [CrossRef]

| Advantages of 3D MSC Spheroids | Disadvantages of Employing 3D Spheroids |
|---|---|
| Molecular and immunophenotypic characteristics of MSCs are enhanced | Size variability based on spheroid generation technique |
| Improved stemness and differentiation potential | Variabilities in the nutrients, waste, and oxygen concentration depending on spheroid size |
| Improved in vivo survival and homing after infusion | The possibility of necrotic spheroid core development |
| Improved secretion of anti-inflammatory, anti-fibrotic, anti-apoptotic, mitogenic, and angiogenic factors | The necessity of easy, repeatable, and affordable methods for the large-scale manufacturing of MSC spheroids to facilitate therapeutic needs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolinska, V.; Harsanyi, S.; Bohac, M.; Danisovic, L. Exploring the Three-Dimensional Frontier: Advancements in MSC Spheroids and Their Implications for Breast Cancer and Personalized Regenerative Therapies. Biomedicines 2024, 12, 52. https://doi.org/10.3390/biomedicines12010052
Smolinska V, Harsanyi S, Bohac M, Danisovic L. Exploring the Three-Dimensional Frontier: Advancements in MSC Spheroids and Their Implications for Breast Cancer and Personalized Regenerative Therapies. Biomedicines. 2024; 12(1):52. https://doi.org/10.3390/biomedicines12010052
Chicago/Turabian StyleSmolinska, Veronika, Stefan Harsanyi, Martin Bohac, and Lubos Danisovic. 2024. "Exploring the Three-Dimensional Frontier: Advancements in MSC Spheroids and Their Implications for Breast Cancer and Personalized Regenerative Therapies" Biomedicines 12, no. 1: 52. https://doi.org/10.3390/biomedicines12010052






